|
|
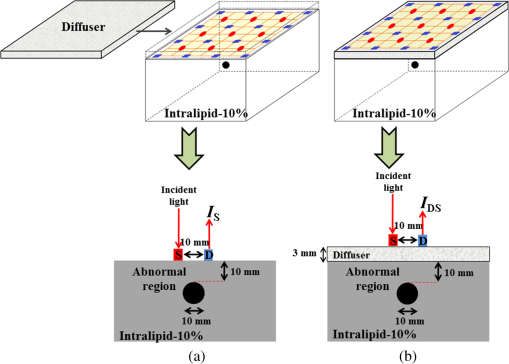
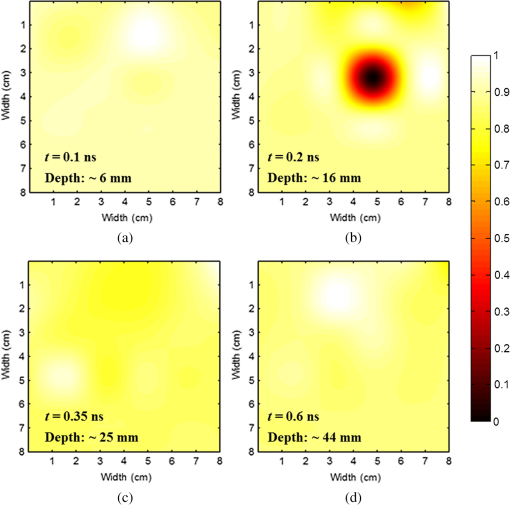
1.IntroductionThe greatest increase of the incidence of breast cancer has been in Asian countries over the last several decades. The incidence of breast cancer is rising globally and is associated with the increased mortality. The breast cancer had become the first cause of death for the female populations in the developed countries.1,2 For clinical detection, it mainly relies on the detection of morphological signs and angiogenesis phenomenon with the modern medical imaging technologies such as mammography, sonography, magnetic resonance imaging (MRI), and positron emission tomography (PET).3,4 However, mammography tends to be difficult to detect malignant lesions in dense breast tissue, which consequently leads to a lower sensitivity for using mammograms to find breast cancers in dense breasts. The detection efficiency of breast sonography strongly depends on the experience and skill of the operators and lack of standardized scanning protocols. Although the active MRI for the “oxygenation dynamics” and the PET for the “gluco-dynamics” have the potential for early detection of breast cancers, they are both very costly and the limitation of a huge size of instruments cannot usually be used for screening or a first line of defense during the regular checkup. Besides, the large size imaging systems cannot provide the diagnosis with completely patient-oriented measurement in clinic. Over the past decade, diffuse optical tomography (DOT) with near-infrared light illumination has been shown to be an effective tool for measuring the biological properties of breast tissue that can provide both functional and structural information with several benefits such as being noninvasive, less expensive, nonionizing radiation imaging, real-time measurement, long-time monitoring, easy operation, compact design, and completely patient-oriented measurement.5–11 For human breast imaging, DOT provides pathological tumor contrast directly in vascularity, hemoglobin concentration, and tissue absorption/scattering properties. DOT detects the abnormality of scattering and absorption, i.e., to detect the breast tumor in normal breast background. The process of image reconstruction in DOT depends on the technical algorithms for solving the forward and the inverse problems. In previous studies, the optical properties of normal breast tissue (as baseline) are measured from the contralateral of breast tumor in clinical diagnosis.12,13 However, human breast tissues are not identical on both sides, which implied the reference signal from the contralateral of detected breast generates concomitant prediction errors of functional image reconstruction. Therefore, we propose a time-domain diffuser-aided diffuse optical imaging (DADOI) method that can reconstruct the images simpler and faster than the traditional DOT approach by monitoring the breast tumor and detects background signal on the same location. Furthermore, the optical properties of the tissue are inferred from the temporal point spread function (TPSF) in the time-domain operation. Thus, time-domain DADOI is a kind of “time-gating” approach to reveal rich depth information. 2.Materials and MethodsFigure 1 shows the scheme of time-domain DADOI system. The system consists of 1 ps laser diode head (LDH-P-780, PicoQuant, Berlin, Germany) as light sources at 780 nm. A computer controlled multichannel laser diode driver (PDL 828 SEPIA II, PicoQuant, Berlin, Germany) was used. The optical power of laser is with full width at half maximum (FWHM) . The light sources were delivered sequentially to nine locations on the optode by using a 1:9 optical switch (Piezosystem Jena, GmbH, Jena, Germany) for time-division multiplexing. A universal serial bus (USB) interface data acquisition card (NI-DAQ 6343, National Instruments, Austin, Texas) was used for optical switch controlling. The backscattered optical signals from phantom were detected by the use of 16 single photon counting module (SPCM-AQRH-14-FC, Excelitas Technologies, Wiesbaden, Germany) that were controlled and monitored with dual SPCM power supply (DSN 102, PicoQuant, Berlin, Germany). It also provides safety shutdown to prevent SPCM degradation. A multichannel time-correlated single photon counting module (TCSPC) with USB interface was used for acquisition of distribution of times of flight of photons (HydraHarp 400, PicoQuant, Berlin, Germany). The optode array consists of array of sources and array of detectors in a square geometry with minimum distances of source-detector separation 10 mm. Thus, the whole measurement area was approximately with source-detector pairs corresponding to 36 channels. Fig. 1Schematic of time-domain diffuser-aided diffuse optical imaging (DADOI) system setup. SEPIA II is the computer controlled multichannel diode laser driver, LDH is the picosecond laser diode head, SPCM is the single photon counting module, DSN is the dual SPCM power supply, and HydraHarp is the USB interface board for time-correlated single photon counting. 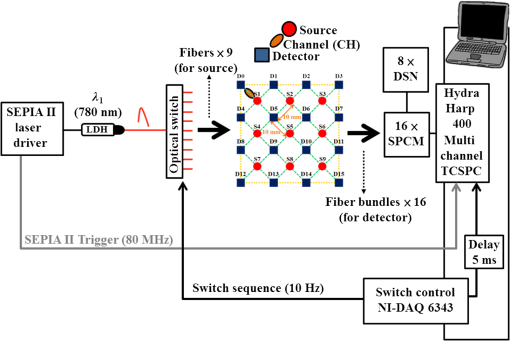 The real-time imaging capability of the time-resolved DADOI system was tested on a liquid phantom. The phantom geometry and the principle of time-resolved DADOI method are shown in Fig. 2. The phantom setup consisted of tank that filled with the solution of Intralipid and black ink. The scattering coefficient of diluted Intralipid-10% could be similar to breast tissue with given concentration. A 10-mm diameter solid spheroid made of resin was embedded in the tank as an absorbing inhomogeneity. The absorbing inclusion was placed below the middle between the source S5 and the detector D6. The depth from the surface of the absorbing inclusion to the surface of the phantom was 10 mm. The spatial distribution of the detected backscattering light from the surface of phantom without diffuser used was defined as [Fig. 2(a)] and the detected light with a 3-mm thick diffuser plate between the optode and the surface of phantom was defined as [Fig. 2(b)]. The adopted diffuser provides much stronger scattering such that can be a background signal with blurred image information. The signal of time-resolved DADOI can be obtained by calculating the modified optical density with time correlated as Eq. (1). All the backscattering lights and are detected by each source–detector pairs of the optobe (as shown in Fig. 1), then the time-correlated variation of modified optical density was calculated for mapping of spatial distribution 3.Results and DiscussionsFigure 3 shows the imaging result of time-domain DADOI method. In an experimental result, the temporal responses of received intensity and were summed as an integral intensity to verify the feasibility of DADOI method. Figure 3(a) shows the backscattering image without diffuser plate and Fig. 3(b) shows the backscattering image with inserted diffuser plate between optode and phantom. The calculation of the modified optical density with time correlated is to substitute the complex algorithms of inverse problem of conventional DOT measurement for mapping of spatial distribution. Figure 3(c) demonstrates the contrast image of DADOI method. According to the contrast comparison in Fig. 3(d), the better contrast image can be observed clearly in the DADOI result. Fig. 3The DADOI results from the phantom study. (a) Imaging without diffuser [image mapping of ]; (b) imaging with diffuser [image mapping of ]; (c) imaging by DADOI method; and (d) contrast comparison among (a), (b), and (c). 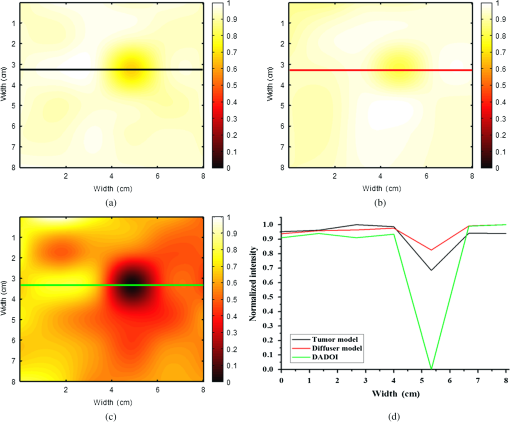 In time-domain DADOI, the depth information can be obtained from the arrival delay of detected photon (time-of-flight signal). In this study, the tomography of depth information was reconstructed simply by using temporal responses of photon migration. Figure 4 shows the tomographic images from four different depths. The depth information was evaluated with source-detector separation, refractive index, velocity of light, and TPSF. With the experimental result of phantom study, the time-domain DADOI method reveals a good feasibility for clinical diagnosis of breast cancer. 4.ConclusionsIn conclusion, time-domain DADOI method can provide better imaging contrast and simpler imaging than the conventional DOT measurement. However, the bottleneck of the DOT is still limited by the spatial resolution. In future study, the multiwavelength sources could be applied to map the images for analyzing and quantifying the vascular and tissue properties such as oxy-hemoglobin, deoxy-hemoglobin, water, and lipids of the breast tissue. AcknowledgmentsThis work was supported in part by the Taiwan National Science Council under Grant NSC 101-2628-E-009-026-MY3, NSC 102-2622-E-009-007-CC3, NSC 102-2321-B-009-002, NSC 102-3011-P-010-003, and NSC 102-2627-E-010-001. ReferencesB. O. AndersonR. Jakesz,
“Breast cancer issues in developing countries: an overview of the breast health global initiative,”
World J. Surg., 32
(12), 2578
–2585
(2008). http://dx.doi.org/10.1007/s00268-007-9454-z WJSUDI 0364-2313 Google Scholar
A. Jemalet al.,
“Global cancer statistics,”
CA Cancer J. Clin., 61
(2), 69
–90
(2011). http://dx.doi.org/10.3322/caac.v61:2 CAMCAM 0007-9235 Google Scholar
W. A. Berget al.,
“Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer,”
JAMA, 299
(18), 2151
–2163
(2008). http://dx.doi.org/10.1001/jama.299.18.2151 JAMAAP 0098-7484 Google Scholar
W. T. Yanget al.,
“Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings,”
Breast Cancer Res. Treat., 109
(3), 417
–426
(2008). http://dx.doi.org/10.1007/s10549-007-9671-z BCTRD6 0167-6806 Google Scholar
S. FantiniA. Sassaroli,
“Near-infrared optical mammography for breast cancer detection with intrinsic contrast,”
Ann. Biomed. Eng., 40
(2), 398
–407
(2012). http://dx.doi.org/10.1007/s10439-011-0404-4 ABMECF 0090-6964 Google Scholar
R. Al abdiet al.,
“Optomechanical imaging system for breast cancer detection,”
J. Opt. Soc. Am. A, 28
(12), 2473
–2493
(2011). http://dx.doi.org/10.1364/JOSAA.28.002473 JOAOD6 0740-3232 Google Scholar
J. Wanget al.,
“Near-infrared tomography of breast cancer hemoglobin, water, lipid, and scattering using combined frequency domain and cw measurement,”
Opt. Lett., 35
(1), 82
–84
(2010). http://dx.doi.org/10.1364/OL.35.000082 OPLEDP 0146-9592 Google Scholar
P. Taroniet al.,
“Noninvasive assessment of breast cancer risk using time-resolved diffuse optical spectroscopy,”
J. Biomed. Opt., 15
(6), 060501
(2010). http://dx.doi.org/10.1117/1.3506043 JBOPFO 1083-3668 Google Scholar
H. Soliman1et al.,
“Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer,”
Clin. Cancer Res., 16
(9), 2605
–2614
(2010). http://dx.doi.org/10.1158/1078-0432.CCR-09-1510 CCREF4 1078-0432 Google Scholar
C. M. Carpenteret al.,
“Image-guided optical spectroscopy provides molecular-specific information in vivo: MRI-guided spectroscopy of breast cancer hemoglobin, water, and scatterer size,”
Opt. Lett., 32
(8), 933
–935
(2007). http://dx.doi.org/10.1364/OL.32.000933 OPLEDP 0146-9592 Google Scholar
T. Durduranet al.,
“Diffuse optical measurement of blood flow in breast tumors,”
Opt. Lett., 30
(21), 2915
–2917
(2005). http://dx.doi.org/10.1364/OL.30.002915 OPLEDP 0146-9592 Google Scholar
B. J. Tromberget al.,
“Diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy,”
Breast Cancer Res., 7
(6), 279
–285
(2005). http://dx.doi.org/10.1186/bcr1358 BCTRD6 0167-6806 Google Scholar
C. Zhouet al.,
“Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy,”
J. Biomed. Opt., 12
(5), 051903
(2007). http://dx.doi.org/10.1117/1.2798595 JBOPFO 1083-3668 Google Scholar
BiographyChia-Wei Sun received the BSc degree in electrical engineering from National Cheng Kung University in Tainan, Taiwan, in 1997, and the MSc degree in biomedical engineering from National Yang-Ming University, Taipei, Taiwan, in 1999. He received the PhD degree in Institute of Photonics and Optoelectronics from National Taiwan University, Taipei, Taiwan, in 2003. He is currently an assistant professor with the Department of Photonics, National Chiao Tung University, Hsingchu, Taiwan. He has contributed to more than 40 peer-reviewed journal papers. His current research foci are diffuse optical tomography (DOT), near-infrared spectroscopy (NIRS), optical coherence tomography (OCT), neurophotonics, and clinical applications based on biomedical optical imaging techniques. Ching-Cheng Chuang received the BS and MS degrees in biomedical engineering from Chung Yuan Christian University, Chung Li, Taiwan, in 2003 and 2005. He received his PhD degree in the Institute of Biomedical Engineering, National Taiwan University (NTU), Taipei, Taiwan, in 2012. His research interests include biophotonics, neuroimaging, biooptical signal/image processing and optical device design. Chia-Yen Lee received the BS degree in biomedical engineering from Chung Yuan Christian University, Chung Li, Taiwan, in July 2004. She received the MS and PhD degrees in the biomedical engineering from the National Taiwan University (NTU), Taipei, Taiwan, in 2005 and 2011. Her research has been mainly focusing on two major topics, namely intensity inhomogeneity correction for segmenting breast sonograms and quantitative dual-spectrum infrared system for chemotherapy assessment. Chung-Ming Chen received the PhD degree in electrical engineering in 1993 from Cornell University. He has worked on various topics: biomedical image processing, medical imaging computer-aided detection, bioinformatics analysis, biochips and analysis and protein analysis and prediction. Currently, he holds a professorship at the Institute of Biomedical Engineering, National Taiwan University. |