|
|
|
Cell transfection is one of the most important techniques in life sciences. It allows a specific modification of the gene expression of a cell. This is often achieved by the transient expression of a gene after DNA transfection. The delivery of small interfering RNA (siRNA) or Morpholinos allows the transient blockage of the translation of a protein of interest. Thereby, a knockdown of a gene of interest is achieved. The injection of proteins enables direct manipulation without modifications on the genetic level. A versatile alternative to common transfection techniques, like electroporation and lipofection, is based on the interaction of laser irradiation and gold nanoparticles.1–3 The routine procedure consists of the incubation of cells with gold nanoparticles with sizes of 80 to 200 nm for 3 to 6 h, which is sufficient for sedimentation for these large particles.1,2,4 Alternatively, smaller antibody-conjugated particles are used.3 Irradiation of the gold nanoparticles can be performed at various pulse lengths and laser wavelengths. Yao et al. applied nanosecond and picosecond laser pulses at 532 nm to deliver 10-kDa dextrans and antibodies into cells. Antibody conjugated 30-nm nanospheres were used in their study.3 Heinemann et al.1 presented a fully automated setup employing 850-ps pulses at 532 nm. The delivery of siRNA, Morpholinos, and dextrans of sizes up to 2000 kDa was demonstrated using this setup.1,4,5 For this purpose, 80 to 200-nm gold nanoparticles were analyzed. Baumgart et al.2 used femtosecond laser pulses to deliver DNA to cells by the irradiation of 100-nm gold nanoparticles. The interactions, which lead to membrane permeabilization, are based on heating of the particle or nanocavitation due to the near-field enhancement.6,7 The main disadvantage of all techniques is the incubation with the gold nanoparticles. First, it is time-consuming, which limits the high-throughput applicability in routine usage. Second, it might affect the safety of the procedure, especially for sensitive cells. Both issues can be overcome using gold nanostructures as demonstrated by Wu et al.8 and Schomaker et al.9 This requires the complex production of plasmonic structures. Furthermore, they need to fit standard cell culture plates for routine usage, which is probably difficult to achieve. In this study, we aim to present a new and very simple technique to achieve an immobilization of spherical gold nanoparticles on glass and surface-treated cell culture surfaces in combination with gold nanoparticle-mediated laser transfection. It is based on the attachment of gold nanoparticles to surfaces after silanization.10 It is applied for nanoparticles of different sizes. The delivery of 10-kDa dextrans is demonstrated. A comparison to the standard procedure of gold nanoparticle mediate laser transfection with 3 h of incubation is given. The fully automatized optical setup and the standard perforation parameters with about six particles on the cell surface are described by Heinemann et al.1 and Kalies et al.4 Briefly, it consists of an 850-ps laser operating at 532 nm and at a repetition rate of 20.25 kHz, two galvanometer scanning mirrors, an attenuator, focusing optics, and a moveable microscopy stage. In this study, a fixed radiant exposure of , a scanning velocity of , and a spot size of were applied. This leads to a speed of 8 s per single well of a 96 well plate. Spherical gold nanoparticles of sizes 30, 60, 80, and 200 nm were tested. The standard protocol with incubation consists of three steps. First, about 60,000 cells at the day of the experiment are incubated with 200-nm gold nanoparticles in a 96 well plate.1 S, 10-kDa fluorescein isothiocyanate (FITC) dextrans as an indicator of perforation efficiency are added in new medium. The perforation efficiency combines the number of perforated cells with the fluorescence per single cell. The concentration is . In the last step, the perforation efficiency is evaluated by the readout of the fluorescence per well with a microplate reader at a 520-nm emission wavelength. Furthermore, the viability is tested with the viability indicator QBlue which is based on the reduction of resorufin in metabolically active cells 1 h after laser treatment. Heinemann et al.1,4,5 also give a more detailed protocol. The proposed immobilization protocol of this study makes use of the silane (3-aminopropyl)-trimethoxysilane (APTMS).10 In a process called silanization, hydroxyl groups on glass replace the alkoxy groups on the silane. This leads to the formation of a covalent ─Si─O─Si─ bond. A self-assembled monolayer is created. It yields free amino groups, which can electrostatically bind the gold nanoparticles.10 The binding to the used polystyrene cell culture surfaces (black, clear-bottom 96 well plates, Corning, Pittston, Pennsylvania GmbH) occurs because the surfaces are routinely surface-modified by the manufacturer to enhance cell adhesion. This modification leads to the addition of oxygen containing chemical groups to the surface. These can be used equally for the described hydroxyl groups on glass surfaces. The 96 well plates or glass cover slips are treated for 16 h with 1% APTMS in ethanol. Then, the gold nanoparticles are added for 2 h with the given surface concentrations. The wells were washed with distilled water. Finally, 30,000 ZMTH3 cells were seeded in the wells for another 24 h. As in the standard procedure, 10 kDa dextrans served as a perforation indicator. Laser treatment can be started without the need for further incubation steps. If not stated separately, all experiments were conducted in triplicate on three different days and the standard error of the mean is shown. In the first set of experiments, a parameter study was conducted to explore the optimal particle concentration for different particle sizes. We determined that the concentrations of 0.05 to were all suited for membrane perforation dependent on the particle size [see Fig. 1(a)]. In all experiments, 30-nm gold nanoparticles yielded the lowest perforation. For the concentrations of 0.1 and , good perforation efficiencies were achieved with 60 and 80-nm particles. The highest values were yielded for , where almost every cell was perforated. The efficiency for 200-nm particles was best at 0.5 and . These concentrations correspond well to the concentrations needed with 3 h of incubation in the standard procedure. The viability decreased strongly with higher concentrations for gold nanoparticles with sizes lower than 200 nm [see Fig. 1(b)]. For these particles, only a slight decrease could be observed. The decrease in viability is in good agreement with the perforation measurements, as dead cells do not retain the 10-kDa dextrans after perforation. Probably, the total number of particles per cell for a given surface concentration, which is different for each size, leads to these results. This needs to be investigated in further studies. In the following experiments, we used 80-nm particles with a concentration of and 200-nm particles with a concentration of . Fig. 1Influence of different nanoparticle sizes and concentrations on perforation efficiency (a) and cell viability (b) in gold nanoparticle-mediated laser transfection with immobilized particles on the cell culture surface. Fluorescence is normalized to the highest value. 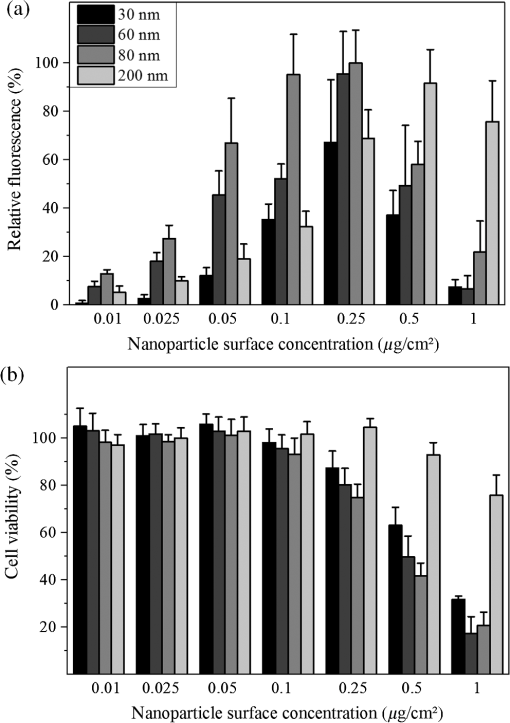 Next, we analyzed whether the newly proposed method is on par with the standard procedure of gold nanoparticle-mediated laser transfection. We incubated cells with 200-nm gold nanoparticles for 3 h and compared the perforation efficiency to the previously mentioned parameters. The obtained efficiencies were equal or slightly better than those with the standard procedure [see Fig. 2(a)]. Therefore, the immobilization of the nanoparticles does not affect the perforation efficiency. The location of particles beneath or next to the cells seems to have a minor influence on the delivery of small molecules. This might be explained by the formation of vapor bubbles due to heating, which perforate cell parts that are accessible by the external medium. The viability for 80-nm particles at was about 60% compared to the better viability with 200-nm particles at of almost 90%. This is in good agreement with our previous studies, which demonstrated a viability of about 90% in gold nanoparticle-mediated laser transfection, and with Fig. 1(b).1,4,5 Fig. 2Comparison of the proposed immobilization technique to the standard procedure of gold nanoparticle-mediated laser perforation. 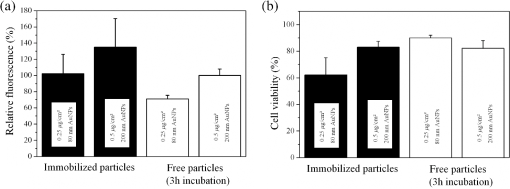 We also investigated whether the procedure in conjunction with laser perforation influences the long-term viability of the cells (see Fig. 3). For this purpose, 8000 cells were seeded on the day before the experiment. The viability after 24 and 48 h was comparable to the viability 2 h after laser treatment. In nonirradiated samples, no impact on the viability was observed. Therefore, only the irradiation of the particles but not the new immobilization procedure influences viability. Fig. 3Long-term viability after laser transfection with immobilized particles on cell culture surfaces. Nonirradiated samples showed no decrease in viability, the particle containing and the irradiated samples showed a decrease. 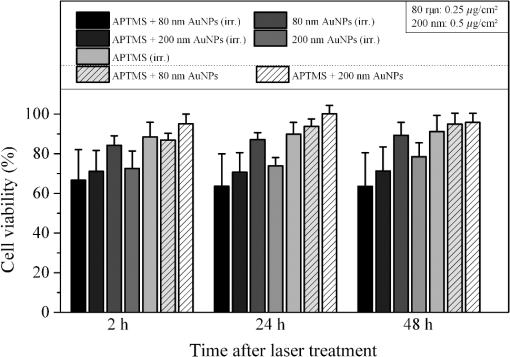 We used dark-field microscopy and ImageJ11 to count the particles in one field-of-view on cover slips with immobilized gold nanoparticles, with irradiated immobilized particles, and with (irradiated) cells and immobilized particles. The cells were detached by trypsinization before counting. No significant difference in 15 samples was found (). Finally, the results of our study lead us to the conclusion that the handling of gold nanoparticle-mediated laser transfection can be improved using immobilized particles. This can be achieved by the simple method described in this study. The efficiency of perforation and viability of the cells is comparable to the results previously published with incubation of particles. Therefore, this new study is of high importance for research on laser transfection with gold nanoparticles. AcknowledgmentsWe would like to thank the German Research Foundation (DFG) and the REBIRTH Cluster of Excellence for funding. ReferencesD. Heinemannet al.,
“Gold nanoparticle mediated laser transfection for efficient siRNA mediated gene knock down,”
PLoS One, 8
(3), e58604
(2013). http://dx.doi.org/10.1371/journal.pone.0058604 1932-6203 Google Scholar
J. Baumgartet al.,
“Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells,”
Biomaterials, 33
(7), 2345
–2350
(2012). http://dx.doi.org/10.1016/j.biomaterials.2011.11.062 BIMADU 0142-9612 Google Scholar
C. Yaoet al.,
“Influence of laser parameters on nanoparticle-induced membrane permeabilization,”
J. Biomed. Opt., 14
(5), 054034
(2009). http://dx.doi.org/10.1117/1.3253320 JBOPFO 1083-3668 Google Scholar
S. Kalieset al.,
“Plasmonic laser treatment for Morpholino oligomer delivery in antisense applications,”
J. Biophotonics,
(2013). http://dx.doi.org/10.1002/jbio.201300056 JBOIBX 1864-063X Google Scholar
S. Kalieset al.,
“Enhancement of extracellular molecule uptake in plasmonic laser perforation,”
J. Biophotonics, 7
(7), 474
–482
(2014). http://dx.doi.org/10.1002/jbio.201200200 JBOIBX 1864-063X Google Scholar
G. BiskerD. Yelin,
“Noble-metal nanoparticles and short pulses for nanomanipulations: theoretical analysis,”
J. Opt. Soc. Am. B, 29
(6), 1383
–1393
(2012). http://dx.doi.org/10.1364/JOSAB.29.001383 JOBPDE 0740-3224 Google Scholar
E. BoulaisR. LachaineM. Meunier,
“Plasma mediated off-resonance plasmonic enhanced ultrafast laser-induced nanocavitation,”
Nano Lett., 12
(9), 4763
–4769
(2012). http://dx.doi.org/10.1021/nl302200w NALEFD 1530-6984 Google Scholar
T.-H. Wuet al.,
“Image patterned molecular delivery into live cells using gold particle coated substrates,”
Opt. Express, 18
(2), 938
–946
(2010). http://dx.doi.org/10.1364/OE.18.000938 OPEXFF 1094-4087 Google Scholar
M. Schomakeret al.,
“Ultrashort laser pulse cell manipulation using nano- and micro- materials of different shapes,”
Proc. SPIE, 7762 7623G
(2010). http://dx.doi.org/10.1117/12.869054 PSISDG 0277-786X Google Scholar
B. S. Flavelet al.,
“Adhesion of chemically and electrostatically bound gold nanoparticles to a self-assembled silane monolayer investigated by atomic force volume spectroscopy,”
J. Nanoparticle Res., 11
(8), 2013
–2022
(2009). http://dx.doi.org/10.1007/s11051-008-9562-1 JNARFA 1388-0764 Google Scholar
C. A. SchneiderW. S. RasbandK. W. Eliceiri,
“NIH Image to ImageJ: 25 years of image analysis,”
Nat. Methods, 9 671
–675
(2012). http://dx.doi.org/10.1038/nmeth.2089 Google Scholar
|


