|
|
1.IntroductionThe use of low doses of laser as a laser phototherapy (LPT) tool to improve healing of oral ulcers and chemotherapy- and radiotherapy-induced mucositis, bone necrosis, and to reduce the clinical course of herpes simplex outburst has dramatically increased in the last decade. The clinical benefits of LPT in numerous clinical conditions and oral diseases derive from clinical observations and reports that were recently validated by well-controlled in vitro and in vivo studies1–5 reviewed in Ref. 6. Although the LPT has clinical advantages, the molecular mechanisms involved in accelerated healing and the safety concerns associated with using LPT on normal cells are poorly understood. We recently characterized the involvement of mTOR signaling in the process of accelerated epithelial healing mediated by LPT.7 In this study, we found that LPT induced accelerated epithelial migration and activation of the PI3K/mTOR signaling pathway in addition to increased polarization of F-actin cytoskeleton filaments. Recent reports have suggested that reactive oxygen species (ROS) is a key molecular circuitry activated during LPT.8–11 Accumulation of ROS has been reported in skeletal muscles during early tissue repair,10 in mouse embryonic fibroblasts,11 and in several cell lines derived from preadipocytes, prechondrocytes, myoblasts, mesenchymal stromal cells, lung cancer cells, insulinoma cells, fibroblasts, human cervix adenocarcinoma cells, macrophages, and rat basophilic leukemia cells.8 In contrast, downregulation of ROS has been reported in murine cortical neurons.12 Collectively, these data suggest that the effects of LPT on ROS are tissue specific. The in vivo effects of ROS accumulation are debated in the literature. Dramatic accumulation of ROS is directly associated with the progression of multiple diseases, such as cardiovascular disease13 (reviewed in Ref. 14), vascular pathology,15 neurodegenerative and inflammatory conditions,1,16–18 and the free radical theory of aging.19 These effects may be caused by high intracellular toxicity20 that results in elevated genotoxic effects and cell death.21,22 Deregulated accumulation of ROS may also be associated with carcinogenesis and tumor progression due to its role in increasing genomic instability.20,23,24 Notably, physiological levels of ROS are associated with crucial mechanisms involved in the protection and maintenance of pluripotent cells, including hematopoietic and neural stem cells.25–27 In addition, physiological ROS regulates several intracellular signaling pathways by triggering mitogenic activated protein kinases, c-Jun amino-terminal kinases, and the Nuclear Factor Kappa B (NF-κB) transcription factor28 (reviewed in Ref. 29), suggesting a much broader role for ROS in controlling cellular functions and homeostasis. The clinical benefits of LPT for oral lesions are not completely clear due to our lack of understanding of the physiological effects of laser irradiation on oral epithelial cells. In this study, we examined the effect of LPT on the accumulation of ROS, genomic stability, and deoxyribose nucleic acid (DNA) damage in oral epithelial cells. We discuss the potential therapeutic advantages of using low doses of laser therapy to stimulate oral mucosa healing. 2.Materials and Methods2.1.Cell Lineages and ReagentsNormal oral epithelial keratinocytes (NOK-SI) cell line30 was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B, as previously reported.31 Cells were maintained in a 5% -humidified incubator at 37°C. NOK-SI cells were kindly provided by Dr. J. Silvio Gutkind (National Institute of Dental and Craniofacial Research, NIH, Bethesda, Maryland). 2.2.Laser PhototherapyThree groups with different LPT parameters were established: Sham (), , and laser groups (Table 1). The selected parameters used herein are based on the previous studies in which low energy densities of irradiation result in better wound healing compared with high energy densities.32–35 Sham group received identical treatment conditions but with the laser equipment switched off. To prevent cross-irradiation between samples, each culture condition was seeded in separated culture dishes or plates. Table 1Irradiation patterns of LPT.
The irradiations were performed using continuous wave indium–gallium–aluminum–phosphide (InGaAlP) diode laser with an output power of 40 mW, output density of , and a wavelength of 660 nm (Twin Laser, MM Optics, Sao Paulo, Brazil) in a punctual (spot size of ) mode. Laser was applied perpendicularly and in contact with the tissue-culture plates. The energy densities (fluency) used were 4 and corresponding to 4 and 20 s of exposure time, respectively. Each well received three sessions of irradiations with 6-h intervals (0, 6 h, and 12 h). The output power of the equipment was tested using a power meter (Laser Check; MM Optics LTDA, Sao Paulo, Brazil). Because the distance between the laser source and the surface of application is critical, the LPT was administered through the bottom of the optically clear plates. The irradiation occurred in partially dark conditions without the influence of other light sources. 2.3.ROS AssayROS assay was performed after cells received three sessions of irradiations with 6-h intervals (0, 6 h, and 12 h). Intracellular levels of ROS were detected using chloromethyl CM-H2DCFDA (Molecular Probes/Life technologies, Grand Island, New York) and measured at a wavelength of 517 to 527 nm. ROS were detected after intracellular esterases removed the acetate groups upon cellular oxidation. Briefly, cells were resuspended in phosphate-buffered saline (PBS) containing CM-H2DCFDA and incubated for 30 min at room temperature. Negative control cells received vehicle only and baseline fluorescence intensity was determined using sham irradiation. Positive controls received hydrogen peroxide (100 μM). Grayscale images were captured separately after fluorescence excitation as well as after Hoechst 33342 staining to determine the total number of cells. 2.4.Comet AssayNOK-SI received three sessions of LPT using the protocol. Comet assay was performed immediately after the third LPT application at 0 min, 5 min, 30 min, and 24 h time points (recovery period). NOK-SI cells were embedded in 0.75% low-melting point agarose and allowed to solidify on glass slides. Cells were then placed in lysis buffer (2.5 M NaCl, 100 mM ethylenediamine tetra-acetic acid (EDTA), and 10 mM Tris; pH 10.0 to 10.5) containing fresh 1% (v/v) Triton X-100 and 10% (v/v) dimethyl sulfoxide (DMSO) for 1 h. Following the exposure to alkaline buffer (300 mM NaOH and 1 mM EDTA; ) for 20 min, DNA was electrophoresed using 25 V () and 300 mA. The slides were neutralized in 400 mM Tris (pH 7.5) and stained with Hoechst 33342 (Invitrogen). Cells were analyzed using the TriTek CometScore TM software (TriTek, Sumerduck, Virginia). NOK-SI cells treated with hydrogen peroxide (100 μM) served as positive controls. Three output parameters were measured, including the percentage of tail DNA, tail length, and tail moment. Tail moment was used for statistical analysis.24,25 2.5.ImmunofluorescenceCells were placed on glass coverslips in 12-well plates and submitted to three sessions of LPT using the protocol. At the end of the last LPT session, the immunofluorescences were performed at 0 min, 5 min, 30 min, and 24 h time points (recovery period). The cells were fixed with absolute methanol at for 5 min. Cells were blocked with 0.5% (v/v) Triton X-100 in PBS and 3% (w/v) bovine serum albumin and incubated with anti-phospho-Histone H2A.X (Ser139) (Millipore, Billerica, California), anti-BRCA1 (C-20) (Santa Cruz Biotechnology, Dallas, Texas) and anti-phospho-BRCA1 (Ser1524, Cell Signaling Technology, Danvers, Massachusetts) antibodies as indicated. Cells were then washed three times, incubated with FITC or TRITC-conjugated secondary antibodies, and stained with Hoechst 33342 for visualization of DNA content. Images were captured using a QImaging ExiAqua monochrome digital camera attached to a Nikon Eclipse 80i Microscope (Nikon, Melville, New York) and visualized with QCapturePro software, as previously described.31 Grayscale images were captured separately after fluorescence excitation using FITC_HYQ and TRITC_HYQ filters. The number of cells was quantified using grayscale images captured following Hoechst 33342, γ-H2AX, or BRCA1 staining. 2.6.Statistical AnalysisStatistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, California). Statistical analyses of the Comet assay, γ-H2AX, and BRCA1 stains were performed by one-way analysis of variance followed by Tukey’s multiple comparison tests. Asterisks denote statistical significance (*, **, ***, and ns ). 3.Results3.1.LPT Induces Intracellular Accumulation of ROS in Human Oral Epithelial CellsEmerging clinical studies have revealed the benefits of using LPT for healing diseases and wounds.6,36–38 Further dissection of the molecular signaling associated with laser therapy-induced accelerated healing revealed the involvement of signaling networks, including the activation and accumulation of intracellular ROS that was identified by our group.8,12 Interestingly, although accumulation of ROS has long been associated with toxic buildup of byproducts derived from anaerobic respiration, emerging evidence suggests a role for physiological levels of ROS in signal transduction.28,29,39,40 It remains unclear how LPT induces accumulation of ROS in oral epithelial cells and whether there are safety concerns with using LPT. To evaluate the effects of LPT on ROS accumulation, genomic instability, and DNA damage in oral epithelial cells, we exposed NOK-SI cells to 4 and irradiation doses of laser. Unexpectedly, we found that cells irradiated with generated higher levels of ROS (***) compared to cells receiving (ns ) [Figs. 1(a) and 1(b)]. Because accumulation of ROS is associated with increased genomic instability, which may cause chromosome rearrangements, and accumulation of DNA breaks41,42 (reviewed in Ref. 24), we examined whether LPT () induced increased DNA fragmentation (T0). We also monitored these cells for an additional 24 h (T5 min to 24 h) [Figs. 2(a) and 2(b)] using the Comet assay. LPT did not induce DNA fragmentation (NS ) even when used at optimal conditions that promote accumulation of ROS () (Fig. 1). Hydrogen peroxide was used as a positive control (***). These findings suggest that LPT activates physiological levels of ROS in oral epithelial cells without interfering with genomic stability. Fig. 1Reactive oxygen species (ROS) levels in human oral keratinocytes receiving laser phototherapy (LPT). Representative immunofluorescence microphotographs of CM-H2DCFDA-positive oral epithelial cells. (a) Cells that received of laser energy density show accumulation of ROS compared to cells receiving of laser energy density or sham irradiation. (b) Quantification of the intracellular levels of ROS (CM-H2DCFDA) following administration of 4 or of laser. Administration of results in significant accumulation of ROS (***) compared to administration of that is not statistically different from sham irradiation (NS ). was used as a positive reaction for the experiment (*). Scale bars represent 50 μm. 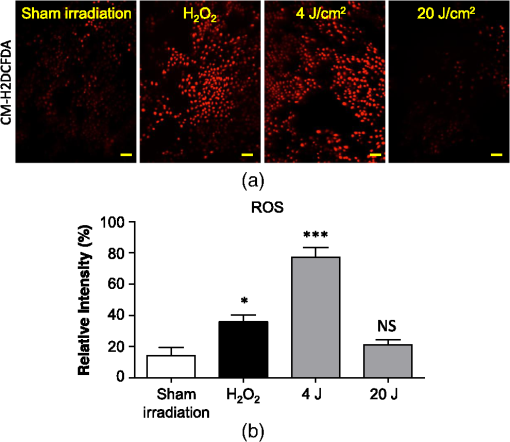 Fig. 2LPT does not induce genomic instability. (a) Representative microphotographs of the alkaline comet assay depict deoxyribose nucleic acid (DNA) fragmentation in response to sham irradiation, hydrogen peroxide, and of laser irradiation in NOK-SI cells. The DNA damage recovery phase was established after LPT irradiation and followed for 24 h. Note that the undamaged NOK-SI cells following sham irradiation and (comet head only) and fragmentation of DNA in positive control cells (—100 μM; tail formation of the comet). (b) Comet assay quantification shows DNA damage exclusively in cells that received (***). Note that the lack of DNA damage in NOK-SI cells that received of laser (no comet tail) (NS ) (; error bar: ). 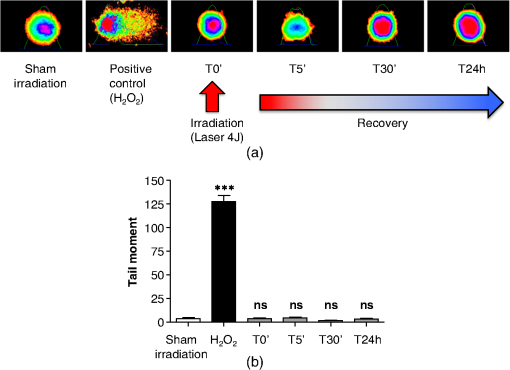 3.2.Low Doses of LPT do not Induce DNA Double-Strand Breaks in Normal Oral Epithelial CellsApplication of different types of laser irradiation results in the generation and accumulation of a wide variety of DNA damage in various tissues.43,44 Because laser therapy involves low doses of irradiation, we investigated whether LPT induces DNA strand breaks. We used the γ-H2AX DNA double-strand break marker as a tool for identifying DNA breaks. The histone H2AX is involved in assembling the DNA damage response complex following genomic injury. γ-H2AX is phosphorylated at serine 139 by Ataxia Telangiectasia Mutated (ATM) in response to DNA double-strand breaks. Phosphorylation of γ-H2AX results in recruitment of several components of the DNA damage response machinery, including BRCA1, BRCA2, Rad51, Mre11, NBS1, FANCD2, and p53.45,46 Furthermore, the continuous presence of phosphorylated γ-H2AX in the chromatin denotes continuous double-strand break repair.45,46 To access the effect of LPT on genomic material in epithelial cells, we analyzed the number of phosphorylated γ-H2AX foci at different time points (0 min, 5 min, 30 min, and 24 h) [Fig. 3(a)]. Surprisingly, we found that LPT did not induce double-strand breaks at any time points, similar to results observed in the sham irradiation group (NS ) [Fig. 3(b)]. The hydrogen peroxide positive control cells showed greater accumulation of DNA double-strand break foci compared to sham irradiated cells (***) [Fig. 3(a), arrow]. Fig. 3LPT does not induce DNA strand breaks. (a) Microphotographs of representative examples of immunofluorescence staining for γ-H2AX at different time points in NOK-SI cells. (b) Graphic representations of time course quantification of γ-H2AX foci per cell following irradiation with of energy density. Note that the amount of γ-H2AX foci formation does not change following LPT and remains similar to basal levels observed in the sham irradiation control group (NS ). The positive control group () shows the significant accumulation of γ-H2AX foci (***) ( point; error bar: mean±SEM). Scale bars represent 25 μm. 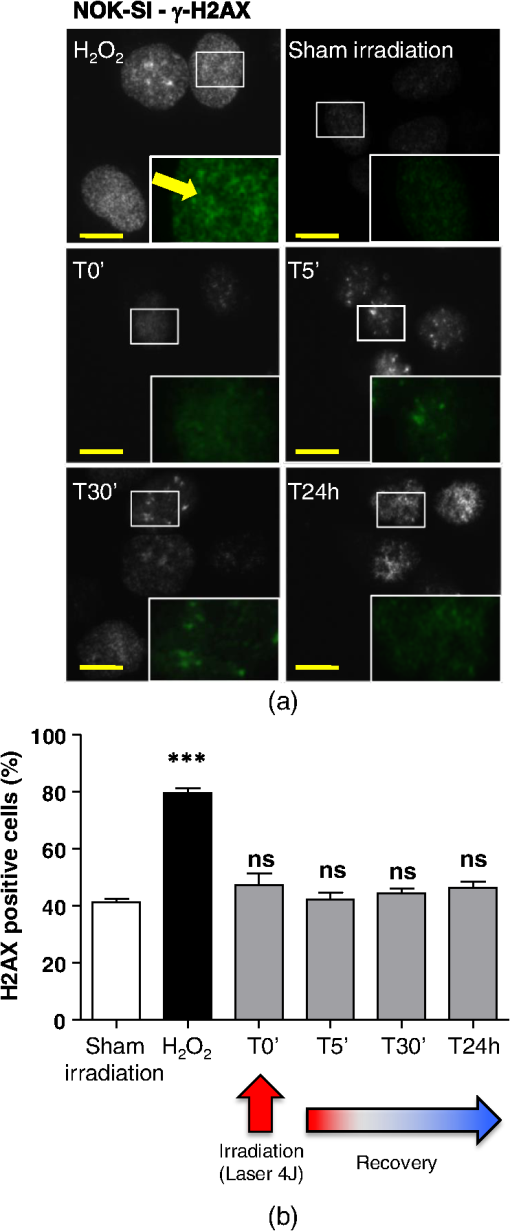 3.3.Low Energy of LPT does not Trigger DNA Damage Repair (DDR) MachineryThe maintenance of chromatin integrity requires constant repair of DNA damage that is mediated by several molecules, including the breast cancer type 1 susceptibility protein (BRCA1). The protein encoded by the BRCA1 tumor suppressor gene protects the genome by initiating cell cycle checkpoints and actively participating in DNA repair by interacting with RAD51 following DNA damage.47 ATM-dependent phosphorylation of serine 1524 causes BRCA1 nuclear translocation.48 Administration of the genotoxic hydrogen peroxide resulted in increased phosphorylation of BRCA1 at serine 1524 and nuclear translocation [Fig. 4(a)]. Additionally, hydrogen peroxide caused high levels of nuclear foci formation and colocalization of phospho-BRCA1 and phospho-γ-H2AX, as observed in Fig. 4(b) (bright foci). In agreement with our previous findings, irradiation of NOK-SI cells with did not trigger nuclear accumulation of BRCA1. LPT resulted in low levels of phospho-γ-H2AX, similar to results from the sham irradiated control group [Fig. 4(a)] but phospho-γ-H2AX failed to colocalize with phospho-BRCA1 [Fig. 4(b)]. LPT did not induce DNA damage, as revealed by the number of cells with nuclear-localized BRCA1 and the level of phosphorilated BRCA1 at serine 1524 phosphorylation (p-BRCA1) following irradiation (ns ) [Fig. 4(c)]. These findings suggest that LPT may be a safe therapeutic strategy for lesions and ulcers from the oral mucosa. Furthermore, our analyses that used two independent molecular markers for genomic integrity showed that LPT did not trigger accumulation of DNA double-strand breaks or activate DDR. Fig. 4DNA damage repair machinery is not activated by LPT. (a) Immunofluorescent staining localization of p-BRCA1 and γ-H2AX in NOK-SI cells that received at different time points (5 min to 24 h). Note that the nuclear accumulation of p-BRCA1 and γ-H2AX exclusively in the positive control group. Similar to the sham irradiation group, cells that received do not activate DDR. Nuclei were stained with Hoechst 33342. (b) Double staining of p-BRCA1 and γ-H2AX show colocalization of both markers in foci. LPT does not increase the accumulation of p-BRCA1 and γ-H2AX above basal levels. Note that the two markers do not colocalize in the LPT group. (c) BRCA1 and phospho-BRCA1 quantification. LPT did not increase BRCA1 or phospho-BRCA1 levels compared to sham irradiation (NS ). Treatment with induces the accumulation of BRCA1 and phospho-BRCA1 compared to sham irradiation (***). Scale bars represent 25 μm. 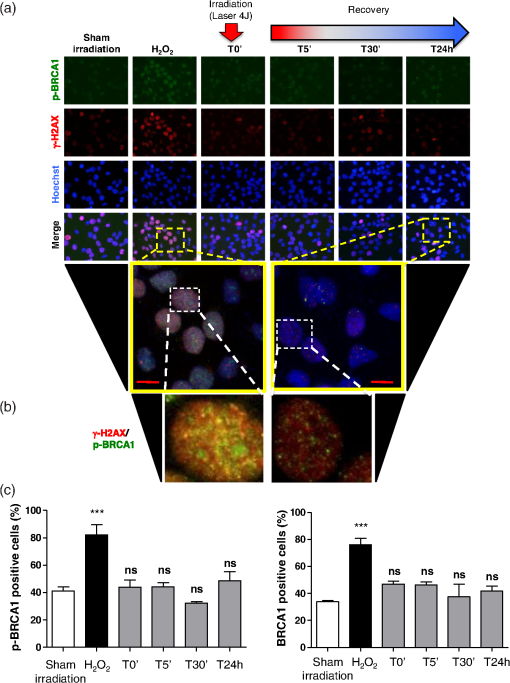 4.DiscussionThe use of light sources as therapy for human diseases dates from the early 20th century with research from Niels Ryberg Finsen. Among several reports, the 1903 Nobel Prize winner published “On the effects of light on the skin” and “The use of concentrated chemical light rays in medicine,” which served as the foundation for understanding the use of light sources as effective therapeutic strategies for certain human diseases ( http://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/finsen-bio.html). Several years later, Albert Einstein established the field of quantum mechanics with the landmark publication of “On the Quantum Theory of Radiation” describing light as bundles of photons, which laid the groundwork for our current knowledge on Light Amplification by Stimulated Emission of Radiation (LASER). Since this time, the interest in using different light sources, including laser, in medicine has progressively increased. Emerging clinical evidences have associated the use of LPT with better clinical outcomes and reduced morbidity of soft tissue diseases and conditions. LPT has shown the promising results in skin ulcers36 that may be associated with the clinical evolution of diabetes,4,37 herpes simplex outbreaks,38 and oral mucositis triggered by chemotherapy and radiotherapy.49–52 Although these publications have focused on different laser parameters (i.e., type of laser, energy density, dose) and emphasized clinical outcomes, the fundamental biological effects of laser on cellular and molecular mechanisms of irradiated tissues remain largely unexplored. Mechanistically, the effect of LPT on cells has been attributed to the accelerated respiratory metabolism,53,54 the significant increase of mitochondrial membrane potential,55 and increase mitochondrial respiration and ATP synthesis,56 which result in cellular proliferation, prevention of cell death,57 reestablishment of cellular metabolism,58 and reduction of pain and inflammation.59–61 In the context of cell specific signaling, administration of LPT is a beneficial therapy for neuron-induced formate and a preventive strategy for chemical-induced neurotoxicity.62,63 LPT is also effective in nerve repair in animal models64 and accelerates epithelial migration and wound healing in vitro7,65,66 and in vivo.67 ROS is the most well-studied pathway modulated by LPT in normal and pathological conditions.8,10,55 The generation and accumulation of intracellular levels of ROS in normal cells play a critical role in the oxidation of various cellular components, including nucleic acids, proteins, and lipids.68 Here, we demonstrate that administration of LPT at low energy density () is sufficient to induce rapid accumulation of ROS in normal human oral keratinocytes. Indeed our findings suggest that low energy density parameters of LPT promote a beneficial effect on normal epithelial cells as previously demonstrated by us and other in in vitro and in vivo experimental settings under similar low energy density.2,6,7,67,69 These beneficial effects include enhanced cellular proliferation of tenocytes,70 accelerated oral epithelial migration,7 and augmented wound healing.67 Therefore, accumulation of ROS levels within physiological levels may act as “second messengers” in response to different stimuli including LPT.71 In fact, increasing body of evidences suggests that LPT using red and near-infrared light is absorbed by cytochrome c oxidase leading to increase on mitochondrial membrane potential, enhanced ATP production and ROS accumulation. LPT, therefore, can impact the levels of cellular energy availability and activate molecular circuitries involved in light/tissue interaction.10,55,71–74 The production and consumption of energy result in intracellular buildup of toxic byproducts that is often associated with genomic damage (reviewed in Ref. 20). Although ROS accumulation is often associated with cellular metabolism, physical agents, such as ionizing or ultraviolet radiation, also induce accumulation of ROS.20 We have found that laser is no exception. Our findings showed that low doses of LPT induced rapid accumulation of ROS in human oral keratinocytes. In contrary to our expectation, ROS accumulation did not induce genomic instability, DNA breaks or activate DNA repair machinery. Our findings suggest that administration of laser at a low energy density () promotes accumulation of physiological levels of ROS without inducing DNA damage. Similar to our findings, physiological levels of ROS have been associated with maintaining a genomically stable population of stem cells.75 Collectively, our work reveals two important findings: (1) administration of laser at a low energy density () efficiently induces the accumulation of safe levels of ROS that could be associated with laser bioestimulation effects25 and (2) LPT appears to be a safe therapeutic strategy for epithelial cells when used at low energy densities. These findings correlate with our overall understanding of the safety of using LPT in clinical applications and lead to new exciting questions about the molecular circuitry involved in LPT-induced accelerated healing. AcknowledgmentsThis work was funded in part by the University of Michigan, School of Dentistry startup and by the Universidade Federal do Rio Grande do Sul (UFRGS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. There was no industrial funding for the study. ReferencesZ. I. Alamet al.,
“Oxidative DNA damage in the Parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra,”
J. Neurochem., 69
(3), 1196
–1203
(1997). http://dx.doi.org/10.1046/j.1471-4159.1997.69031196.x JONRA9 0022-3042 Google Scholar
F. G. Bassoet al.,
“Biostimulatory effect of low-level laser therapy on keratinocytes in vitro,”
Lasers Med. Sci., 28
(2), 367
–374
(2013). http://dx.doi.org/10.1007/s10103-012-1057-8 LMSCEZ 1435-604X Google Scholar
C. D’Arcangeloet al.,
“A preliminary study of healing of diode laser versus scalpel incisions in rat oral tissue: a comparison of clinical, histological, and immunohistochemical results,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 103
(6), 764
–773
(2007). Google Scholar
A. Schindlet al.,
“Diabetic neuropathic foot ulcer: successful treatment by low-intensity laser therapy,”
Dermatology, 198
(3), 314
–316
(1999). http://dx.doi.org/10.1159/000018140 DERMEI 0742-3217 Google Scholar
A. Schindlet al.,
“Low-intensity laser irradiation improves skin circulation in patients with diabetic microangiopathy,”
Diabetes Care, 21
(4), 580
–584
(1998). http://dx.doi.org/10.2337/diacare.21.4.580 DICAD2 0149-5992 Google Scholar
P. V. PeplowT. Y. ChungG. D. Baxter,
“Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models,”
Photomed. Laser Surg., 28
(3), 291
–325
(2010). http://dx.doi.org/10.1089/pho.2008.2446 PLDHA8 1549-5418 Google Scholar
A. C. Pellicioliet al.,
“Laser phototherapy accelerates oral keratinocyte migration through the modulation of the mammalian target of rapamycin signaling pathway,”
J. Biomed. Opt., 19
(2), 28002
(2014). http://dx.doi.org/10.1117/1.JBO.19.2.028002 JBOPFO 1083-3668 Google Scholar
T. Kushibikiet al.,
“Blue laser irradiation generates intracellular reactive oxygen species in various types of cells,”
Photomed. Laser Surg., 31
(3), 95
–104
(2013). http://dx.doi.org/10.1089/pho.2012.3361 PLDHA8 1549-5418 Google Scholar
F. M. de Limaet al.,
“Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion,”
Photochem. Photobiol., 89
(1), 179
–188
(2013). http://dx.doi.org/10.1111/j.1751-1097.2012.01214.x PHCBAP 0031-8655 Google Scholar
L. Luoet al.,
“Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-beta1 in skeletal muscle during the repair process,”
Lasers Med. Sci., 28
(3), 725
–734
(2013). http://dx.doi.org/10.1007/s10103-012-1133-0 LMSCEZ 1435-604X Google Scholar
A. C. Chenet al.,
“Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts,”
PLoS One, 6
(7), e22453
(2011). http://dx.doi.org/10.1371/journal.pone.0022453 1932-6203 Google Scholar
Y. Y. Huanget al.,
“Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro,”
J. Biophotonics, 6
(10), 829
–838
(2013). http://dx.doi.org/10.1002/jbio.201200157 JBOIBX 1864-063X Google Scholar
A. R. Collinset al.,
“Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates,”
FASEB J., 12
(13), 1397
–1400
(1998). FAJOEC 0892-6638 Google Scholar
M. T. Elnakishet al.,
“Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: important role of Rac/NADPH oxidase,”
J. Pathol., 231
(3), 290
–300
(2013). http://dx.doi.org/10.1002/path.4255 JPTLAS 0022-3417 Google Scholar
A. Konioret al.,
“NADPH oxidases in vascular pathology,”
Antioxid. Redox Signaling,
(2013). ARSIF2 1523-0864 Google Scholar
M. A. LovellS. P. GabbitaW. R. Markesbery,
“Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF,”
J. Neurochem., 72
(2), 771
–776
(1999). http://dx.doi.org/10.1046/j.1471-4159.1999.0720771.x JONRA9 0022-3042 Google Scholar
V. S. DhillonM. Fenech,
“Mutations that affect mitochondrial functions and their association with neurodegenerative diseases,”
Mutat. Res., 759 1
–13
(2013). http://dx.doi.org/10.1016/j.mrrev.2013.09.001 MUREAV 0027-5107 Google Scholar
H. WisemanB. Halliwell,
“Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer,”
Biochem. J., 313
(Pt 1), 17
–29
(1996). BIJOAK 0264-6021 Google Scholar
D. Harman,
“Aging: a theory based on free radical and radiation chemistry,”
J. Gerontol., 11
(3), 298
–300
(1956). http://dx.doi.org/10.1093/geronj/11.3.298 JOGEA3 0022-1422 Google Scholar
M. S. Cookeet al.,
“Oxidative DNA damage: mechanisms, mutation, and disease,”
FASEB J., 17
(10), 1195
–1214
(2003). http://dx.doi.org/10.1096/fj.02-0752rev FAJOEC 0892-6638 Google Scholar
J. H. Booet al.,
“Accumulation of phosphorylated beta-catenin enhances ROS-induced cell death in presenilin-deficient cells,”
PLoS One, 4
(1), e4172
(2009). http://dx.doi.org/10.1371/journal.pone.0004172 1932-6203 Google Scholar
H. M. ShenS. Pervaiz,
“TNF receptor superfamily-induced cell death: redox-dependent execution,”
FASEB J., 20
(10), 1589
–1598
(2006). http://dx.doi.org/10.1096/fj.05-5603rev FAJOEC 0892-6638 Google Scholar
M. BenharD. EngelbergA. Levitzki,
“ROS, stress-activated kinases and stress signaling in cancer,”
EMBO Rep., 3
(5), 420
–425
(2002). http://dx.doi.org/10.1093/embo-reports/kvf094 ERMEAX 1469-221X Google Scholar
K. Nakaet al.,
“Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells,”
Antioxid. Redox Signal., 10
(11), 1883
–1894
(2008). http://dx.doi.org/10.1089/ars.2008.2114 ARSIF2 1523-0864 Google Scholar
T. FinkelN. J. Holbrook,
“Oxidants, oxidative stress and the biology of ageing,”
Nature, 408
(6809), 239
–247
(2000). http://dx.doi.org/10.1038/35041687 NATUAS 0028-0836 Google Scholar
M. M. Juntillaet al.,
“AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species,”
Blood, 115
(20), 4030
–4038
(2010). http://dx.doi.org/10.1182/blood-2009-09-241000 BLOOAW 0006-4971 Google Scholar
J. E. Le Belleet al.,
“Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner,”
Cell Stem Cell, 8
(1), 59
–71
(2011). http://dx.doi.org/10.1016/j.stem.2010.11.028 CSCEC4 1875-9777 Google Scholar
T. Finkel,
“Oxygen radicals and signaling,”
Curr. Opin. Cell Biol., 10
(2), 248
–253
(1998). http://dx.doi.org/10.1016/S0955-0674(98)80147-6 COCBE3 0955-0674 Google Scholar
T. Finkel,
“Signal transduction by reactive oxygen species,”
J. Cell Biol., 194
(1), 7
–15
(2011). http://dx.doi.org/10.1083/jcb.201102095 JCLBA3 0021-9525 Google Scholar
R. M. Castilhoet al.,
“Rac1 is required for epithelial stem cell function during dermal and oral mucosal wound healing but not for tissue homeostasis in mice,”
PLoS One, 5
(5), e10503
(2010). http://dx.doi.org/10.1371/journal.pone.0010503 1932-6203 Google Scholar
F. S. Giudiceet al.,
“Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer,”
PLoS One, 8
(3), e58672
(2013). http://dx.doi.org/10.1371/journal.pone.0058672 1932-6203 Google Scholar
H. H. van BreugelP. R. Bar,
“Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro,”
Lasers Surg. Med., 12
(5), 528
–537
(1992). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. N. Pereiraet al.,
“Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts,”
Lasers Surg. Med., 31
(4), 263
–267
(2002). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. V. Corazzaet al.,
“Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources,”
Photomed. Laser Surg., 25
(2), 102
–106
(2007). http://dx.doi.org/10.1089/pho.2006.2011 PLDHA8 1549-5418 Google Scholar
A. R. Medradoet al.,
“Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts,”
Lasers Surg. Med., 32
(3), 239
–244
(2003). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Kubota,
“Defocused diode laser therapy (830 nm) in the treatment of unresponsive skin ulcers: a preliminary trial,”
J. Cosmet. Laser Ther., 6
(2), 96
–102
(2004). http://dx.doi.org/10.1080/14764170410014983 1476-4172 Google Scholar
D. G. Minatelet al.,
“Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies,”
Lasers Surg. Med., 41
(6), 433
–441
(2009). http://dx.doi.org/10.1002/lsm.v41:6 LSMEDI 0196-8092 Google Scholar
R. Navarroet al.,
“Low-level-laser therapy as an alternative treatment for primary herpes simplex infection: a case report,”
J. Clin. Pediatr. Dent., 31
(4), 225
–228
(2007). JCPDEX 1053-4628 Google Scholar
T. I. KaruL. V. PyatibratN. I. Afanasyeva,
“A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation,”
Photochem. Photobiol., 80
(2), 366
–372
(2004). http://dx.doi.org/10.1562/2004-03-25-RA-123.1 PHCBAP 0031-8655 Google Scholar
M. Sundaresanet al.,
“Requirement for generation of for platelet-derived growth factor signal transduction,”
Science, 270
(5234), 296
–299
(1995). http://dx.doi.org/10.1126/science.270.5234.296 SCIEAS 0036-8075 Google Scholar
S. Raguet al.,
“Oxygen metabolism and reactive oxygen species cause chromosomal rearrangements and cell death,”
Proc. Natl. Acad. Sci. U. S. A., 104
(23), 9747
–9752
(2007). http://dx.doi.org/10.1073/pnas.0703192104 PNASA6 0027-8424 Google Scholar
D. K. Wooet al.,
“Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice,”
Am. J. Pathol., 180
(1), 24
–31
(2012). http://dx.doi.org/10.1016/j.ajpath.2011.10.003 AJPAA4 0002-9440 Google Scholar
C. Dinantet al.,
“Activation of multiple DNA repair pathways by sub-nuclear damage induction methods,”
J. Cell Sci., 120
(15), 2731
–2740
(2007). http://dx.doi.org/10.1242/jcs.004523 JNCSAI 0021-9533 Google Scholar
A. El-HusseinM. HarithH. Abrahamse,
“Assessment of DNA damage after photodynamic therapy using a metallophthalocyanine photosensitizer,”
Int. J. Photoenergy, 2012 1
–10
(2012). IJPNBU 1110-662X Google Scholar
C. X. Deng,
“Tumorigenesis as a consequence of genetic instability in Brca1 mutant mice,”
Mutat. Res., 477
(1–2), 183
–189
(2001). http://dx.doi.org/10.1016/S0027-5107(01)00119-1 MUREAV 0027-5107 Google Scholar
K. W. KinzlerB. Vogelstein,
“Cancer-susceptibility genes. Gatekeepers and caretakers,”
Nature, 386
(6627), 761
–763
(1997). http://dx.doi.org/10.1038/386761a0 NATUAS 0028-0836 Google Scholar
S. J. Boulton,
“Cellular functions of the BRCA tumour-suppressor proteins,”
Biochem. Soc. Trans., 34
(Pt 5), 633
–645
(2006). BCSTB5 0300-5127 Google Scholar
D. Cortezet al.,
“Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks,”
Science, 286
(5442), 1162
–1166
(1999). http://dx.doi.org/10.1126/science.286.5442.1162 SCIEAS 0036-8075 Google Scholar
A. P. Gautamet al.,
“Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy—a randomized controlled trial,”
Supportive Care Cancer, 21
(5), 1421
–1428
(2013). http://dx.doi.org/10.1007/s00520-012-1684-4 0941-4355 Google Scholar
A. P. Gautamet al.,
“Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients—a triple blinded randomized controlled trial,”
Radiother. Oncol., 104
(3), 349
–354
(2012). http://dx.doi.org/10.1016/j.radonc.2012.06.011 RAONDT 0167-8140 Google Scholar
P. A. Carvalhoet al.,
“Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients,”
Oral Oncol., 47
(12), 1176
–1181
(2011). http://dx.doi.org/10.1016/j.oraloncology.2011.08.021 EJCCER 1368-8375 Google Scholar
A. Kuhnet al.,
“Low-level infrared laser therapy in chemotherapy-induced oral mucositis: a randomized placebo-controlled trial in children,”
J. Pediatr. Hematol. Oncol., 31
(1), 33
–37
(2009). http://dx.doi.org/10.1097/MPH.0b013e318192cb8e JPHOFG 1077-4114 Google Scholar
B. Beauvoitet al.,
“Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors,”
Anal. Biochem., 226
(1), 167
–174
(1995). http://dx.doi.org/10.1006/abio.1995.1205 ANBCA2 0003-2697 Google Scholar
C. E. CooperR. Springett,
“Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy,”
Philos. Trans. R. Soc., B, 352
(1354), 669
–676
(1997). http://dx.doi.org/10.1098/rstb.1997.0048 PTRBAE 0962-8436 Google Scholar
E. Alexandratouet al.,
“Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy,”
Photochem. Photobiol. Sci., 1
(8), 547
–552
(2002). http://dx.doi.org/10.1039/b110213n PPSHCB 1474-905X Google Scholar
S. Passarellaet al.,
“Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser,”
FEBS Lett., 175
(1), 95
–99
(1984). http://dx.doi.org/10.1016/0014-5793(84)80577-3 FEBLAL 0014-5793 Google Scholar
M. S. Moreiraet al.,
“Effect of laser phototherapy on wound healing following cerebral ischemia by cryogenic injury,”
J. Photochem. Photobio. B, 105
(3), 207
–215
(2011). http://dx.doi.org/10.1016/j.jphotobiol.2011.09.005 JPPBEG 1011-1344 Google Scholar
M. M. Marqueset al.,
“Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts,”
Lasers Surg. Med., 34
(3), 260
–265
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
R. T. Chowet al.,
“Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials,”
Lancet, 374
(9705), 1897
–1908
(2009). http://dx.doi.org/10.1016/S0140-6736(09)61522-1 LANCAO 0140-6736 Google Scholar
E. S. Boschiet al.,
“Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat,”
Lasers Surg. Med., 40
(7), 500
–508
(2008). http://dx.doi.org/10.1002/lsm.v40:7 LSMEDI 0196-8092 Google Scholar
A. C. Alveset al.,
“Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation,”
Arthritis Res. Ther., 15
(5), R116
(2013). http://dx.doi.org/10.1186/ar4296 ARTRCV 1478-6354 Google Scholar
J. T. Eellset al.,
“Therapeutic photobiomodulation for methanol-induced retinal toxicity,”
Proc. Natl. Acad. Sci. U. S. A., 100
(6), 3439
–3444
(2003). http://dx.doi.org/10.1073/pnas.0534746100 PNASA6 0027-8424 Google Scholar
H. L. Lianget al.,
“Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity,”
Neuroscience, 153
(4), 963
–974
(2008). http://dx.doi.org/10.1016/j.neuroscience.2008.03.042 0306-4522 Google Scholar
J. J. AndersS. GeunaS. Rochkind,
“Phototherapy promotes regeneration and functional recovery of injured peripheral nerve,”
Neurol. Res., 26
(2), 233
–239
(2004). http://dx.doi.org/10.1179/016164104225013914 NRESDZ 0161-6412 Google Scholar
F. P. Eduardoet al.,
“Cultured epithelial cells response to phototherapy with low intensity laser,”
Lasers Surg. Med., 39
(4), 365
–372
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Y. Leeet al.,
“Effect of low-level laser therapy on oral keratinocytes exposed to bisphosphonate,”
Lasers Med. Sci.,
(2013). LMSCEZ 1435-604X Google Scholar
V. P. Wagneret al.,
“Influence of different energy densities of laser phototherapy on oral wound healing,”
J. Biomed. Opt., 18
(12), 128002
(2013). http://dx.doi.org/10.1117/1.JBO.18.12.128002 JBOPFO 1083-3668 Google Scholar
F. Gueraudet al.,
“Chemistry and biochemistry of lipid peroxidation products,”
Free Radic. Res., 44
(10), 1098
–1124
(2010). http://dx.doi.org/10.3109/10715762.2010.498477 FRARER 1071-5762 Google Scholar
P. V. PeplowT. Y. ChungG. D. Baxter,
“Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies,”
Photomed. Laser Surg., 28
(Suppl. 1), S3
–S40
(2010). PLDHA8 1549-5418 Google Scholar
W. C. Tsaiet al.,
“Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression,”
PLoS One, 7
(5), e38235
(2012). http://dx.doi.org/10.1371/journal.pone.0038235 1932-6203 Google Scholar
X. GaoD. Xing,
“Molecular mechanisms of cell proliferation induced by low power laser irradiation,”
J. Biomed. Sci., 16
(4), 1
–16
(2009). http://dx.doi.org/10.1186/1423-0127-16-4 JBCIEA 1021-7770 Google Scholar
M. J. Jouet al.,
“Critical role of mitochondrial reactive oxygen species formation in visible laser irradiation-induced apoptosis in rat brain astrocytes (RBA-1),”
J. Biomed. Sci., 9
(6 Pt 1), 507
–516
(2002). http://dx.doi.org/10.1007/BF02254977 JBCIEA 1021-7770 Google Scholar
Y. Y. Huanget al.,
“Biphasic dose response in low level light therapy—an update,”
Dose Response, 9
(4), 602
–618
(2011). http://dx.doi.org/10.2203/dose-response.11-009.Hamblin DOSECX 1559-3258 Google Scholar
T. De Marchiet al.,
“Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress,”
Lasers Med. Sci., 27
(1), 231
–236
(2012). http://dx.doi.org/10.1007/s10103-011-0955-5 LMSCEZ 1435-604X Google Scholar
T. S. LiE. Marban,
“Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells,”
Stem Cells, 28
(7), 1178
–1185
(2010). http://dx.doi.org/10.1002/stem.438 STCEDI 1066-5099 Google Scholar
BiographyCaroline Siviero Dillenburg received her DDS from the Pontifical Catholic University of Rio Grande do Sul, Brazil. She is a master’s degree student at the Department of Oral Pathology, School of Dentistry, Federal University of Rio Grande do Sul, Brazil. Her personal research interest is focused on the clinical effects of laser phototherapy in wound repair. Luciana Oliveira Almeida received her PhD degree from the University of Sao Paulo, Brazil. She is a postdoctoral fellow at the Laboratory of Epithelial Biology at the University of Michigan, School of Dentistry. Her research interest is focused on genetic and epigenetic alterations that change behavior of epithelial cells and its malignant counterpart. Manoela Domingues Martins received her DDS from the Federal University of Rio Grande do Sul, Brazil, and PhD degrees University of Sao Paulo, Brazil. She is a full professor at the Department of Oral Pathology, School of Dentistry, Federal University of Rio Grande do Sul, Brazil. Her personal research interest is the clinical and basic aspects of laser phototherapy in oral lesions and tissue regeneration. Cristiane Helena Squarize received her DDS from the Pontifical Catholic University of Campinas, Brazil and PhD degree from the University of Sao Paulo, Brazil. She is an assistant professor at the Department of Periodontics and Oral Medicine from the University of Michigan, School of Dentistry. Her interests are the study of the PI3K molecular circuitry with focus in wound healing and epithelial biology. Rogerio Moraes Castilho received his DDS from the Pontifical Catholic University of Campinas, Brazil, and PhD degree from the University of Sao Paulo, Brazil. He is an assistant professor at the Department of Periodontics and Oral Medicine from the University of Michigan, School of Dentistry and the principal investigator of the Laboratory of Epithelial Biology. His interest is in the molecular mechanism responsible for epigenetically regulate histone modifications and influence the behavior of epithelial cells. |

