|
|
1.IntroductionEmotion is a key component in the regulatory system of stress responses, and the same environmental stressor can produce different somatic and psychological responses across different subjects.1 The prefrontal cortex (PFC) plays important roles in emotion and emotional regulation.2 According to the valence asymmetry hypothesis, the left/right asymmetry of the PFC activity is correlated with specific emotional responses to stressors and personality traits.3–5 Electroencephalography (EEG) has demonstrated that subjects with greater relative left PFC activity exhibit more positive and less negative dispositional mood6 than their right-dominant counterparts. In contrast, right frontally activated subjects respond more to negative affective challenges and less to positive affective challenges than their left dominant counterparts.7 Near-infrared spectroscopy (NIRS) has been established as a noninvasive method for monitoring neuronal activity by measuring changes of oxyhemoglobin (oxy-Hb) and deoxyhemoglobin (deoxy-Hb) concentrations in cerebral vessels.8 Changes in total hemoglobin (sum of oxy-Hb and deoxy-Hb; t-Hb) reflect cerebral blood volume changes,9 while changes in oxy-Hb correlate with changes in regional cerebral blood flow (rCBF).10 NIRS has been applied for various functional studies in normal adults, newborn infants, and patients with brain disorders.11–27 It was shown that differential regulation of somatic and psychological stress responses is mediated by asymmetry of PFC activity, based on NIRS evaluation of the right/left asymmetry of PFC activity in terms of the laterality index of activity {i.e., of oxy-Hb changes; LIA}. Significant positive relationships have been found between LIA and somatic and psychological stress responses.16–19,26 A number of studies have demonstrated correlations between electrical neural activity and change in blood oxygenation measured by NIRS. Simultaneous recording and analysis of NIRS and visual-evoked potential signals in humans has indicated that there is a strong linear correlation between hemodynamic changes and evoked potential amplitude.28 In addition, simultaneous measurement of NIRS and EEG in a resting condition demonstrated that an increase of oxy-Hb was associated with an increase of neuronal activity whereas a decrease of oxy-Hb was associated with a decrease of neuronal activity.29,30 In the present study, we hypothesized that asymmetry of NIRS-measured oxy-Hb changes at rest in the PFC may reflect asymmetry in PFC activity at rest and predict emotional state. In order to test this hypothesis, we have developed a new parameter, laterality index at rest (LIR), for assessment of the asymmetry of oxy-Hb changes in the PFC at rest. We investigated the correlation between LIR and anxiety level evaluated with the State-Trait Anxiety Inventory (STAI) test. We found that subjects with right-dominant oxy-Hb changes at rest showed higher STAI scores, while those with left-dominant oxy-Hb changes at rest showed lower STAI scores. Finally, we evaluated the relation between left/right asymmetry of the PFC activity at rest and during mental stress tasks and found a significant positive correlation between LIR and LIA. 2.Materials and Methods2.1.Subjects and Experimental ProtocolSubjects were 39 healthy adults (29 females; 10 males); 19 were 20 to 24 years old (13 females, 6 males) and 20 were 60 to 79 years old (16 females, 4 males), who had normal or corrected-to-normal vision. They completed questionnaires that provided information on history of drug use and physical and mental health status. Potential subjects who self-reported a substance abuse problem or other psychiatric disorder were excluded from the study. They were all right-handed as judged by the Edinburgh Handedness Inventory. Written informed consent was obtained from each subject on forms approved by the ethical committee of Nihon University School of Medicine. The ethical committee of Nihon University College of Engineering also approved this study. To avoid the influence of environmental stress, the subjects were seated in a comfortable chair in a regular room with good air conditioning throughout the experiments. One trial consisted of the following steps. First, each subject was instructed to think about nothing in particular for 3 min, and then to fill in the STAI; the STAI measures two types of anxiety; state anxiety (STAI-1) and trait anxiety (STAI-2). Higher scores are positively correlated with higher levels of anxiety. Next, after a preparation and calibration period (1 min), NIRS measurements were carried out for 3 min. 2.2.NIRS MeasurementsWe used a NIRS system (Pocket NIRS, Hamamatsu Photonics K.K., Japan) for measurements of the concentration changes of oxy-Hb, deoxy-Hb, and t-Hb in the PFC. This device employs a wireless communication system (Bluetooth®); the subject could move freely within a range of from the personal computer. It uses light emitting diodes of 3 different wavelengths (735, 810, and 850 nm) as light sources and one photo-diode as a detector, and has two channels. Two AAA batteries allow up to 8 h of continuous measurement for two-probe operation. The sampling rate was 61.3 Hz (i.e., the sampling time was about 16.3 ms). The concentration changes of hemoglobin were expressed in arbitrary units (a.u.). The NIRS probes were set symmetrically on the forehead with a flexible fixation pad, so that the midpoint between the emission and detection probes was 3 cm above the centers of the upper edges of the bilateral orbital sockets; the distance between the emitter and detector was set at 3 cm. This positioning is similar to positions Fp1 (left) and Fp2 (right) of the international electroencephalographic 10–20 system. Magnetic resonance imaging (MRI) confirmed that the emitter-detector was located over the dorsolateral and frontopolar areas of the PFC. 2.3.Data AnalysisNIRS signals were transformed to concentration changes of oxy-Hb, deoxy-Hb, and t-Hb using the modified Beer–Lambert law. In order to analyze left/right asymmetry of PFC activity at rest, we calculated the LIR as follows: Consider where and denote oxy-Hb concentration changes of the right and the left PFC in the resting condition. Taking into acount Eqs. (1) and (2), observe that and are always non-negative. Based on these quantities, we defined the LIR as follows:The numerator of Eq. (3) consists of the difference between the oxy-Hb concentration changes of the right and the left PFC summed over the analysis period (3 min). This quantity was normalized by the sum, instead of the difference of oxy-Hb concentration changes of the right and the left PFC. It should be noted that if we had used and per se, instead of the variations from their minimum values, then the denominator could be zero or near zero where the target quantity diverges. If the denominator becomes negative, it would be difficult to interpret the meaning of the parameter. The index defined by Eq. (3) provides values in the range of [, ]. A positive LIR indicates that the right PFC is more active at rest than the left PFC, on average, while a negative LIR indicates that the left PFC is more active at rest than the right PFC, on average. We then analyzed the relation between LIR and STAI-1 and -2 scores. Finally, we evaluated the relation between left/right asymmetry of the PFC activity at rest and during mental stress tasks in the young group. In order to determine left/right asymmetry of PFC activity during the stress task, we employed a mental arithmetic task as a psychological stressor. The subjects were asked to consecutively subtract a two-digit number from a four-digit number (e.g., 1022–13) as quickly as possible for 60 s. This mental arithmetic task has previously been used to investigate mental stress-induced PFC activity.16–19 In order to investigate the relationships between LIR and during the task and STAI scores, we examined the correlations between them by means of Pearson’s product-moment correlation or Spearman’s rank-order correlation analysis. NIRS data were averaged every second and baseline measurements were normalized to a 140-s segment for each trial. The cerebral blood oxygenation changes in the bilateral PFC were continuously monitored by NIRS during: (1) control conditions for 20 s; (2) the mental arithmetic task for 60 s; and (3) the recovery phase for 60 s. The mean control values (measured during the first 10 s) were subtracted from the mean activation values (measured throughout task performance). In order to determine left/right asymmetry of PFC activity during the stress task, we calculated a laterality index (LIA) for the oxy-Hb concentration changes ; indicates greater activity of the right PFC, while indicates greater activity of the left PFC.16–19 3.ResultsThe NIRS measurements indicated the presence of spontaneous oscillations of oxy-Hb concentration in the bilateral PFC at rest in all subjects. The amplitude of oxy-Hb concentration changes at rest varied among the subjects, with average values of () and () (a.u, , ). The anxiety levels of subjects before the “rest” task also varied among the subjects; the mean STAI-1 and -2 scores were and , respectively. We evaluated the relation between left/right asymmetry of the PFC activity at rest and STAI scores. Figure 1(a) shows the scatter plot of LIR against STAI score for all 39 subjects. There was a significant positive correlation between LIR and STAI-1 scores (, ). This suggests that the right PFC was more active at rest than that the left PFC, corresponding to a higher anxiety level. In contrast, the STAI-2 scores did not correlate with LIR [, , Fig. 1(b)]. Fig. 1Scatter plot of laterality index at rest (LIR) against STAI-1 (a) and STAI-2 (b). There was a significant positive correlation between LIR and STAI-1 scores (, ), but STAI-2 scores did not correlate with LIR (, ). Properties of a random variable may change under coordinate transformation. 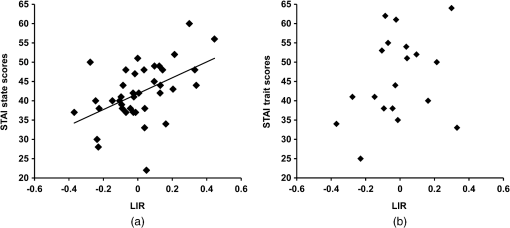 Then, we examined a possible effect of aging on the correlation between LIR and STAI-1 by analyzing the correlation in the young group (, 20 to 24 years) and the old group (, 60 to 79 years) separately. In the young group, there was a significant positive correlation between LIR and STAI-1 score (, ) [Fig. 2(a)]. In the aged group, we found a similar positive correlation between LIR and STAI-1 score (, ) [Fig. 2(b)]. Statistical analysis (Fisher’s -transformation) suggested that aging has no significant effect on the correlation (see Appendix). Fig. 2Effect of aging on the correlation between LIR and STAI-1. Both young (a) and aged (b) groups exhibited significant positive correlations between LIR and STAI-1 scores.  Finally, we evaluated the relation between left/right asymmetry of the PFC activity at rest and during the mental arithmetic task. First, we evaluated the relation between left/right asymmetry of the PFC activity during the mental arithmetic task (i.e., LIA) and STAI-1 scores in the young group [Fig. 3(a)]. There was a significant positive correlation between LIA and STAI-1 scores (, ). Then, we evaluated the relation between left/right asymmetry of the PFC activity at rest and during the mental arithmetic task [Fig. 3(b)]. We found a moderate, though nonsignificant, positive correlation between LIR and LIA (, ) by means of Spearman’s rank-order correlation analysis. Fig. 3(a) Relation between left/right asymmetry of the prefrontal cortex (PFC) activity during mental arithmetic task (i.e., LIA) and STAI-1 scores in the young group. (b) Relation between left/right asymmetry of the PFC activity at rest (i.e., LIR) and during the mental arithmetic task (i.e., LIA). 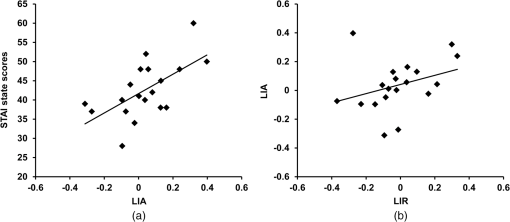 4.DiscussionIn the present study, we evaluated spontaneous oscillation of hemodynamics in the PFC by using NIRS. Spontaneous hemodynamic oscillation in the resting brain has previously been detected not only with NIRS31–34 but also with blood oxygen level-dependent functional MRI (fMRI).35–39 Although the origin and function of the spontaneous oscillation remain unclear, two possibilities have been proposed. First, a part of the spontaneous oscillations in the brain could originate from the global circulation.32–34 For example, Tong et al. found a strong correlation between spontaneous oscillations in the periphery and in the brain with varying time delays, indicating that some portion of the spontaneous oscillation reflect systemic physiological circulatory effects. In the present study, we evaluated asymmetry in the amplitude of oxy-Hb changes in the bilateral PFC in terms of LIR [see Eq. (3)]. Although systemic physiological circulatory conditions, such as heartbeats and respiration, could affect the amplitude of oxy-Hb changes at rest, LIR might not be significantly affected, since systemic physiological circulatory effects should be observed in both the right and left PFC equally. The second possibility is that the spontaneous oscillation of oxy-Hb might reflect resting state neural activity.29,31,35–39 In NIRS activation studies, changes of oxy-Hb during activation imply evoked changes of rCBF in response to neuronal activation, since changes in oxy-Hb are correlated with changes in rCBF.10 In addition, simultaneous measurements of NIRS and EEG at rest demonstrated a relationship between oxy-Hb change and mean EEG peak frequency.29 These observations indicate that changes of oxy-Hb concentration at rest measured by NIRS reflect neuronal activity at rest. In the present study, we evaluated asymmetry of the resting activity in the PFC in terms of LIR of oxy-Hb [see Eq. (3)]. We found a significant positive correlation between LIR and STAI-1 scores, indicating that subjects with right-dominant activity at rest (i.e., positive values of LIR) showed higher STAI scores, while those with left-dominant oxy-Hb changes at rest (i.e., negative values of LIR) showed lower STAI scores. Interestingly, there appeared to be a moderate, though nonsignificant, positive correlation between LIR and LIA, indicating that subjects with left (or right) dominant PFC activity at rest also exhibit left (or right) dominant PFC activity during the mental arithmetic task. This correlation between LIR and LIA was obtained by Spearman’s rank-order correlation analysis, and thus the putative relationship would be monotonic, not linear. Therefore, the trend of left/right asymmetry of PFC activity was maintained similarly at rest and during the mental arithmetic task. During NIRS measurements at rest, the subjects were instructed to rest quietly for 3 min without any task. In addition, in order to avoid the influence of environmental stress, the subjects were seated in comfortable chairs in a regular room with good air conditioning throughout the experiments. However, we cannot exclude the possibility that the subjects might have felt some degree of mental stress during NIRS measurements at rest, since participating in the experiment itself could be a stressor. Indeed, it is difficult to think about nothing during rest periods in task activation studies on mental stress, particularly for subjects with high anxiety levels who are poor at calming themselves during daily life. Interestingly, there was no correlation between LIR and STAI-2 scores, which reflect traits of anxiety. This suggests that asymmetry of PFC activity at rest reflects anxiety level only when PFC activity is measured. Further studies are necessary to evaluate dynamic changes in LIR and its relation with STAI. The present results are consistent with the valence asymmetry hypothesis; the left/right asymmetry of PFC activity is correlated with specific emotional responses to mental stress and personality traits.3–5 That is, induced negative affect increases relative right-sided PFC activation, while induced positive affect elicits an opposite pattern of asymmetric activation.6,7 In addition, patients with major depressive disorder exhibited reduced left frontal EEG activity in the resting state compared with normal controls, suggesting that asymmetry in PFC activity at rest measured by EEG is correlated with the emotional state.40 These results obtained by EEG are consistent with the present results obtained by NIRS study. Furthermore, the present results are also consistent with the hypothesis of a connection between bilateral frontal cortex activity and behavioral activation; i.e., the behavioral activation system and the behavioral inhibition system may be related to anterior asymmetry.41 The PFC plays an important role in mediating somatic responses to stress via projections to neuroendocrine and autonomic centers in the medial hypothalamus.42 Interestingly, right/left asymmetry of PFC activity also plays a role in regulation of stress response, which is similar to regulation of emotional responses. NIRS findings indicate that right dominant PFC activity during mental arithmetic tasks was associated with greater levels of activity of the neuroendocrine and autonomic nervous systems compared with those of left dominant PFC activity.16–19,26 However, it is not yet clear whether right/left asymmetry of PFC activity at rest correlates with the stress responses of the neuroendocrine and autonomic nervous systems. Further studies are needed to clarify these issues. We examined the effect of aging on the correlation between LIR and STAI-1. Both young and aged groups showed a significant positive correlation between LIR and STAI-1 score, and statistical analysis using Fisher’s -transformation suggested that aging has no significant effect on the correlation (see Appendix). Sakatani et al.19 evaluated the effect of aging on the correlation between LIA (i.e., LI of PFC activity during mental arithmetic tasks) and heart rate changes during task performance. They found that right PFC activity predominantly modulates sympathetic effects during the task in both young and aged groups, suggesting that aging did not affect the laterality of PFC activity in modulation of autonomic nervous system function. Finally, limitations of the present study should be discussed. First, NIRS parameter changes may be caused by changes in the blood flow of the scalp, since NIRS measures the blood oxygenation changes within the illuminated area, which includes both intracranial and extracranial tissues. Indeed, a recent study demonstrated that hemodynamic changes in the scalp were associated with functional activation tasks.43 In the present study, however, we evaluated asymmetry of oxy-Hb changes in the right and left PFC. Although blood oxygenation changes in the extracranial tissues could affect the oxy-Hb changes, it would be difficult to explain the relation between asymmetry of the oxy-Hb changes (LIR) and STAI scores only in terms of the extracranial factor. In addition, Tanida et al.18 observed minimal changes in the skin blood flow during mental arithmetic tasks using a thermograph. However, further studies are necessary to clarify whether these effects might have significantly influenced the present NIRS data. Second, NIRS does not allow the measurement of cerebral blood oxygenation changes in the whole brain. In the present study, we measured cerebral blood oxygenation changes only in the PFC; however, this may be reasonable, as the PFC plays important roles in emotion and emotional response. Third, NIRS analysis using the modified Beer–Lambert law only allows us to assess the combined effect of blood oxygenation changes and light path lengths. The observed asymmetry of the prefrontal fluctuation may reflect individual differences of the light path lengths in the right and left prefrontal areas. Although previous studies could not find a significant difference between these path lengths,14 it would be desirable to exclude any effect of the path lengths. Finally, a possible limitation of the present study is concerning the baseline period should be mentioned. Our baseline period (3 min) was relatively short compared with that used in studies on spontaneous hemodynamic oscillation (e.g., in the range of 0.01 to 0.15 Hz), which was evaluated by means of power spectral analysis.35–39 However, it should be noted that we analyzed averaged values of oxy-Hb changes with respect to the minimum values during the baseline period [see Eqs. (1) and (2)], and defined left/right asymmetry of activity (i.e., LIR) based on the averaged values [Eq. (3)]. We consider that this approach would have been adequate to evaluate the relationship between LIR and STAI, but we cannot rule out the possibility that the use of a longer baseline period might have influenced the observed relationship. In summary, we found that asymmetry of spontaneous oscillation of hemodynamic changes measured by NIRS in the PFC is correlated with STAI-1 (state anxiety) score but not STAI-2 (trait anxiety) score. The relation between left/right asymmetry of PFC activity and STAI-1 was maintained similarly at rest and during the mental arithmetic task. In addition, aging had no significant effect on the correlation. Thus, NIRS may be a useful tool for objective assessment of anxiety levels. AppendicesAppendixIn order to compare the two correlations (young group/aged group) in a statistical framework, we considered the following two hypotheses: We examined whether should be retained or rejected based on the data. In many hypothesis test arguments, the joint distribution of the target quantities is assumed to be Gaussian. This is also the assumption in this study. First, we must select a significance level. The value of 0.05 is often chosen and was set as the significance level here also. When sample size is small, which is the case with the present study, the sampling distribution of the Pearson correlation coefficient is skewed and is not Gaussian. A standard procedure to overcome this issue is to transform the correlations into Fisher ’s: where and stand for the correlation coefficients of the first and the second datasets (, ) and ln is the natural logarithm. This gives in the present study,It is known that the standard error of the difference between the two ’s is approximately given by where and are the number of samples for the first and second data sets. Since and , the -score or the test statistic for the present problem is given byFor the 0.05 significance level, the target interval of for is [, ]. The -score is well within this region. We conclude that the difference between the two correlations is not statistically significant. This suggests that aging has no significant effect on the correlation. AcknowledgmentsThis research was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan (B23300247), and grants from Alpha Electron Co., Ltd. (Fukushima, Japan) and Iing Co., Ltd. (Tokyo, Japan). ReferencesR. J. Davidson,
“Affective style and affective disorders: perspectives from affective neuroscience,”
Cognit. Emotion, 12
(3), 307
–330
(1998). http://dx.doi.org/10.1080/026999398379628 COEMEC 0269-9931 Google Scholar
R. J. Davidson,
“Cerebral asymmetry and emotion: conceptual and methodological conundrums,”
Cognit. Emotion, 7
(1), 115
–138
(1993). http://dx.doi.org/10.1080/02699939308409180 COEMEC 0269-9931 Google Scholar
R. J. DavidsonD. C. JacksonN. H. Kalin,
“Emotion, plasticity, cortex, and regulation: perspectives from affective neuroscience,”
Psychol. Bull., 126
(6), 890
–909
(2000). http://dx.doi.org/10.1037/0033-2909.126.6.890 PSBUAI 0033-2909 Google Scholar
T. Canliet al.,
“An fMRI study of personality influences on brain reactivity to emotional stimuli,”
Behav. Neurosci., 115
(1), 33
–42
(2001). http://dx.doi.org/10.1037/0735-7044.115.1.33 BENEDJ 0735-7044 Google Scholar
H. Fischeret al.,
“Right-sided human prefrontal brain activation during acquisition of conditioned fear,”
Emotion, 2
(5), 233
–241
(2002). http://dx.doi.org/10.1037/1528-3542.2.3.233 EBMOEN 1528-3542 Google Scholar
A. J. Tomarkenet al.,
“Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency,”
Psychophysiology, 29
(5), 576
–592
(1992). http://dx.doi.org/10.1111/psyp.1992.29.issue-5 PSPHAF 0048-5772 Google Scholar
R. E. WheelerR. J. DavidsonA. J. Tomarken,
“Frontal brain asymmetry and emotional reactivity: a biological substrate of affective style,”
Psychophysiology, 30
(1), 82
–89
(1993). http://dx.doi.org/10.1111/psyp.1993.30.issue-1 PSPHAF 0048-5772 Google Scholar
F. F. Jöbsis,
“Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,”
Science, 198
(4323), 1264
–1267
(1977). http://dx.doi.org/10.1126/science.929199 SCIEAS 0036-8075 Google Scholar
M. Ferrariet al.,
“Effects of graded hypotension on cerebral blood flow, blood volume, and mean transit time in dogs,”
Am. J. Physiol., 262
(6), H1908
–H1914
(1992). AJPHAP 0002-9513 Google Scholar
Y. HoshiN. KobayashiM. Tamura,
“Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model,”
J. Appl. Physiol., 90
(5), 1657
–1662
(2001). JAPYAA 0021-8987 Google Scholar
C. Hocket al.,
“Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study,”
J. Cereb. Blood Flow Metab., 15
(6), 1103
–1108
(1995). http://dx.doi.org/10.1038/jcbfm.1995.137 JCBMDN 0271-678X Google Scholar
Y. HoshiM. Tamura,
“Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man,”
Neurosci. Lett., 150
(1), 5
–8
(1993). http://dx.doi.org/10.1016/0304-3940(93)90094-2 NELED5 0304-3940 Google Scholar
T. Katoet al.,
“Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy,”
J. Cereb. Blood Flow Metab., 13
(3), 516
–520
(1993). http://dx.doi.org/10.1038/jcbfm.1993.66 JCBMDN 0271-678X Google Scholar
A. Kleinschmidtet al.,
“Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy,”
J. Cereb. Blood Flow Metab., 16
(5), 817
–826
(1996). http://dx.doi.org/10.1097/00004647-199609000-00006 JCBMDN 0271-678X Google Scholar
K. Sakataniet al.,
“Effects of aging on language-activated cerebral blood oxygenation changes of the left prefrontal cortex: near infrared spectroscopy study,”
J. Stroke Cerebrovasc. Dis., 8
(6), 398
–403
(1999). http://dx.doi.org/10.1016/S1052-3057(99)80047-0 1052-3057 Google Scholar
M. Tanidaet al.,
“Relation between asymmetry of prefrontal cortex activities and the autonomic nervous system during a mental arithmetic task: near infrared spectroscopy study,”
Neurosci. Lett., 369
(1), 69
–74
(2004). http://dx.doi.org/10.1016/j.neulet.2004.07.076 NELED5 0304-3940 Google Scholar
M. TanidaM. KatsuyamaK. Sakatani,
“Relation between mental stress-induced prefrontal cortex activity and skin conditions: a near infrared spectroscopy study,”
Brain Res., 1184 210
–216
(2007). http://dx.doi.org/10.1016/j.brainres.2007.09.058 BRREAP 1385-299X Google Scholar
M. TanidaM. KatsuyamaK. Sakatani,
“Effects of fragrance administration on stress-induced prefrontal cortex activity and sebum secretion in the facial skin,”
Neurosci. Lett., 432
(2), 157
–161
(2008). http://dx.doi.org/10.1016/j.neulet.2007.12.014 NELED5 0304-3940 Google Scholar
K. SakataniM. TanidaM. Katsuyama,
“Effects of aging on activity of the prefrontal cortex and autonomic nervous system during mental stress task,”
Adv. Exp. Med. Biol., 662 473
–478
(2010). http://dx.doi.org/10.1007/978-1-4419-1241-1 AEMBAP 0065-2598 Google Scholar
J. H. Meeket al.,
“Regional haemodynamic responses to visual stimulation in awake infants,”
Pediatr. Res., 43
(6), 840
–843
(1998). http://dx.doi.org/10.1203/00006450-199806000-00019 PEREBL 0031-3998 Google Scholar
K. Sakataniet al.,
“Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near-infrared spectroscopy,”
Early Hum. Dev., 55
(3), 229
–236
(1999). http://dx.doi.org/10.1016/S0378-3782(99)00019-5 EHDEDN 0378-3782 Google Scholar
K. Sakataniet al.,
“Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: a near infrared spectroscopy study,”
Stroke, 29
(7), 1299
–1304
(1998). http://dx.doi.org/10.1161/01.STR.29.7.1299 SJCCA7 0039-2499 Google Scholar
N. Fujiwaraet al.,
“Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors,”
NeuroImage, 21
(4), 1464
–1471
(2004). http://dx.doi.org/10.1016/j.neuroimage.2003.10.042 NEIMEF 1053-8119 Google Scholar
Y. Murataet al.,
“Increase in focal concentration of deoxyhemoglobin during neuronal activity in cerebral ischemic patients,”
J. Neurol. Neurosurg. Psychiatry, 73
(2), 182
–184
(2002). http://dx.doi.org/10.1136/jnnp.73.2.182 JNNPAU 0022-3050 Google Scholar
Y. Murataet al.,
“Decreases of blood oxygenation level dependent signal in the activated motor cortex during functional recovery after resection of a glioma,”
Am. J. Neuroradiol., 25
(7), 1242
–1246
(2004). Google Scholar
K. Sakatani,
“Optical diagnosis of mental stress: review,”
Adv. Exp. Med. Biol., 737 89
–95
(2012). http://dx.doi.org/10.1007/978-1-4614-1566-4 AEMBAP 0065-2598 Google Scholar
D.A. Boaset al.,
“Twenty years of functional near-infrared spectroscopy: introduction for the special issue,”
Neuroimage, 85 1
–5
(2014). http://dx.doi.org/10.1016/j.neuroimage.2013.11.033 NEIMEF 1053-8119 Google Scholar
L. Rovatiet al.,
“Optical and electrical recording of neural activity evoked by graded contrast visual stimulus,”
BioMed. Eng. OnLine, 6 28
(2007). http://dx.doi.org/10.1186/1475-925X-6-28 1475-925X Google Scholar
Y. Hoshiet al.,
“Relationship between fluctuations in the cerebral hemoglobin oxygenation state and neuronal activity under resting conditions in man,”
Neurosci. Lett., 245
(3), 147
–150
(1998). http://dx.doi.org/10.1016/S0304-3940(98)00197-9 NELED5 0304-3940 Google Scholar
M. Buttiet al.,
“Multimodal analysis of a sustained attention protocol: continuous performance test assessed with near infrared spectroscopy and EEG,”
in Proc. 28th IEEE Eng. Med. Biol. Soc. Ann. Int. Conf.,
1040
–1043
(2006). Google Scholar
Y. HoshiM. Tamura,
“Fluctuations in the cerebral oxygenation state during the resting period in functional mapping studies of the human brain,”
Med. Biol. Eng. Comput., 35
(4), 328
–330
(1997). http://dx.doi.org/10.1007/BF02534085 MBECDY 0140-0118 Google Scholar
M. L. Pierroet al.,
“Phase-amplitude investigation of spontaneous low-frequency oscillations of cerebral hemodynamics with near-infrared spectroscopy: a sleep study in human subjects,”
NeuroImage, 63
(3), 1571
–1584
(2012). http://dx.doi.org/10.1016/j.neuroimage.2012.07.015 NEIMEF 1053-8119 Google Scholar
L. Minatiet al.,
“Intra- and extra-cranial effects of transient blood pressure changes on brain near-infrared spectroscopy (NIRS) measurements,”
J. Neurosci. Methods, 197
(2), 283
–288
(2011). http://dx.doi.org/10.1016/j.jneumeth.2011.02.029 JNMEDT 0165-0270 Google Scholar
Y. TongB. D. Frederick,
“Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain,”
NeuroImage, 53
(2), 553
–564
(2010). http://dx.doi.org/10.1016/j.neuroimage.2010.06.049 NEIMEF 1053-8119 Google Scholar
Y. Tonget al.,
“Low-frequency oscillations measured in the periphery with near-infrared spectroscopy are strongly correlated with blood oxygen level-dependent functional magnetic resonance imaging signals,”
J. Biomed. Opt., 17
(10), 106004
(2012). http://dx.doi.org/10.1117/1.JBO.17.10.106004 JBOPFO 1083-3668 Google Scholar
B. Biswalet al.,
“Functional connectivity in the motor cortex of resting human brain using echo-planar MRI,”
Magn. Reson. Med., 34
(4), 537
–541
(1995). http://dx.doi.org/10.1002/(ISSN)1522-2594 MRMEEN 0740-3194 Google Scholar
M. J. LoweB. J. MockJ. A. Sorenson,
“Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations,”
NeuroImage, 7
(2), 119
–132
(1998). http://dx.doi.org/10.1006/nimg.1997.0315 NEIMEF 1053-8119 Google Scholar
M. D. Greiciuset al.,
“Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI,”
Proc. Natl. Acad. Sci. U.S.A., 101
(13), 4637
–4642
(2004). http://dx.doi.org/10.1073/pnas.0308627101 PNASA6 0027-8424 Google Scholar
A. Anandet al.,
“Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression,”
Psychiatry Res., 171
(3), 189
–198
(2009). http://dx.doi.org/10.1016/j.pscychresns.2008.03.012 PSRSDR 0165-1781 Google Scholar
A. H. Kempet al.,
“Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder,”
Biol. Psychol., 85
(2), 350
–354
(2010). http://dx.doi.org/10.1016/j.biopsycho.2010.08.001 BLPYAX 0301-0511 Google Scholar
J. Hewiget al.,
“The relation of cortical activity and BIS/BAS on the trait level,”
Biol. Psychol., 71
(1), 42
–53
(2006). http://dx.doi.org/10.1016/j.biopsycho.2005.01.006 BLPYAX 0301-0511 Google Scholar
C. TsigosG. P. Chrousos,
“Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress,”
J. Psychosomatic Res., 53
(4), 865
–871
(2002). http://dx.doi.org/10.1016/S0022-3999(02)00429-4 JPCRAT 0022-3999 Google Scholar
E. Kirilinaet al.,
“The physiological origin of task-evoked systemic artifacts in functional near infrared spectroscopy,”
NeuroImage, 61
(1), 70
–81
(2012). http://dx.doi.org/10.1016/j.neuroimage.2012.02.074 NEIMEF 1053-8119 Google Scholar
BiographyWakana Ishikawa received her BS in electrical engineering and bioscience and her MS in electrical engineering and bioscience from Waseda University, Tokyo, Japan. She has been interested in the analysis of NIRS data for brain-computer-interface based on Bayesian machine learning and prediction algorithms with Monte Carlo implementations. Masakaze Sato received his BS in electrical engineering and bioscience and his MS in electrical engineering and bioscience from Waseda University, Tokyo, Japan. He has been interested in the analysis of NIRS data for brain-computer-interface based on Bayesian machine learning and prediction algorithms with Monte Carlo implementations. Yukikatsu Fukuda received his BS in electrical engineering and bioscience from Waseda University, Tokyo, Japan, in 2012 and is currently pursuing his MS degree. His current research interests include Bayesian machine learning for prediction problems with Monte Carlo implementations, using near infrared spectroscopy time series data. Takashi Matsumoto received his BS in electrical engineering from Waseda University, Tokyo, Japan, his MS in applied mathematics from Harvard University, Cambridge, Massachusetts, and his PhD in electrical engineering, from Waseda. One of his recent interests is analyzing data from humans using statistical machine learning algorithms. In particular, he has been conducting research on analysis of NIRS as well EEG data for brain-computer-interface based on Bayesian learning/prediction algorithms and bootstrapping. NaohiroTakemura received his BA in engineering and his MS in informatics from Kyoto University, Japan. He has been engaged in neural network and computational modeling of human movement. His current interest is investigating mechanisms of human mental states by integrating findings from the fields of neuroscience, psychology, and informatics. Kaoru Sakatani received his MD from Osaka Medical College, Osaka, Japan, in 1981, the PhD in medicine from the graduate school of Osaka Medical College (1987), and the PhD in engineering from the Graduate School of Hokkaido University, Sapporo, Japan, in 1998. He is currently a professor with Nihon University College of Engineering and School of Medicine. He is a board-certified neurosurgeon in Japan and his research interests include biomedical engineering, optical engineering, neuroimaging, and neuroscience |

