|
|
1.IntroductionThe phenomenon of red fluorescence emitted by dental plaque and caries lesions after excitation with light at different spectrum ranges has been extensively studied.1–6 This fluorescence is probably proceeding from porphyrins synthesized by oral microorganisms associated with dental caries.1,5 Several tools proposed to support the clinicians in caries diagnosis process are based on the quantification of the fluorescence emitted by the carious tissue.2,7 Another use of these devices is the possibility of quantifying the red fluorescence of dental plaque in the oral environment.6,8–10 One of those devices is the quantitative light-induced fluorescence (QLF), which emits a blue light at 405 nm and collects the fluorescence at the green and red spectra.7 With regard to the dental plaque, the researchers have first focused on the investigation of individual microorganisms responsible for the emission of the red fluorescence. The authors observed that obligate anaerobic bacteria were the main source of red fluorescence, including microorganisms usually present in thicker plaque and in dentin caries lesions.3,11 The subsequent studies investigated the red fluorescence emitted by the dental plaque, evaluating whether the interactions among the microorganisms could have some influence on it. It was observed that combinations of some bacterium were more associated with the emission of red fluorescence than the individual species of microorganisms,6 and that this phenomenon is supposed to be related to the maturity of the dental plaque.3,6,12 Due to these findings, some authors have speculated that the red fluorescent plaque could be more cariogenic;3,4,13 hence, measuring this fluorescence could be a useful parameter to predict the caries development. To test this hypothesis, we designed the present in situ study aiming to investigate the role of the red fluorescence of dental plaque on the detection of its cariogenicity and potential to induce demineralization. 2.Material and Methods2.1.Subject Selection and Study DesignThis study was approved by the Committee for Ethics in Research of School of Dentistry, University of Sao Paulo, and signed and informed consent was obtained from volunteers. A two-phase crossover in situ study was designed. Each phase lasted for 14 days with a 7-day washout period between them. The sample size was determined using a procedure for paired samples, considering an expected relative difference between the experimental and control groups of 50%, alpha error of 5%, and statistical power of 80%. We performed separate calculations for the different outcomes, leading to 17 volunteers for surface microhardness loss, 16 for the cross-sectional microhardness test, and 11 for the red fluorescence of plaque. Eighteen volunteers (11 females and 7 males) aged 20 to 40 years were selected (). They presented good general and oral health, with no recent caries lesions, and stimulated salivary flow rate higher than . They did not report antibiotic use 2 months before the study and use of the orthodontic appliances. 2.2.Preparation of Enamel Samples and Palatal AppliancesBovine incisors were selected, cleaned with a rotating bristle brush and pumice/water slurry, and washed with tap water. Then, enamel slabs with dimensions of were sectioned from the middle third of the crown using a low-speed saw in a cut machine (Labcut 1010, Extec Corp., London, United Kingdom). These slabs were flattened under water cooling on a rotating polishing machine (Ecomet 3, Buehler, Lake Buff, Illinois) using silicon carbide paper (400, 600, and 1200 grits in sequence) and polished with a 1-μm diamond suspension. Then, the samples were visually checked for the absence of cracks or defects with a loupe ( magnification), and baseline surface microhardness analysis (SMH) was performed. Totally, 288 plane enamel blocks without defects, with SMH mean (standard deviation) values of , were selected and sterilized with gamma radiation (25 kGy). A portion of the surface in each enamel slab (a rectangular portion in the border of the enamel block with around 1 mm of width) was covered with a colorless nail varnish (Revlon, New York, New York, USA) to protect it against the cariogenic challenge. Baseline QLF images were taken in order to guarantee that the next measurements were taken at the same positions. In a pilot study, we observed that this colorless varnish does not emit fluorescence with the QLF. The samples were stored in 100% humidity until the beginning of the study. Individual acrylic palatal appliances containing eight cavities () were made and eight enamel blocks were positioned in each appliance and fixed with wax. A plastic mesh (0.27-mm thickness, squares, nylon monofil, Lauhman, Sumaré, Brazil) was fixed in the acrylic to protect the enamel slabs and to allow plaque accumulation. The subjects were randomly allocated into two groups. In the experimental group, they were orientated to drip a 20% sucrose solution extraorally onto each enamel sample, eight times a day in predetermined time periods.14,15 In the control group, the volunteers followed the same orientations, but purified water was dripped instead of the sucrose solution. The volunteers were orientated to remove the appliances during tooth brushing or during their meals. We recommended that they brush their teeth three times per day. One week before and during all phases of the experiment, the volunteers were orientated to use a nonfluoride dentifrice. Two slabs of each appliance (one for each side) were removed at predetermined periods (4, 7, 10, and 14 days). Random numbers generated by software were used to choose the position of the enamel block that was removed at each period. After the end of the first phase and the washout period, the other eight enamel slabs were positioned and the volunteers started the other phase, in which samples from the other group were tested. 2.3.Quantitative Light-Induced Fluorescence AssessmentsWe used a QLF device to perform the fluorescence-based assessments (QLF Inspektor Pro; Inspektor Research Systems, Amsterdam, The Netherlands), and the images were analyzed using the The Inspektor Pro Software (Inspektor Research Systems, Amsterdam, The Netherlands). Immediately after removal of the device, the specimen covered by the dental plaque was assessed by QLF in order to measure the red fluorescence of the plaque ( %). The plaque formed on the nail varnish was removed in order to expose a sound enamel surface to be used as reference during the red fluorescence assessment. The baseline images were considered to adjust the positions of the subsequent ones. 2.4.Surface and Cross-Sectional Microhardness EvaluationsAfter the QLF assessments, the SMH was evaluated. The evaluations were performed using a Knoop indentor of a microhardness tester (Shimadzu Micro Hardness Tester HMV-2, Shimadzu Corporation, Kyoto, Japan). Five indentations at distances of 100 μm between them were carried out. This row of indentations was performed at 100 μm of distance from the baseline indentations. The indentations were performed using a load of 50 g with a 25 s dwell time. We considered the mean of five indentations and the percentage of microhardness loss (%SML) was calculated considering the SMH values after the demineralization and the baseline SMH values. For cross-sectional microhardness analysis (CSMH), the enamel slabs were perpendicularly sectioned in a cutting machine and the cut surface was polished as mentioned previously. Then, CSMH was evaluated in the polished area using a Knoop indenter with a 25 g load and 5 s of dwell time. Two rows of 13 indentations each were performed from the outer enamel surface. From 10 to 60 μm, the indentations were spaced 10 μm apart. After 60 μm, they were spaced 20 μm, apart until the last indentation, which was positioned at 200 μm from the outer surface. The mean hardness at each distance for the two rows was calculated. Then, we calculated the area under the curve of microhardness versus lesion depth () by numerical integration using the trapezoidal rule. The area under the curve of the demineralized enamel was subtracted from the area of the sound one to obtain the mineral loss ().16 2.5.Statistical AnalysisFor all analyses, the measurements of two specimens removed at the same time from each subject of each group were averaged. The data were initially evaluated by Kolmogorov–Smirnov and Levene tests to check the normality and homogeneity, respectively. The variables presented normal distribution and the variances were homogenous, except for the red fluorescence of the dental plaque. We performed logarithmic transformation of these values (), and then the values reached the normality and homoscedasticity. The baseline values of SMH of the enamel slabs were compared using two-way analysis of variance (ANOVA) considering the groups (control or experimental) and days of dental plaque accumulation (4, 7, 10, and 14 days). After the in situ experiments, the outcomes (red fluorescence of dental plaque, %SML and ) were compared considering three independent variables: groups, days of plaque accumulation, and phase of the study (first or second phase). This last variable was evaluated to check if the washout period was adequate. In case of the presence of a statistical difference between the phases, it could be inferred that the first phase exerted a carryover effect on the second phase, and the values of the second phase should not be considered. However, there were no statistically significant differences between the phases for all outcomes. Therefore, the next analyses were performed considering only the variables groups and days of plaque accumulation, and the data of both phases were used. As all volunteers participated in the two groups, and the enamel blocks were clustered in the volunteers, we performed linear multilevel regression analysis considering two levels: enamel blocks (first level) and volunteers (second level). The coefficients and standard errors were calculated and the maximum-likelihood test was employed to derive the -values. After these comparisons, we performed linear multilevel regression analysis to evaluate the association between the red fluorescence of dental plaque and outcomes related to mineral loss of the teeth (%SML and values). First, a null model was fitted (with no variables). Then, we fitted a model with the red fluorescence of plaque and a third model adding the variable group. The analyses were carried out using the statistical software MLwin 2.10 (Centre for Multilevel Modeling, University of Bristol, Bristol, United Kingdom) and the level of significance was fixed at 5%. 3.ResultsOne volunteer had to take antibiotics during the study and was excluded. Therefore, 17 participants completed both phases of the study (follow-up rate of 94.4%). There were no significant differences on the baseline SMH values of the enamel slabs divided among the groups () and days of plaque accumulation (). The box plot graphics representing the values of red fluorescence of plaque (), and data obtained from the %SML and values of the enamel slabs are shown in Fig. 1. Figure 1(a) exhibits a box plot of the values of the red fluorescence after the logarithmic transformation according to the groups and days of plaque accumulation. We observed significantly higher red fluorescence values with increasing days of plaque accumulation in both groups (), which was more pronounced in the experimental group (). A statistically significant difference between experimental and control groups was observed only after 14 days of plaque accumulation [Figure 1(a)]. Fig. 1Box plots of red fluorescence of dental plaque (a), percentage of enamel surface microhardness loss (b) and mineral loss () (c) in different days of exposure to the oral environment of both experimental and control groups. Different letters indicate statistically significant differences among different days of exposure within the same group (). The superscript number (1) indicates a statistically significant difference between experimental and control groups at that specific time period (). Asterisks are outliers. 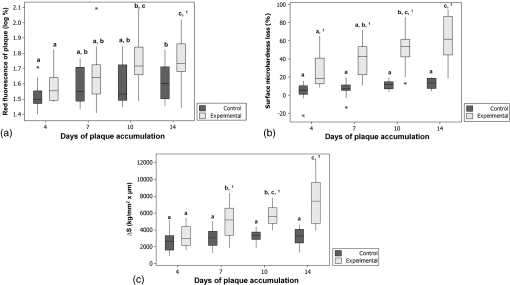 The %SML was significantly higher in the experimental group compared to the control group for all days of exposure. Furthermore, there was a significant increase among the days of plaque accumulation, but only for the experimental group (). In the control group, there was no significant difference among the different days of plaque accumulation [Figure 1(b)]. A similar trend was observed for the values. There was no significant difference among the values obtained with different days of plaque accumulation in the control group, but in the experimental group there was a trend of higher mineral loss with a longer time of sucrose exposure (). Moreover, there was a statistically significant difference between the control and experimental groups considering the different days of plaque accumulation, except after 4 days [Figure 1(c)]. According to the linear multilevel regression analysis to investigate the association of red fluorescence of dental plaque and mineral loss (measured by SMH and CSMH techniques), we observed a significant association between red fluorescence of plaque and percentage of SMH loss (Table 1, model 2), which remained significant when it was adjusted for the sucrose exposure (Table 1, model 2). Moreover, we could observe in the random part of the model that the first level (enamel blocks) was responsible for the major part of the residual variance, since the percentage of variance attributed to the second level was 19.9 in the final model (Table 1). Table 1Multilevel linear regression analysis of the association between red fluorescence of plaque (log ΔR %) and percentage of surface microhardness loss (%SML) adjusted by exposure to sucrose or not.
Note: β=Coefficient of linear regression; SE=Standard error*p-value calculated by maximum-likelihood test In Table 2, when the association of red fluorescence for plaque and values was analyzed, a similar trend was observed. We also found a statistically significant association with this outcome (Table 2, model 2), which was also significant when adjusted for the sucrose exposure (Table 2, model 3). The higher portion of the residual variance (around 90%) was also related to the enamel specimens (Table 2). Table 2Multilevel linear regression analysis of the association between red fluorescence of plaque (log ΔR %) and integrated area of cross-sectional hardness assessment (ΔS) adjusted by exposure to sucrose or not.
β=Coefficient of linear regression; SE=Standard error*p-value calculated by maximum-likelihood test 4.DiscussionPrevious studies have suggested that the red fluorescence of dental plaque is probably related to its maturity and thickness, and some bacterial species related to dental caries are capable of producing this type of fluorescence.3,6,17 Thus, some authors have speculated that dental plaque emitting red fluorescence could be more cariogenic.3,4,13,17 To test this premise, the current experiment was designed, consisting of, to the best of our knowledge, the first in situ study that directly tested the relationship between the red fluorescence of dental plaque and its cariogenicity. We observed that the red fluorescence of dental plaque indicated that this biofilm was actually more mature, but it was not possible to observe if this parameter is directly associated with cariogenicity. For the detection of mineral loss, we employed microhardness techniques. It is well known from the literature that the mineral loss would be greater in the experimental group and with more days of exposure to sucrose.14,18 Both microhardness tests detected this trend. Another methodological aspect in our study was the use of a multilevel approach for the statistical analysis, which presents some advantages when compared to the ANOVA.19 First, since a multilevel approach allows the inclusion of several variables in the regression model,20 we included the phase of the study as an independent variable and we could observe that the first phase of the experiment does not present any carryover effect on the second phase. Moreover, multilevel analysis would allow including participants who have dropped out in the second phase. However, we excluded the volunteer who did not complete our study, because the statistical power of the study was not affected by this exclusion. Another advantage of multilevel analysis is that it allows the evaluation of both the fixed and the random effects of the variables. The fixed effects are related to the measures of central tendency (regression coefficients and their respective standard errors), while the random effects are associated with a variance components model. The analysis of the random effects allows evaluating the magnitude of the residual variance of the model in the different levels.19,20 In the current study, we observed that the residual variance of the final model of around 80% and 90%, considering, respectively, the SMH and values, was related to the enamel samples (first level). This finding emphasizes the advantage of using a crossover experimental design in order to reduce the intersubject variability. We employed the QLF method to quantify the red fluorescence of the dental plaque, which has been related to metabolites produced by bacterium associated with dental caries and periodontal disease.5,17 These metabolites have been shown to be porphyrins;1,7 however, it has also been speculated that this fluorescence would possibly be emitted by extrinsic and intrinsic polysaccharides produced by microorganisms present in mature dental plaque.7 The first reports on this issue have focused on the red fluorescence produced by some anaerobic species of microorganisms cultured in the laboratory, such as some species of Actinomyces and microorganisms associated with periodontal disease.3,11 Regarding dental caries, species of streptococci did not exhibit red fluorescence,3,5,11 but various species of Lactobacilli emitted fluorescence at the red spectrum.11 Recently, a research observed that microorganisms related to dental caries, including Streptococcus mutans, Lactobacillus salivarius, and Bifidobacterium dentium, produced red fluorescence when some nutrients were added to the growth media. This finding suggested that the metabolic products of the oral microorganisms are responsible for emit red fluorescence.17 Other studies were performed to evaluate the fluorescence of dental plaque instead of that of individual species of microorganisms. An in vitro study found that some species only exhibited red fluorescence when they were close to other microorganisms,6 which was confirmed by an in situ study.12 Thus, these authors stated that the architecture of dental plaque was responsible for the emission of the red fluorescence after excitation with a blue light.6,7,12 Recently, some in vitro studies showed that the red fluorescence of biofilm grown with sucrose exposure was correlated with maturity and with some cariogenic properties of this biofilm.13,21 However, the authors did not evaluate the fluorescence of the biofilm formed for the time with no sucrose exposure.13,21 Therefore, it would not be possible to infer if the fluorescence was due to maturity or because of cariogenic characteristics. However, it would seem plausible that the red fluorescent dental plaque is more cariogenic. Therefore, we designed an in situ experiment using enamel specimens with standardized sucrose exposure and compared these to a control group with no cariogenic challenge. If the red fluorescent plaque was really more cariogenic, it would be expected that the red fluorescence of the plaque had followed the same pattern of mineral loss. Thereby, a gradual increase of red fluorescence would occur only in the experimental group. However, we observed this gradual increase with different days of plaque accumulation, but this fact happened in both experimental and control groups, although the increase was more significant in the biofilm exposed to sucrose. This result corroborates the assertion that the red fluorescent plaque is related to its maturity.3,6,7,9,11,12 The statistically significant difference observed between the groups after 14 days of plaque accumulation is understandable since the dental plaque exposed to sucrose is often formed faster.22 However, since there was no significant demineralization in the control group, the red fluorescence of the dental plaque was not directly associated with its cariogenic activity. A possible explanation for our results is that even with no sucrose exposure, the dental plaque that is not disturbed mechanically becomes thicker and harbors anaerobic bacteria species that emit red fluorescence, independently of its cariogenicity.3,11 Therefore, our findings refuted the hypothesis that the red fluorescence of dental plaque could be a single indicator of its cariogenicity. However, we observed in the multiple linear regression analysis that the red fluorescence of dental plaque adjusted for sucrose exposure was associated with the mineral loss of the specimens. Thus, collecting the fluorescence of the dental plaque together with the frequency of sucrose exposure of the patients could be a promising approach to assess the risk for the occurrence of dental caries lesions. Further studies should be conducted to investigate this possibility. In conclusion, the red fluorescence of dental plaque is related to the mature plaque, but this fact is not necessarily associated with its cariogenic activity. AcknowledgmentsWe would like to thank the volunteers who kindly participated in our research. This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Process # 565061/2008-9, 302368/2008-6 and 304545/2011-2) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Process # 2009/16082-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no commercial interests in the products herein mentioned. ReferencesW. Buchalla,
“Comparative fluorescence spectroscopy shows differences in noncavitated enamel lesions,”
Caries Res., 39
(2), 150
–156
(2005). http://dx.doi.org/10.1159/000083162 CAREBK 0008-6568 Google Scholar
T. Gimenezet al.,
“Fluorescence-based methods for detecting caries lesions: systematic review, meta-analysis and sources of heterogeneity,”
PloS One, 8
(4), e60421
(2013). http://dx.doi.org/10.1371/journal.pone.0060421 1932-6203 Google Scholar
L. Coulthwaiteet al.,
“The microbiological origin of fluorescence observed in plaque on dentures during QLF analysis,”
Caries Res., 40
(2), 112
–116
(2006). http://dx.doi.org/10.1159/000091056 CAREBK 0008-6568 Google Scholar
R. Heinrich-Weltzienet al.,
“Quantitative light-induced fluorescence (QLF)—a potential method for the dental practitioner,”
Quintessence Int., 34
(3), 181
–188
(2003). Google Scholar
K. KonigG. FlemmingR. Hibst,
“Laser-induced autofluorescence spectroscopy of dental caries,”
Cell. Mol. Biol., 44
(8), 1293
–1300
(1998). CMBID4 0145-5680 Google Scholar
M. H. van der Veenet al.,
“Red autofluorescence of dental plaque bacteria,”
Caries. Res., 40
(6), 542
–545
(2006). http://dx.doi.org/10.1159/000095655 CAREBK 0008-6568 Google Scholar
E. D. de Josselin de Jonget al.,
“Quantified light-induced fluorescence, review of a diagnostic tool in prevention of oral disease,”
J. Appl. Phys., 105
(10), 102031
(2009). http://dx.doi.org/10.1063/1.3116138 JAPIAU 0021-8979 Google Scholar
L. Coulthwaiteet al.,
“QLF is not readily suitable for in vivo denture plaque assessment,”
J. Dent., 37
(11), 898
–901
(2009). http://dx.doi.org/10.1016/j.jdent.2009.07.002 JDENAB 0300-5712 Google Scholar
D. P. Raggioet al.,
“Reliability and discriminatory power of methods for dental plaque quantification,”
J. Appl. Oral Sci., 18
(2), 186
–193
(2010). http://dx.doi.org/10.1590/S1678-77572010000200014 1678-7757 Google Scholar
I. A. Prettyet al.,
“Quantification of dental plaque in the research environment,”
J. Dent., 33
(3), 193
–207
(2005). http://dx.doi.org/10.1016/j.jdent.2004.10.017 JDENAB 0300-5712 Google Scholar
A. M. Lennonet al.,
“The ability of selected oral microorganisms to emit red fluorescence,”
Caries Res., 40
(1), 2
–5
(2006). http://dx.doi.org/10.1159/000088898 CAREBK 0008-6568 Google Scholar
R. Z. Thomaset al.,
“Bacterial composition and red fluorescence of plaque in relation to primary and secondary caries next to composite: an in situ study,”
Oral Microbiol. Immunol., 23
(1), 7
–13
(2008). http://dx.doi.org/10.1111/j.1399-302X.2007.00381.x OMIMEE 0902-0055 Google Scholar
E. S. Leeet al.,
“Association between the cariogenicity of a dental microcosm biofilm and its red fluorescence detected by quantitative light-induced fluorescence-digital (QLF-D),”
J. Dent., 41
(12), 1264
–1270
(2013). http://dx.doi.org/10.1016/j.jdent.2013.08.021 JDENAB 0300-5712 Google Scholar
C. P. Aireset al.,
“Effect of sucrose concentration on dental biofilm formed in situ and on enamel demineralization,”
Caries Res., 40
(1), 28
–32
(2006). http://dx.doi.org/10.1159/000088902 CAREBK 0008-6568 Google Scholar
J. A. Curyet al.,
“Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose,”
Caries Res., 34
(6), 491
–497
(2000). http://dx.doi.org/10.1159/000016629 CAREBK 0008-6568 Google Scholar
P. A. Anaet al.,
“Effect of Er,Cr:YSGG laser and professional fluoride application on enamel demineralization and on fluoride retention,”
Caries Res., 46
(5), 441
–451
(2012). http://dx.doi.org/10.1159/000333603 CAREBK 0008-6568 Google Scholar
C. M. Volgenantet al.,
“Effect of metalloporphyrins on red autofluorescence from oral bacteria,”
Eur. J. Oral Sci., 121
(3 Pt 1), 156
–161
(2013). http://dx.doi.org/10.1111/eos.2013.121.issue-3pt1 EJOSFY 0909-8836 Google Scholar
G. C. Valeet al.,
“Temporal relationship between sucrose-associated changes in dental biofilm composition and enamel demineralization,”
Caries Res., 41
(5), 406
–412
(2007). http://dx.doi.org/10.1159/000105764 CAREBK 0008-6568 Google Scholar
H. GoldsteinW. BrowneJ. Rasbash,
“Multilevel modelling of medical data,”
Stat. Med., 21
(21), 3291
–3315
(2002). http://dx.doi.org/10.1002/(ISSN)1097-0258 SMEDDA 1097-0258 Google Scholar
A. V. Diez-Roux,
“Multilevel analysis in public health research,”
Ann. Rev. Pub. Health, 21 171
–192
(2000). http://dx.doi.org/10.1146/annurev.publhealth.21.1.171 AREHDT 0163-7525 Google Scholar
Y. S. Kimet al.,
“Monitoring the maturation process of a dental microcosm biofilm using the quantitative light-induced fluorescence-digital (QLF-D),”
J. Dent., 42
(6), 691
–696
(2014). http://dx.doi.org/10.1016/j.jdent.2014.03.006 JDENAB 0300-5712 Google Scholar
R. A. Ccahuana-Vasquezet al.,
“Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride,”
Caries Res., 41
(1), 9
–15
(2007). http://dx.doi.org/10.1159/000096100 CAREBK 0008-6568 Google Scholar
BiographyDaniela G. Bittar is a dentist and a specialist in pediatric dentistry. She concluded her Master of Science course in 2012 in the School of Dentistry, University of Sao Paulo (USP). The present manuscript is related to her Master of Science thesis. Nowadays, she is attending a specialization course in orthodontics. Laura Regina A. Pontes is a final year undergraduate student in the School of Dentistry, USP. She has participated in the program of training in research for undergraduate students for 3 years and she is recipient of a scholarship for undergraduate students from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Ana Flávia B. Calvo is a dentist and is a PhD student in the School of Dentistry, USP. She is also a specialist in pediatric dentistry and concluded her Master of Science course in the Piracicaba Dental School, University of Campinas (UNICAMP). Tatiane F. Novaes is a dentist and finished her Master of Science and PhD course student in the School of Dentistry, University of Sao Paulo. Nowadays, she is participant of the postdoctoral program in the University Cruzeiro do Sul, São Paulo, Brazil. Mariana M. Braga is a dentist and a senior lecturer in the Department of Pediatric Dentistry in the School of Dentistry, USP. She is PhD in dental sciences for the same university. She is recipient of the Research Productivity Scholarship–CNPq. Patricia M. Freitas is a dentist and obtained her PhD degree in the Piracicaba Dental School, UNICAMP. Nowadays, she is an associate professor at the Department of Restorative Dentistry, School of Dentistry, USP. She is recipient of the Research Productivity Scholarship – CNPq. Cinthia P. M. Tabchoury is a graduate in pharmacy and biochemistry in the Federal University of Alfenas, and she concluded her Master of Science and PhD course in pharmacology in the Piracicaba Dental School, UNICAMP. She is an associate professor of biochemistry and cariology in the Piracicaba Dental School, UNICAMP. She is also recipient of the Research Productivity Scholarship–CNPq. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

