|
|
|
After the first use of a surgical microscope in clinics by otolaryngologists in the early 20th century, the surgical microscope was regarded as an essential tool in an operating room.1 Despite significant advances in the surgical microscope technique, typically, the magnified surface image can only be provided by the microscope, resulting in missing subsurface information. However, noninvasive visualization of the subsurface during surgeries is crucial in some clinical applications, such as ophthalmic surgery and neurosurgery.2,3 However, due to the limitations, current surgical procedures heavily rely on the surgeons’ experience. For instance, during penetrating keratoplasty, surgeons typically incise the cornea at 560 μm from the epithelium layer under the guidance of a conventional surgical microscope. Because the surgeons are not able to visualize the clear corneal layers using the surgical microscope, severe side effects, such as corneal perforation and consequent poor eyesight recovery, can be caused if the cross-section area of cornea is inhomogeneous.4,5 Optical coherence tomography (OCT) was first introduced from MIT in the early 1990s.6 The principle of OCT is based on a low-coherence Michelson interferometer, and thus, OCT can provide cross-sectional images of microstructures in biological tissues. OCT offers high-resolution, noninvasive, nondestructive, and real-time imaging capabilities. Many studies have shown that OCT is a powerful tool in ophthalmology, cardiology, gastroenterology, oncology, dermatology, and dentistry.7 As another application, OCT has been used to overcome and/or assist the restriction of the current surgical microscope. In 2005, OCT was utilized as a surgical tool for anterior segment surgery.8 In addition, a handheld OCT probe has been used for macular surgery in 2009.9 However, these systems have only been used to monitor the operating regions not during the surgeries but before and after, resulting in missing the real-time information of lesions. Recently, an integrated OCT and surgical microscope system has been developed to guide viteroretinal surgery in real time.10,11 Further, a real-time intraoperative OCT system with a graphics processing unit (GPU) was utilized in microsurgery guidance.12,13 Although these integrated systems offered an opportunity to monitor the surgical process in real time, they did not provide the OCT and microscope images at the same time on the same view. Thus, an additional display tool for the OCT images was required, which was cumbersome during the operation. In this paper, we developed virtual intraoperative surgical OCT (VISOCT) by combining commercial clinical surgical microscope and spectral-domain OCT (SD-OCT). The VISOCT system could simultaneously acquire, process, and display OCT images through a GPU. The processed OCT images were projected back onto the microscope view plane via our homemade optical systems, and the images were visualized through the ocular lenses mounted on the microscope, not through a tabletop display. In our approach, no additional display to show OCT images is required, and the surgical procedures can be much simpler and truly real-time compared to the existing approach. We have successfully monitored and conducted stimulated penetrating keratoplasty of a live rabbit using the VISOCT system in vivo. Potentially, our VISOCT system can accurately guide surgeries in real time in clinics. Figure 1(a) shows the experimental setup of our VISOCT system. First, the SD-OCT system was composed of an optical fiber-based Michelson interferometer using a broadband superluminescent diode [(SLD), SLD-34-HP, Superlum, Carrigtwohill, Ireland] with a center wavelength of 850 nm and a bandwidth of 50 nm. The SLD light was split into reference and sample arms through a optical fiber coupler (FC850-40-10-APC, Thorlabs, Newton, New Jersey). A spectrometer consisted of a transmission type diffraction grating (1800 Ipmm, Wasatch photonics, Logan, Utah), a focusing lens (AC508-075-B, Thorlabs), and a collimator. Interference OCT signals were acquired by a 12-bit line scan CMOS camera with 4096 pixels (Sprint SPL4096-140K, Balser, Ahrensburg, Germany). We utilized a full-range k-linearization method to compensate for the spectrometer’s nonlinearity.14 The OCT images were acquired by a frame grabber (PCIe-1429, National Instruments, Austin, Texas). Axial and lateral resolutions were 8.7 and 30.2 μm, respectively. Fig. 1(a) Experimental setup of a real-time virtual intraoperative surgical optical coherence tomography (VISOCT) microscope. (b) Photograph of the VISOCT probe. OL, objective lens; L, lens; PC, polarization controller; DM, dichroic mirror; G, galvo scanner; BS, beam splitter; C, collimator; M, mirror; NF, neutron-density filter; BP, beam projector; SM, surgical microscope. 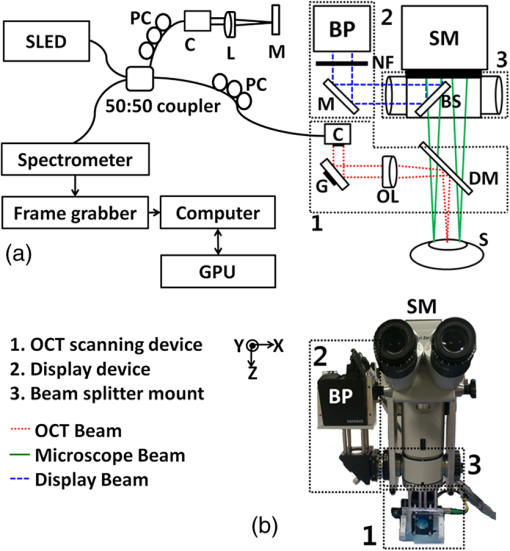 Then, we built the VISOCT microscope by adapting a commercial ophthalmic surgical microscope as shown in Fig. 1(a). The VISOCT system comprised three main parts: (1) OCT scanning, (2) display, and (3) beam splitting subsystems. Each subsystem is indicated with numbers 1, 2, and 3, respectively. The corresponding photograph is shown in Fig. 1(b). The OCT scanning subsystem 1 contained a collimator, galvo scanner (GVS001, Thorlabs), objective lens (AC508-075-B, Thorlabs), and dichroic mirror (NT55-233, Edmund, Barrington, New Jersey). The dichroic mirror (750 to 1125 nm) was designed to reflect near-infrared light (i.e., OCT light). Thus, the reflected visible light easily transmitted through the dichroic mirror and an ocular lens, and was visualized by eyes. The display subsystem 2 containing a beam projector (SP-H03, Samsung, Seoul, South Korea) and the beam splitting subsystem 3 were designed to project the OCT image back onto the microscopic view plane via the microscope ocular lens. A beam splitter was located inside a custom-made mount adapted with a standard microscopy mount. To display the OCT images in real time, we coded image processing software based on both a GPU (Geforce GTX480, NVIDIA, Santa Clara, California) and central processing unit (Core 2 Quad Processor Q8200, Intel, Santa Clara, California). The image processing duties, such as k-domain linearization, background removal, fast Fourier transformation, and log scaling processes, were performed in the GPU using 480 Compute Unified Device Architecture processors. It was programmed by a C++ programming language. The OCT images with along and direction, respectively, were recorded at a frame rate of 102 Hz.15 To demonstrate the performance of the real-time VISOCT system in vivo, we performed simulated penetrating keratoplasty by incising with a surgical blade [Figs. 2(a) and 2(b), Video 1] and suturing with a surgical needle and thread [Figs. 2(c) to 2(f), Video 2 and 3] in the cornea of a rabbit. All animal experimental procedures were conducted under the laboratory animal protocol permitted by the institutional animal care and use committee. A healthy rabbit (Oryctolagus cuniculus) weighing was utilized for the in vivo animal experiments. The rabbit was anesthetized by intravenous injection of ketamine ( body weight). The scanning range of one B-scan OCT image is 20 mm along the direction, while the field of view of the conventional microscopy images is along the and axes, respectively. Fig. 2Real-time VISOCT in vivo for stimulated penetrating keratoplasty. (a), (c), and (e) are the screenshots, acquired via ocular lens, of overlaid OCT and surgical microscopy images of the presented stimulated keratoplasty procedure in the right cornea of the rabbit (Video 1, MPEG, 3.92 MB) [URL: http://dx.doi.org/10.1117/1.JBO.19.3.030502.1], (Video 2, MPEG, 6.17 MB) [URL: http://dx.doi.org/10.1117/1.JBO.19.3.030502.2], and (Video 3, MPEG, 5.62 MB) [URL: http://dx.doi.org/10.1117/1.JBO.19.3.030502.3]). (b), (d), and (f) are the magnified OCT B-scan image cut from (a), (c), and (e), respectively. MIC, microscope; EP, epithelium; STR, stroma; EN, endothelium; IS, incision site; I, iris; F, forceps; N, needle; ST, surgical thread. 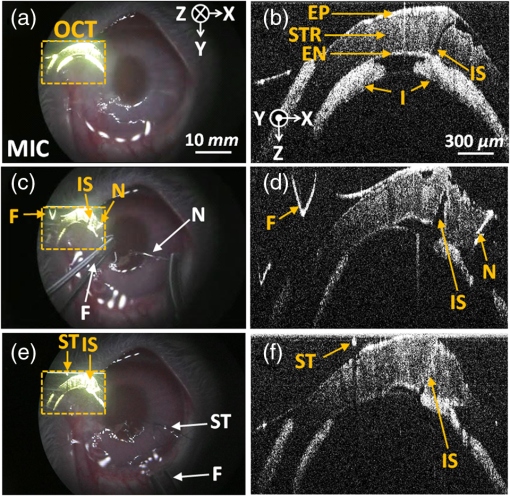 Figures 2(a), 2(c), and 2(e) indicate the screenshots obtained via the ocular lens during the surgery, while Figs. 2(b), 2(d), and 2(f) show the magnified OCT images of them, respectively. First, the cornea incision was processed by the surgical blade. As shown in Video 1, the OCT image was clearly back-projected onto the left side in the microscopic view plane in real time, and we easily incised the rabbit cornea while simultaneously monitoring the cornea structures and the magnified microscope images. After incising the cornea, watery fluid of the anterior chamber came out immediately. Then, an iris and cornea were attached. Moreover, the condition of the incised area was clearly visualized in the OCT image in real time [Figs. 2(a) and 2(b)]. Second, the incised area was sutured by the surgical needle and thread. As shown in Video 2, thanks to the real-time OCT image in the surgical view, we correctly aligned a forceps on the incised cornea layer. Then, we punctured the cornea layer using the surgical needle and thread [Figs. 2(c) and 2(d)]. The OCT image provided the correct position information of the cornea layer and precisely guided the needle insertion. Finally, under the guidance of real-time OCT imaging and display, the surgical thread was precisely strung together. As shown in Video 3, the OCT image showed the alignment of the cornea layer by tightening the threads [Figs. 2(e) and 2(f)]. We could control the strength of a knot based on the real-time OCT and finished the surgery by cutting the remaining thread. Note that the OCT image provided subsurface information of the cornea structures and the movement of the surgical instruments, whereas the microscopy image supplied only surface information. These results imply that our VISOCT system will be extremely useful in the practical surgical operating situation by minimizing unnecessary procedures during the surgeries. In this study, we demonstrated a new concept of real-time VISOCT system for real-time OCT image projection on the microscopic view plane via the surgical ocular lens. The performance of VISOCT system was validated by showing a simple surgery in vivo. We believe that these developments will be crucial to enhance the usability of the surgical OCT system in the real surgical operating environment. Currently, our VISOCT system shows only two-dimensional B-mode OCT images. In this case, it is possible to miss the correct surgical position of the needle of blade. Future possible solutions are as follows: (1) We will add an aiming beam to guide the needle or blade intervention. (2) We will provide real-time three-dimensional OCT images by enhancing the image acquisition and display rates. Then, the VISOCT system would be significantly beneficial in neuroscience and ophthalmology. AcknowledgmentsThis work was supported in part by the Korea healthcare technology R&D Project (A102024-1011-0000200), Ministry of Health & Welfare, Leading Industry Development for Economic Region Project, MKE, KAIT, Dae-Gyeong Leading Industry Office, and National Institute of Health (NIBIB Bioengineering Research Partnership R01 EB013723, S. A. B) to J.K. This work was supported in part by NRF grant of Korea government (MSIP, Ministry of Science, ICT and Future Planning) (2011-0030075), and MSIP under the “IT Consilience Creative Program” (NIPA-2013-H0203-13-1001) supervised by the National IT Industry Promotion Agency to C.K. ReferencesT. C. KrissV. M. Kriss,
“History of the operating microscope: from magnifying glass to microneurosurgery,”
Neurosurgery, 42
(4), 899
–907
(1998). http://dx.doi.org/10.1097/00006123-199804000-00116 NEQUEB 0148-396X Google Scholar
S. A. Boppartet al.,
“Optical coherence tomography for neurosurgical imaging of human intracortical melanoma,”
Neurosurgery, 43
(4), 834
–841
(1998). http://dx.doi.org/10.1097/00006123-199810000-00068 NEQUEB 0148-396X Google Scholar
P. Hahnet al.,
“The use of optical coherence tomography in intraoperative ophthalmic imaging,”
Ophthalmic Surg. Lasers Imaging, 42
(4), S85
–94
(2011). http://dx.doi.org/10.3928/15428877-20110627-08 1542-8877 Google Scholar
O. Pineroset al.,
“Long-term results after penetrating keratoplasty for Fuchs’ endothelial dystrophy,”
Arch. Ophthalmol., 114
(1), 15
–18
(1996). http://dx.doi.org/10.1001/archopht.1996.01100130013002 AROPAW 0003-9950 Google Scholar
G. R. Melleset al.,
“Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy,”
Am. J. Ophthalmol., 127
(3), 340
–341
(1999). http://dx.doi.org/10.1016/S0002-9394(98)00324-9 AJOPAA 0002-9394 Google Scholar
D. Huanget al.,
“Optical coherence tomography,”
Science, 254
(5035), 1178
–1181
(1991). http://dx.doi.org/10.1126/science.1957169 SCIEAS 0036-8075 Google Scholar
B. E. BoumaG. J. Tearney, Handbook of Optical Coherence Tomography, Marcel Dekker, New York
(2002). Google Scholar
G. Geerlinget al.,
“Intraoperative 2-dimensional optical coherence tomography as a new tool for anterior segment surgery,”
Arch. Ophthalmol., 123
(2), 253
–257
(2005). http://dx.doi.org/10.1001/archopht.123.2.253 AROPAW 0003-9950 Google Scholar
P. N. Dayaniet al.,
“Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery,”
Retina, 29
(10), 1457
–1468
(2009). http://dx.doi.org/10.1097/IAE.0b013e3181b266bc RETIDX 0275-004X Google Scholar
J. P. Ehlerset al.,
“Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging,”
Invest. Ophthalmol. Vis. Sci., 52
(6), 3153
–3159
(2011). http://dx.doi.org/10.1167/iovs.10-6720 IOVSDA 0146-0404 Google Scholar
Y. K. Taoet al.,
“Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery,”
Opt. Lett., 35
(20), 3315
–3317
(2010). http://dx.doi.org/10.1364/OL.35.003315 OPLEDP 0146-9592 Google Scholar
K. ZhangJ. U. Kang,
“Real-time intraoperative 4D full-range FD-OCT based on the dual graphics processing units architecture for microsurgery guidance,”
Biomed. Opt. Express, 2
(4), 764
–770
(2011). http://dx.doi.org/10.1364/BOE.2.000764 BOEICL 2156-7085 Google Scholar
J. U. Kanget al.,
“Real-time three-dimensional Fourier-domain optical coherence tomography video image guided microsurgeries,”
J. Biomed. Opt., 17
(8), 081403
(2012). http://dx.doi.org/10.1117/1.JBO.17.8.081403 JBOPFO 1083-3668 Google Scholar
M. Jeonet al.,
“Full-range k-domain linearization in spectral-domain optical coherence tomography,”
Appl. Opt., 50
(8), 1158
–1163
(2011). http://dx.doi.org/10.1364/AO.50.001158 APOPAI 0003-6935 Google Scholar
H. Jeonget al.,
“Ultra-fast displaying spectral domain optical Doppler tomography system using a graphics processing unit,”
Sensors, 12
(6), 6920
–6929
(2012). http://dx.doi.org/10.3390/s120606920 SNSRES 0746-9462 Google Scholar
|


