|
|
1.IntroductionNonmelanoma skin cancer (NMSC) is a term used to encompass skin cancer forms other than malignant melanoma, and it most commonly refers to squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). BCC is the most common malignancy in humans and its incidence is on the rise with considerable public health implications.1 The optimal management of skin cancer relies on early and accurate diagnosis, appropriate treatment, and monitoring for potential relapse. Noninvasive treatments are increasingly being used for the management of BCC. Recent advances in the molecular pathophysiology of BCC have opened the way for new exciting targeted therapies, including oral hedgehog signalling inhibitors, in order to avoid the need of extensive, repetitive, or mutilating surgery.2 A plethora of new developments in optical imaging techniques is available for the noninvasive diagnosis (photodiagnosis) of NMSC including fluorescence, diffuse reflectance, Raman and near-infrared spectroscopies, optical coherence tomography, and multiphoton and confocal laser scanning microscopies.3,4 More interestingly, optical methods are also increasingly being used to monitor clearance of skin cancer after traditional treatments and screen for early relapse detection. In this review, we will present the basic principles of laser-induced fluorescence (LIF) for the management of BCC, and we will discuss its use in early and advanced BCC diagnosis, the use in determining surgical margins, and the ability to detect residual cancer or tumor relapse. 2.LIF: Basic Principles Made SimpleIn LIF, nonionizing radiation is delivered and collected with optical fibers that are placed in contact with the skin surface. The excitation light passes through and explores the tissue under the probe noninvasively, and fluorescence light is emitted back to the surface where it is collected by the fibers at a fixed distance away from the source fiber. These optical measurements depend on the morphology, function and, biochemical composition of the tissue. They are also influenced by the fluorophore distribution, and by the tissue’s optical properties, especially at the maximum imaging depth. Quantitative optical spectroscopy techniques constitute an objective diagnostic methodology, as they do not rely on the operator’s experience. Fluorescence emission from a skin lesion is excited with an irradiation source including coherent or incoherent broadband lights, e.g., laser, light-emitting diode (LED), a xenon lamp, or a halogen lamp. Fluorescence originates either from endogenous fluorophores (autofluorescence) or from exogenously administered fluorophores (photosensitizers). Skin tissue autofluorescence, by near-ultraviolet (UV) and blue excitations, originates from endogenous fluorophores such as reduced nicotinamide adenine dinucleotide (NADH),5–9 collagen, elastin,9,10 and tryptophan.8 Light-induced autofluorescence spectroscopy with laser source can be used to detect and quantify differences between healthy tissue and pathological lesions in vivo, in real time, with easy-to-use methodology for measurements, lack of need for contrast agents, precision, selectivity, and specificity (Table 1).11–13 The use of lasers, instead of incoherent light sources in autofluorescence spectroscopy, has the advantage of using an exact wavelength and can deliver the excitation light with fibers to the selected body site, without significant loss of light power, and derive, in real time, medical information from naturally occurring endogenous fluorophores, without adding external (exogenous) fluorescent markers.14 Tissue fluorescence can be improved by the application of exogenous fluorophores (photosensitizers) with selective absorption and fluorescence properties, which preferentially accumulate in cancer cells and, after the irradiation with light of specific wavelength, emit characteristic fluorescence delineating the site of NMSC.15 Clinical studies have demonstrated that photosensitizer-induced skin fluorescence has significant diagnostic advantages compared with skin autofluorescence due to the increase in the fluorescence intensity by using exogenous fluorophores accumulating in the malignancy and the consequent better demarcation between the malignant and normal tissues (Table 2). Table 1Laser-induced fluorescence (LIF): pros and cons.
Table 2Differences in fluorescence between BCC and healthy skin depending on excitation wavelengths and autofluorophores.
The use of exogenous fluorophores has been integrated in the clinical practice with the topical photodynamic therapy (PDT). Topical 5-aminolevulinic acid (ALA) and methyl aminolevulinic acid (MAL) are the most commonly used topical agents in PDT, while second-generation photosensitizers are under investigation. MAL-PDT has been approved by the European Medicines Agency as a treatment for actinic keratoses on the face and scalp when other therapies are considered less appropriate, for superficial and/or nodular BCC unsuitable for other available therapies, such as lesions on the mid-face or ears, lesions on severely sun damaged skin, large lesions, or recurrent lesions, and for SCC in situ (Bowen’s disease) when surgical excision is considered less appropriate. PDT is increasingly being investigated in dermatology for a wide range of inflammatory and infectious cutaneous diseases.16 It is based on the selective destruction of cancerous or affected cells without damaging the surrounding healthy tissue by combining three elements: oxygen, light, and a photosensitizing agent. Light sources for PDT are coherent and incoherent broadband lights. Lasers as coherent sources are metal vapor lasers (copper and gold vapors), dye-pumped tunable (-dye and Nd:YAG-dye) lasers, and diode lasers. Their advantages include the possibility to treat the lesion without affecting the surrounding healthy skin, the short illumination time without heating the surrounding tissue, and the use of monochromatic light.17–22 Laser’s capacity to emit high-flux monochromatic light and its focal precision, allows for small, demarcated lesions to be selectively treated within a short-time interval.19,21,22 The advantage of dye lasers is the possibility to change the dye and thus the emission wavelength, making it possible to use the same laser in combination with various photosensitizers. Because of their large beam cross-section (typically 1 to ), the metal vapor lasers can be applied for PDT of large lesions, such as those occurring in the skin, without the need to use a beam expander and the alignment with the dye module is not critical as for argon lasers.23 Diode lasers, besides having a convenient size, are also reliable, cheaper, and easy to use.24 Incoherent light sources are fluorescent lamps, LEDs, filtered xenon arc, and metal halide light. LED are broadband sources, cheaper, more compact, and convenient for therapy of wider areas, larger or multiple tumors, and field cancerization therapy.19,20,24–27 Photodynamic diagnosis (PDD) is a method for tumor demarcation that is based on the visualization of the fluorophore accumulating in the tumor tissue, by the use of fluorescence imaging. 3.Laser-Induced Autofluorescence for the Diagnosis of BCCNoninvasive treatments are increasingly being used for the management of BCC, the predominant type of NMSC, making the development of noninvasive diagnostic technologies highly relevant for clinical practice. Lesional biopsy and histopathologic evaluation (Fig. 1) have been the gold standard for the diagnosis of NMSC and the differential diagnosis of SCC and BCC. However, the biopsy is an invasive method; it requires histopathologic evaluation from a trained dermatopathologist, and the result may take some days to be available or the result may not be conclusive due to an error of biopsy sampling or mishandling of the tissue. Also, not every clinically suspect NMSC lesion turns out to be cancerous. LIF spectroscopy is a very attractive diagnostic technique for early diagnosis and demarcation of basal skin carcinoma due to its high sensitivity, user-friendly methodology for real-time measurements, and noninvasiveness. Fig. 1Histopathologic images of (a) basal cell carcinoma (BCC) and (b) squamous cell carcinoma (SCC). 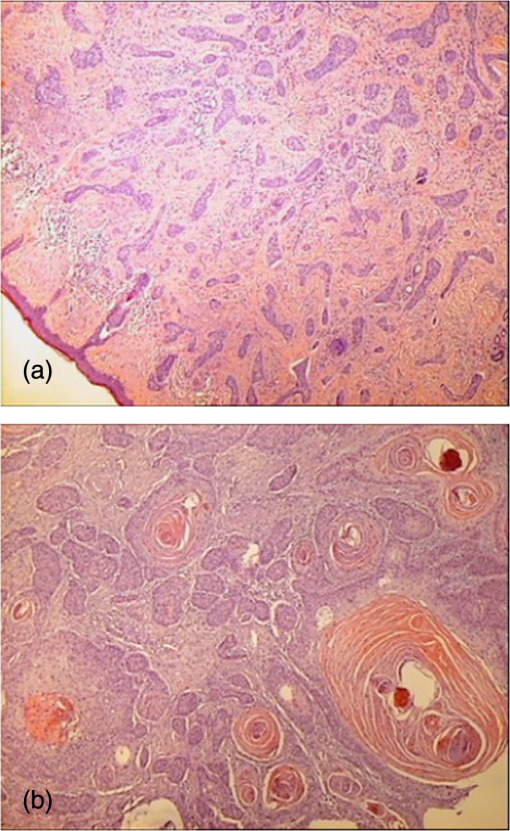 Skin tissues under excitation with UV light (in the spectral region 260 to 400 nm) demonstrate differences in their biochemical content and metabolic state, with higher autofluorescence intensities in healthy skin compared with BCC, allowing diagnostic differentiation. In malignant tissue, fluorescence spectral changes are due to a decrease in collagen and elastin and a decrease in NADH levels, mainly due to the shifted equilibrium between the highly fluorescent NADH and the less-fluorescent oxidized-form in the malignant tissue. In the spectral region of 500 to 600 nm, the reduction of the fluorescence is attributed to hemoglobin absorption.28–32 Similar autofluorescence patterns have been shown for both superficial and nodular BCCs.28 The feasibility of autofluorescence spectroscopy for skin cancer detection using excitation at 375 nm has been investigated. No significant differences in the shape of fluorescent spectra or in fluorescence intensity values between tumor and normal skin has been found.33 Autofluorescence spectra from BCC lesions, excited with HeCd laser at 442 nm, showed decreased fluorescence intensity as compared with the surrounding normal skin, a trend that has also been confirmed by in vivo autofluorescence imaging of BCC lesions.34 More promising results reported higher fluorescence intensity in nonmelanoma tumors (BCC and SCC) compared with healthy skin using UV excitation at 295 nm for the tryptophan residues, which could be a result of epidermal thickening in tumor site. In contrast, the fluorescence intensity associated with collagen cross-links was lower in tumors, because of the erosion and degradation of the connective tissue after excitation with 350 nm.35 Similar results have been shown with lower fluorescence signal in BCC compared with normal skin.36 Fluorescence spectroscopy with a nitrogen/dye laser tuned at 410-nm excitation has been used for the detection of BCC in vivo.37 A correlation was found between the cancer detection diagnostic accuracy and the skin phototype in 49 patients. The diagnostic accuracy for tumor detection was lower in patients with darker skin types, with a diagnostic accuracy of 93% for phototype I, while it was 78% for phototype III. Spectroscopic assessments of autofluorescence during experiments on unstained human skin samples (BCC, SCC, and healthy tissues) were carried out with a homemade nitrogen laser ().37 The fluorescence intensity of malignant tumor was weaker than that of the normal tissue, especially in the skin tissue with SCC, which showed a larger displacement in the red spectrum. Moreover, there were significant differences in the spectral signatures between BCC and SCC, which facilitated their differential diagnosis. Another laser-induced spectroscopic method used in skin tissues is the investigation of the fluorescence lifetime of endogenous fluorophores. Time-resolved autofluorescence spectra of BCC have been studied with in vivo measurements prior to surgical excision.38 This method is relatively independent of the factors of fluorophore concentration and signal attenuation by the sample and has been shown that both ex vivo and in vivo effectively differentiate between healthy and affected tissues in various types of skin cancer.38–40 The paired difference in fluorescence lifetime in each spectral channel between healthy skin and BCC has been investigated. The fluorescence lifetime did not vary significantly with emission wavelength over the spectral range containing the peak fluorescent signal (425 to 540 nm for UV excitation and 475 to 550 nm for blue excitation). There was no significant difference in the mean emission wavelength between healthy skin and BCC for either excitation wavelengths. There was no clear trend in the lifetime shifts in BCC observed with UV excitation; however, for blue light excitation (445 nm), the fluorescence lifetime in BCC was consistently lower than that of the surrounding nonaffected peri-lesional skin (i.e., ).38 Tissue fluorescence analysis with a 442-nm HeCd laser light was used to illuminate and excite unstained skin tissue sections in order to demarcate the normal and BCC areas.41 The fluorescence images of the samples were recorded by a CCD camera though a microscope. In the cancerous regions, the epidermis showed a very weak fluorescence signal, while the stratum corneum exhibited fluorescence emissions peaking at about 510 nm. In the dermis, the basal cell islands and a band of surrounding areas showed a very weak fluorescence signal, while distal dermis above and below the basal cell island showed a greater fluorescence signal with different spectral shapes. Wide-field false-color images of fluorescence lifetimes of unstained biopsies of 25 BCCs were studied, following excitation of autofluorescence with a 355-nm pulsed UV laser to clearly discriminate areas of BCC from the surrounding uninvolved skin and to allow localization and delineation of the malignant areas.39 The maximum visual information could be obtained by merging the lifetime and intensity images to produce intensity-weighted lifetime maps. The resultant image combined two complementary dimensions of information and allowed the lifetime information to be related to the anatomical intensity image without loss of information from either parameters.39 The excitation wavelengths, autofluorophores, and differences in fluorescence between healthy and cancer tissues are summarized in Table 2. 4.PDD for BCCLIF by exogenous fluorophore agents has been used in the context of PDT as a diagnostic method. PDD is a method for tumor demarcation that is based on the visualization of a fluorophore, with the ability to accumulate in tumor tissue, by the use of fluorescence imaging. ALA and MAL are nonfluorescent precursors of fluorescent protoporphyrin IX (PpIX). Visualization of PpIX represents the basis of the PDD for skin tumors, and it may be used with fluorescence imaging systems. Image-processing methods widely used for demarcation of BCC after ALA-induced PpIX include the ratio imaging method and threshold-based imaging.42 Ratio imaging method uses the division of either autofluorescence intensity versus PpIX fluorescence intensity after ALA application, or the fluorescence intensity of tumor marked area versus the ALA-treated normal skin area, or the fluorescence intensities between red over other spectral areas after administration of ALA-induced PpIX.42–44 Threshold imaging method uses either a total emission photon count in fluorescence spectra as the discriminating index where a threshold value is calculated to separate normal tissue indices from indices of cancer tissues or an intensity ratio between normal and control skin tissues’ fluorescence spectra.37,42 This threshold divides the signal intensity scale into two zones: normal and cancer zones. The optimal threshold value, however, depends on the intensity of the acquired fluorescence image.42 Those two methods can reduce geometric effects, influence from the incident light distribution, and provide a quantitative measurement for correlation with subsequent histological assessments, although when intensity of the fluorescence is fluctuating, the threshold-based methods introduce errors in tumor demarcation.42,44 Five superficial and 10 nodular BCCs in 15 patients were studied with fluorescence measurements prior to the topical application of ALA, 2-, 4-, and 6-h post-ALA application, immediately post-PDT ( at 635 nm), and 2 h after treatment. Superficial BCC showed a maximum PpIX fluorescence 6 h post ALA application, whereas in nodular BCC the maximum occurred 2 to 4 h after the application.45 This variability of the fluorescence intensity may be due to the duration of the ALA/MAL application time, the concentration of the ALA/MAL cream, and/or the intensity of the illuminating light. There are various approaches to achieve robust tumor demarcation with derivation of novel unsupervised image segmentation methods that are not dependent on the variability of the fluorescence intensity, and LIF may be used for tumor demarcation, i.e., determination of the tumor boundaries.42 Fluorescence diagnosis allows an accurate assessment of BCC borders to determine excision margins prior to surgical removal. LIF with a diode laser at 633 nm was used to monitor the buildup of the ALA/methyl-esterified δ-ALA (ALA-ME)-induced PpIX in BCCs.46 A clear demarcation between the lesion and the normal skin was detected with LIF for both PpIX precursors before PDT treatment.46 Pharmacokinetic studies investigated the borders of tumor growth and the intensity of accumulation of radachlorin in 32 patients with BCC and the intensity of accumulation of Photosense in 81 patients.47 The discrimination between normal and malignant tissues was done by spectral-fluorescent complex and spectra analyzer LESA-01 (He-Ne-laser, ). There was fluorescence from all tumors, and additional fluorescence zones were found, while a cytological confirmation of BCC was available in most cases. Two-dimensional images of fluorescence signs of radachlorin in normal skin were detectable up to 5 days after injection. In an attempt to increase this PpIX-based fluorescence tissue contrast in normal skin in seven patients with nodular BCC, a multichannel fluorescence imaging system was developed to collect PpIX (635 nm), autofluorescence (470 and 600 nm), and photobleached products (670 nm) emission from both cancerous lesions and surrounding normal skin before and after PDT, after excitation with a small sealed-off nitrogen laser pumping a dye laser emitting at 405 nm.48,49 The photosensitizer’s fluorescence was monitored to distinguish normal skin from malignant lesions, as well as to track the accumulation of photodegraded products during PDT. The malignant region 1 week after PDT was limited to the area delineated by the multicolor fluorescence imaging system, suggesting that the tumor did not, to a large extent, infiltrate the surrounding tissue. Optical spectroscopy may noninvasively monitor disease progression in real time based on its ability to collect optical parameters that correspond to distinct morphology, function, and biochemical composition of the tissue, which may change over time.50 LIF may be used to follow up after PDT in deciding whether the repetition of the treatment is necessary when the response to therapy is difficult to ascertain. 5.Optimizing Results: Processing and Interpretation of Spectral DataIn general, the LIF spectra of normal and malignant tissues exhibit certain differences at several wavelengths. However, it is difficult to observe subtle but consistent differences in the raw data, because these differences are often masked by large variations in intensity. Intrapatient variability in the fluorescence intensity response is typically large and affects the diagnostic accuracy.51,52 In addition, the autofluorescence spectra of malignant tissues have usually very low intensity of fluorescence radiation emitted by endogenous fluorophores, above excitation of 300 nm.3,15,34,36,53 Fluorescence is a highly promising and attractive technique for the diagnosis and demarcation of BCC. However, the multitude and the variability of clinical forms and fluorescence properties of benign and malignant skin lesions pose issues that limit its specificity. This is also the case with BCC that may present as nodular, superficial, morpheic, cystic, or ulcerative type.13 Also, the interpretation of results may depend on patient-related characteristics such as skin age and phototype.54,55 In order to account for intrapatient and intralesion variations in the evaluation of the results from LIF spectra, mathematical models and specific statistical analysis techniques have been applied. Some researchers focused on the spectral profile of each spectrum containing specific characteristics that are more consistent. These have been amplified and compared by the use of effective diagnostic algorithms with some form of normalization. This is especially important for malignant tissues which normally exhibit weak fluorescence with small features that are difficult to observe.56 Nevertheless, the simple normalization, namely, the division with respect to the integrated intensity of the entire spectrum, is redundant and inefficient.52,57 Fluorescence spectra of NMSC37 underwent statistical process using total emission photon count as the discriminating index. A threshold value was calculated to separate normal tissue indices from indices of cancer tissues. The classification accuracy of each data point was determined using the threshold value. Rajaram et al.50 fit the observed spectra to models and were able to extract optical parameters of the tissue such as the absorption and scattering coefficients, hemoglobin concentration, and the relative contributions of the constituent fluorophores. Using these parameters in a leave-one-out cross-validation, they were then able to diagnose BCCs with a sensitivity of 94% and a specificity of 89%.50 Various data analysis methods have been devised and employed to differentiate between fluorescence spectra of normal and cancerous tissues for the purpose of cancer diagnosis. There are several methods for the analysis of autofluorescence tissue spectra. Evaluated methods include principle peak ratio, differential normalized fluorescence (DNF), bivariate DNF (2-D-DNF), principal component analysis, and correlation coefficient mapping.56–64 Among these methods, DNF is a simple, straightforward method and provides excellent classification.56 In the DNF analysis, the diagnostic features are extracted from the difference between the averaged cancerous and averaged normal tissue spectra. Thus, in this analysis,53,65 first, a normalization process is utilized in order to amplify and compare the spectral characteristics of normal and malignant tissues. The LIF spectra of normal and tumor tissues can be normalized to 1, at a particular wavelength, where the normalization wavelengths can be chosen at the point corresponding to maximum fluorescence intensity. The resulting normalized intensity for each spectrum has a dimensionless value and consequently becomes less dependent on the intensity factor. In addition, a baseline curve, as the mean average of normalized fluorescence spectra from a reference set of normal tissue samples, can be determined, due to the fact that the normalized spectra of normal tissues have similar spectral profiles. Finally, a DNF curve for a specific tissue sample can calculated as the difference between the normalized fluorescence spectrum and the baseline curve. An excellent feature of the DNF method is its efficiency in extracting the diagnostic index. The DNF index, which is the spectral intensity of each tissue spectrum at the feature wavelength subtracted by the averaged normal tissue spectrum at the same wavelength, serves as the diagnostic index in cancer detection. The accuracy of the DNF method in a proper discrimination of BCC and normal skin tissues can be specified by calculating the percentage of correctly classified spectra, according to histopathology results.53 The advantages of the DNF method, especially how to best perform cancer diagnosis based on the rich information in the extracted spectral features, have not been fully exploited. In DNF analysis of tissue spectra, usually two or more spectral features become apparent. In practice, either one of them was used in diagnosis or two spectral features were used independently to yield their own results. The sensitivity and specificity obtained for LIF in skin malignancies by DNF depend on the selected wavelength in the peak-near areas and can reach values from 80% to 98%,64 whereas in other tissue malignancies, like tissue pathologies of the esophagus, authors reported a sensitivity of 100% and a specificity of 98%.10 6.Conclusions and Future PerspectivesLaser technology based on optical spectroscopy is a diagnostic tool for BCC, emerging to be integrated in the clinical setting. LIF spectroscopy is a very attractive diagnostic technique for early diagnosis and demarcation of BCC due to its high sensitivity, user-friendly methodology for real-time measurements, and noninvasiveness. The measured optical properties and fluorophore contributions of normal skin and NMSCs are significantly different from each other and correlate well with tissue histology (Table 3). Table 3LIF: take home pearls.
In patients with BCC, PDD may be used for detecting subclinical disease and determining surgical margins and following up for residual tumor or BCC relapse. Further research may investigate new photosensitizers and laser sources for the accurate diagnosis and follow-up monitoring of BCC in clinical practice. The combination of optical techniques such as LIF spectroscopy and diffuse optical spectroscopy offer promise as a useful multimodal approach with considerable superiority for differentiating between normal and malignant tissues than each method alone. AcknowledgmentsThis work was supported by the European Commission under the 7th Framework Programme 2007-2013 through the Sectoral Operational Programmes focus on human resource development and human resources, Contract No. 2007RO051PO001, Educational Program: POSDRU/63/3.2/5/318: International Training in Dermato-cosmetics for Romanian Medics. We would like to gratefully acknowledge Dr. A. A. Serafetinides and Dr. M. Makropoulou, Professors of the Physics Department, School of Applied Mathematical and Physical Sciences, National Technical University of Athens, Greece, for providing access to their facility and for their significant contribution with the spectroscopic measurements and the data interpretation. We also thank A. Tsenga for her help in the histopathology examination of the samples. ReferencesC. Dessiniotiet al.,
“Basal cell carcinoma: what’s new under the sun,”
Photochem. Photobiol., 86
(3), 481
–491
(2010). http://dx.doi.org/10.1111/php.2010.86.issue-3 PHCBAP 0031-8655 Google Scholar
C. DessiniotiC. AntoniouA. J. Stratigos,
“New targeted approaches for the treatment and prevention of nonmelanoma skin cancer,”
Expert Rev. Dermatol., 6
(6), 625
–634
(2011). http://dx.doi.org/10.1586/edm.11.70 1746-9872 Google Scholar
E. Drakakiet al.,
“Spectroscopic methods for the photodiagnosis of nonmelanoma skin cancer,”
J. Biomed. Opt., 18
(6), 061221
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061221 JBOPFO 1083-3668 Google Scholar
S. Seidenariet al.,
“Multiphoton laser microscopy and fluorescence lifetime imaging for the evaluation of the skin,”
Dermatol. Res. Pract., 2012 1
–8
(2012). http://dx.doi.org/10.1155/2012/810749 1687-6105 Google Scholar
M. MycekB. W. Pogue, Handbook of Biomedical Fluorescence, Marcel Dekker, New York
(2003). Google Scholar
R. R. AndersonJ. A. Parrish,
“The optics of human skin,”
J. Invest. Dermatol., 77
(1), 13
–19
(1981). http://dx.doi.org/10.1111/jid.1981.77.issue-1 JIDEAE 0022-202X Google Scholar
Z. Volynskayaet al.,
“Diagnosing breast cancer using diffuse reflectance spectroscopy and intrinsic fluorescence spectroscopy,”
J. Biomed. Opt., 13
(2), 024012
(2008). http://dx.doi.org/10.1117/1.2909672 JBOPFO 1083-3668 Google Scholar
I. BliznakovaE. BorisovaL. Avramov,
“Laser- and light-induced autofluorescence spectroscopy of human skin in dependence on excitation wavelengths,”
Acta Phys. Pol. A, 112
(5), 1131
–1136
(2007). ATPLB6 0587-4246 Google Scholar
E. Borisovaet al.,
“Optical biopsy of human skin—a tool for cutaneous tumours’ diagnosis,”
Int. J. Bioautom., 16
(1), 53
–72
(2012). Google Scholar
R. Richards-Kortumet al.,
“Spectroscopic diagnosis of colonic dysplasia,”
Photochem. Photobiol., 53
(6), 777
–786
(1991). PHCBAP 0031-8655 Google Scholar
J. BigioJ. R. Mourant,
“Ultraviolet and visible spectroscopies for tissue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy,”
Phys. Med. Biol., 42
(5), 803
–814
(1997). http://dx.doi.org/10.1088/0031-9155/42/5/005 PHMBA7 0031-9155 Google Scholar
L. Bachmannet al.,
“Fluorescence spectroscopy of biological tissues—a review,”
Appl. Spectrosc. Rev., 41
(6), 575
–590
(2006). http://dx.doi.org/10.1080/05704920600929498 APSRBB 0570-4928 Google Scholar
E. Borisovaet al.,
“Qualitative optical evaluation of malignancies related to cutaneous phototype,”
Proc. SPIE, 7563 75630X
(2010). http://dx.doi.org/10.1117/12.852791 PSISDG 0277-786X Google Scholar
P. Pavlovaet al.,
“Investigation of relations between skin cancer lesions’ images, and their reflectance, and fluorescent spectra,”
Melanoma in the Clinic—Diagnosis Management and Complications of Malignancy, 87
–104 InTech, Rijeka, Croatia
(2011). Google Scholar
M. A. Calinet al.,
“Optical techniques for the non-invasive diagnosis of skin cancer,”
J. Cancer Res. Clin. Oncol., 139
(7), 1083
–1104
(2013). http://dx.doi.org/10.1007/s00432-013-1423-3 JCROD7 1432-1335 Google Scholar
P. G. LangJ. C. Maize,
“Basal cell carcinoma,”
Cancer of the Skin, 101
–132 Elsevier Saunders, Philadelphia, PA
(2005). Google Scholar
L. BrancaleonH. Moseley,
“Laser and non-laser light sources for photodynamic therapy,”
Lasers Med. Sci., 17
(3), 173
–186
(2002). http://dx.doi.org/10.1007/s101030200027 0268-8921 Google Scholar
E. S. MarmurC. D. SchmultsD. J. Goldberg,
“A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer,”
Dermatol. Surg., 30
(s2), 264
–271
(2004). http://dx.doi.org/10.1111/dsu.2004.30.issue-s2 DESUFE 1076-0512 Google Scholar
M. B. EricsonA. M. WennbergO. Larkφ,
“Review of photodynamic therapy in actinic keratosis and basal cell carcinoma,”
Ther. Clin. Risk Manag., 4
(1), 1
–9
(2008). Google Scholar
K. Kostovićet al.,
“Photodynamic therapy in dermatology: current treatments and implications,”
Coll. Antropol., 36
(4), 1477
–1481
(2012). COANDS 0350-6134 Google Scholar
I. Wang,
“Photodynamic therapy and laser-based diagnostic studies of malignant tumours,”
Lund University,
(1999). Google Scholar
M. C. IssaM. Manela-Azulay,
“Photodynamic therapy: a review of the literature and image documentation,”
An. Bras. Dermatol., 85
(4), 501
–504
(2010). http://dx.doi.org/10.1590/S0365-05962010000400011 ABDEB3 0365-0596 Google Scholar
M. A. Soleret al.,
“Photodynamic therapy of superficial basal cell carcinoma with 5-aminolevulinic acid with dimethylsulfoxide and ethylendiaminetetraacetic acid: a comparison of two light sources,”
Photochem. Photobiol., 71
(6), 724
–729
(2000). http://dx.doi.org/10.1562/0031-8655(2000)071<0724:PTOSBC>2.0.CO;2 PHCBAP 0031-8655 Google Scholar
N. C. ZeitouniA. R. OseroffS. Shieh,
“Photodynamic therapy for nonmelanoma skin cancers. Current review and update,”
Mol. Immunol., 39
(17–18), 1133
–1136
(2003). http://dx.doi.org/10.1016/S0161-5890(03)00083-X MOIMD5 0161-5890 Google Scholar
Y. A. El-Sayed Ahmed,
“Photodynamic laser therapy of some skin tumors,”
Zagazig University,
(2005). Google Scholar
P. Babilaset al.,
“Photodynamic therapy in dermatology—an update,”
Photodermatol. Photoimmunol. Photomed., 21
(3), 142
–149
(2005). http://dx.doi.org/10.1111/ppp.2005.21.issue-3 PPPHEW 0905-4383 Google Scholar
C.A. Mortonet al.,
“European guidelines for topical photodynamic therapy. Part 2: emerging indications- field cancerization, photorejuvenation and inflammatory/infective dermatoses,”
J. Eur. Acad. Dermatol. Venereol., 27
(6), 672
–679
(2013). http://dx.doi.org/10.1111/jdv.2013.27.issue-6 JEAVEQ 0926-9959 Google Scholar
C. A. Klinteberg,
“On the use of light for the characterization and treatment of malignant tumours,”
Lund University,
(1999). Google Scholar
N. Ramanujam,
“Fluorescence spectroscopy of neoplastic and nonneoplastic tissues,”
Neoplasia, 2
(1–2), 89
–117
(2000). http://dx.doi.org/10.1038/sj.neo.7900077 1522-8002 Google Scholar
S. G. KongM.E. MartinT. Vo-Dinh,
“Hyperspectral fluorescence imaging for mouse skin tumor detection,”
ETRI J., 28
(6), 770
–776
(2006). http://dx.doi.org/10.4218/etrij.06.0106.0061 1225-6463 Google Scholar
N. A. Bashkatovet al.,
“Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm,”
J. Phys. D Appl. Phys., 38
(15), 2543
–2555
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/004 JPAPBE 0022-3727 Google Scholar
Q. Liu,
“Role of optical spectroscopy using endogenous contrasts in clinical cancer diagnosis,”
World J. Clin. Oncol., 2
(1), 50
–63
(2011). http://dx.doi.org/10.5306/wjco.v2.i1.50 2218-4333 Google Scholar
H. Sterenborget al.,
“In vivo fluorescence spectroscopy and imaging of human skin tumours,”
Lasers Med. Sci., 9
(3), 191
–201
(1994). http://dx.doi.org/10.1007/BF02590223 0268-8921 Google Scholar
H. Zenget al.,
“Autofluorescence of basal cell carcinoma,”
Proc. SPIE, 3245 314
–317
(1998). http://dx.doi.org/10.1117/12.312300 PSISDG 0277-786X Google Scholar
L. Brancaleonet al.,
“In vivo fluorescence spectroscopy of nonmelanoma skin cancer,”
Photochem. Photobiol., 73
(2), 178
–183
(2001). http://dx.doi.org/10.1562/0031-8655(2001)073<0178:IVFSON>2.0.CO;2 PHCBAP 0031-8655 Google Scholar
R. NaI. StenderH. Wulf,
“Can autofluorescence demarcate basal cell carcinoma from normal skin? A comparison with protoporphyrin IX fluorescence,”
Acta Derm. Venerol., 81
(4), 246
–249
(2001). http://dx.doi.org/10.1080/00015550152572859 ADVEA4 0001-5555 Google Scholar
M. Panjehpouret al.,
“Laser-induced fluorescence spectroscopy for in vivo diagnosis of non-melanoma skin cancers,”
Lasers Surg. Med., 31
(5), 367
–373
(2002). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Thompsonet al.,
“In vivo measurements of diffuse reflectance and time-resolved autofluorescence emission spectra of basal cell carcinomas,”
J. Biophotonics, 5
(3), 240
–254
(2012). http://dx.doi.org/10.1002/jbio.201100126 JBOIBX 1864-063X Google Scholar
N. P. Galletlyet al.,
“Fluorescence lifetime imaging distinguishes basal cell carcinoma from surrounding uninvolved skin,”
Br. J. Dermatol., 159
(1), 152
–161
(2008). http://dx.doi.org/10.1111/j.1365-2133.2008.08577.x BJDEAZ 1365-2133 Google Scholar
J. Mcgintyet al.,
“Wide-field fluorescence lifetime imaging of cancer,”
Biomed. Opt. Express, 1
(2), 627
–640
(2010). http://dx.doi.org/10.1364/BOE.1.000627 BOEICL 2156-7085 Google Scholar
Q. Heet al.,
“Microscopic fluorescence spectral analysis of basal cell carcinomas,”
Proc. SPIE, 6534 653414
(2007). http://dx.doi.org/10.1117/12.741593 PSISDG 0277-786X Google Scholar
I. Koprivaet al.,
“Robust demarcation of basal cell carcinoma by dependent component analysis-based segmentation of multi-spectral fluorescence images,”
J. Photochem. Photobiol. B Biol., 100
(1), 10
–18
(2010). http://dx.doi.org/10.1016/j.jphotobiol.2010.03.013 JPPBEG 1011-1344 Google Scholar
H. J. Sterenborget al.,
“Evaluation of spectral correction techniques for fluorescence measurements on pigmented lesions in vivo,”
J. Photochem. Photobiol. B, 35
(3), 159
–165
(1996). http://dx.doi.org/10.1016/S1011-1344(96)07320-4 JPPBEG 1011-1344 Google Scholar
W. Zhenget al.,
“Optimal excitation-emission wavelengths for autofluorescence diagnosis of bladder tumors,”
Int. J. Cancer, 104
(4), 477
–481
(2003). http://dx.doi.org/10.1002/(ISSN)1097-0215 IJCNAW 1097-0215 Google Scholar
C. af Klinteberget al.,
“Kinetic fluorescence studies of 5-aminolevulinic acid-induced protoporphyrin IX accumulation in basal cell carcinomas,”
J. Photochem. Photobiol. B Biol., 49
(2–3), 120
–128
(1999). http://dx.doi.org/10.1016/S1011-1344(99)00045-7 JPPBEG 1011-1344 Google Scholar
M. S. Thompsonet al.,
“Photodynamic therapy and diagnostic measurements of basal cell carcinomas using esterified and non-esterified delta-aminolevulinic acid,”
J. Porphyrins Phthalocyanines, 5
(2), 147
–153
(2001). http://dx.doi.org/10.1002/jpp.325 JPPHFZ 1088-4246 Google Scholar
E. G. Vakulovskayaet al.,
“Photodynamic therapy and fluorescent diagnostics of skin cancer with radochlorine and photosense: comparing efficacy and toxicity,”
Proc. SPIE, 5315 148
–151
(2004). http://dx.doi.org/10.1117/12.537787 PSISDG 0277-786X Google Scholar
S. Andersson-Engelset al.,
“Multi-colour fluorescence imaging in connection with photodynamic therapy of minolevulinic acid (ALA) sensitised skin malignancies,”
Bioimaging, 3
(3), 134
–143
(1995). http://dx.doi.org/10.1002/1361-6374(199509)3:3<134::AID-BIO4>3.3.CO;2-T BOIMEL 0966-9051 Google Scholar
S. Andersson-Engelset al.,
“Clinical recording of laser-induced fluorescencespectra for evaluation of tumour demarcation feasibility in selected clinical specialities,”
Lasers Med. Sci., 6
(4), 415
–424
(1991). http://dx.doi.org/10.1007/BF02042464 0268-8921 Google Scholar
N. Rajaramet al.,
“Pilot clinical study for quantitative spectral diagnosis of non-melanoma skin cancer,”
Lasers Surg. Med., 42
(10), 716
–727
(2010). http://dx.doi.org/10.1002/lsm.21009 LSMEDI 0196-8092 Google Scholar
E. Drakakiet al.,
“Laser-induced fluorescence and reflectance spectroscopy for the discrimination of basal cell carcinoma from the surrounding normal skin tissue,”
Skin Pharmacol. Physiol., 22
(3), 158
–165
(2009). http://dx.doi.org/10.1159/000211912 SPPKE6 1660-5527 Google Scholar
A. I. Deevet al.,
“Age dependence on skin autofluorescence,”
Bull. Exp. Biol. Med., 127
(3), 317
–319
(1999). http://dx.doi.org/10.1007/BF02433369 BEXBAN 0007-4888 Google Scholar
R. Naet al.,
“Autofluorescence spectrum of skin: component bands and body site variations,”
Skin Res. Technol., 6
(3), 112
–117
(2000). http://dx.doi.org/10.1034/j.1600-0846.2000.006003112.x 0909-752X Google Scholar
T. Vo-Dinhet al.,
“In vivo cancer diagnosis of the esophagus using differential normalized fluorescence (DNF) indices,”
Lasers Surg. Med., 16
(1), 41
–47
(1995). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
C. A. Klinteberget al.,
“Laser-induced fluorescence diagnostics of basal cell carcinomas of the skin following topical ALA application,”
Proc. SPIE, 2926 32
–40
(1996). http://dx.doi.org/10.1117/12.260817 PSISDG 0277-786X Google Scholar
E. Bossuet al.,
“Determination of endogenous porphyrins and the maximal HpD tumor/normal skin ratio in SKH-1 hairless mice by light induced fluorescence spectroscopy,”
Artif. Cells Blood Substitutes Immobil. Biotechnol., 27
(2), 109
–117
(1999). http://dx.doi.org/10.3109/10731199909117686 ABSBE4 1073-1199 Google Scholar
C. Y. Wanget al.,
“A probability-based multivariate statistical algorithm for autofluorescence spectroscopic identification of oral carcinogenesis,”
Photochem. Photobiol., 69
(4), 471
–477
(1999). http://dx.doi.org/10.1111/php.1999.69.issue-4 PHCBAP 0031-8655 Google Scholar
M. B. Ericsonet al.,
“Fluorescence contrast and threshold limit: implications for photodynamic diagnosis of basal cell carcinoma,”
J. Photochem. Photobiol. B, 69
(2), 121
–127
(2003). http://dx.doi.org/10.1016/S1011-1344(02)00413-X JPPBEG 1011-1344 Google Scholar
S. K. Majumderet al.,
“Nonlinear pattern recognition for laser-induced fluorescence diagnosis of cancer,”
Lasers Surg. Med., 33
(1), 48
–56
(2003). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
Y. A. Skvortsova,
“Simulation of tissues for biomedical applications,”
University of Iowa,
(2009). Google Scholar
C. Sandberget al.,
“Fluorescence diagnostics of basal cell carcinomas comparing methyl-aminolaevulinate and aminolaevulinic acid and correlation with visual clinical tumour size,”
Acta Derm. Venereol., 91
(4), 398
–403
(2011). http://dx.doi.org/10.2340/00015555-1068 ADVEA4 1651-2057 Google Scholar
E. Drakakiet al.,
“Laser induced fluorescence spectroscopy for ex vivo diagnosis and classification of basal cell carcinoma,”
in Proc. 6th European Symp. Biomedical Engineering (ESBME),
(2008). Google Scholar
BiographyEleni Drakaki is a research associate (PhD) in UOA, NTUA, and TEI at Athens, Greece. She has considerable experience in several R&D projects. Her research interests, in the area of light-tissue interactions, include clinical studies, spectroscopic measurements on tissue simulators and human tissue samples in vivo and in vitro, photodynamic therapy, development of optical systems and theoretical study of light propagation. She has more than 50 publications in scientific journals and conference proceedings. Clio Dessinioti is a dermatologist and a clinical/research fellow at the Department of Dermatology of the University of Athens, in Andreas Sygros Hospital, Athens, Greece, since 2007. In 2011, she completed her PhD degree on epidemiologic and genetic risk factors of patients with basal cell carcinoma. She has participated as subinvestigator in 20 multicenter clinical trials. Special research interests include skin cancer and photocarcinogenesis. Alexander J. Stratigos is a professor of dermatology-venereology at the Department of Dermatology, Andreas Sygros Hospital, University of Athens, Athens, Greece. His clinical and research interests include melanoma and nonmelanoma skin cancers, with particular emphasis on their epidemiology, prevention, and treatment. He has authored or coauthored more than 120 articles in peer-reviewed journals. He is currently the secretary of the European Association of Dermato-Oncology (EADO). Carmen Salavastru is A/Professor at “Carol Davila” University of Medicine and Pharmacy, Colentina Hospital, Bucharest. She has published 8 books as co-author, and 15 articles in ISI indexed journals, while she has 10 research projects based on a national or international agreement. Member of EADV, since 2001, she is also ESDR member, and AAD member. Her research interests are based in pediatric dermatology, photoaging and UV-induced carcinoma, clinical applications of lasers in dermatology and PDT. Christina Antoniou is professor and chair in the Department of Dermatology, University of Athens, Andreas Sygros Hospital, Athens, Greece. Her interests include photodermatoses, psoriasis, and T-cell lymphoma. She has been a visiting professor and invited speaker at many national and international venues. She has written more than 120 articles in peer-reviewed journals, and she is a member of the editorial board of the European Academy of Dermatology and Venereology (EADV). |

