|
|
|
Rapid development in terahertz (THz) source and receiver technologies has enabled applications in the fields of imaging and spectroscopy.1 The THz frequency range, located midway between the microwave and infrared region, has become increasingly important for biological applications due to its nonionizing nature and sensitivity to water content.2 Recently, colon and skin studies (at 584 GHz frequency) have indicated that reflection-based polarization sensitive continuous-wave (CW) THz imaging is capable of detecting intrinsic contrast between cancerous and normal tissues based on water content combined with structural changes.3,4 Endoscopy is a minimally invasive diagnostic medical procedure to examine the interior surfaces of an organ or tissue without surgery. Besides conventional endoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) are current diagnostic imaging modalities for the detection of local and distant relapse of cancer. CT is a noninvasive technique that provides quick tomographic images of the tissue, but it uses a series of cross sectional x-rays that are ionizing and cannot detect tumors smaller than 5-mm diameter.5 MRI is very sensitive for detecting lesions larger than 10 mm, but uses liquid enema for contrast which is more expensive.5 Although PET provides high sensitivity and specificity, it presents poor resolution unless the tumor is metabolically active.6 As THz rays are nonionizing and offer intrinsic contrast between normal and abnormal tissues, a THz endoscope integrated into a conventional endoscopic system can be used as a potential tool for the examination and detection of cancerous or precancerous regions of biological tissue. Endoscopic imaging systems provide high flexibility not only in biomedical technology but also in remote detection. Current THz endoscopic systems use either optical fibers or THz polymer fibers to propagate the electromagnetic wave. A recently developed fiber guided THz endoscopic system used two channels to measure the reflected signals from the specimen, and relied on optical fibers for pulse propagation.7 Here, the optical fiber is connected with a photo-conductive antenna and is necessarily operated under high voltage which is undesirable for in vivo applications. Another study based on antiresonant hollow-core waveguides used a polymer tube for the transmission; since the radiation is not confined inside the bent tube, it limits the guiding capability and possesses high bending losses.8 A recent near-field study that distinguished breast cancer from normal tissue also relied on an uncoated polymer tube to propagate the THz beam with an attached bull’s-eye structure to obtain field enhancement.9 The imaging setup used in the study works only in transmission modality. In the current study, we proposed a prototype single-channel THz endoscopic system to transmit and collect the intrinsic THz signal reflected from the sample, unlike the other imaging techniques that require at least two channels (and contrast agents) for the measurement. In addition, the current technique operates both in transmission and reflection modalities to fulfill the in vivo imaging criteria to overcome the higher THz absorption associated with the tissue. The present study uses a CW as opposed to conventional pulsed THz imaging systems. CW systems provide spectrally selective high-resolution imagery with high signal-to-noise ratio (SNR) values. The single-channel endoscopic system uses a optically pumped far-infrared (FIR) gas laser operating at 584 GHz frequency with 33 mW output power. As depicted in Fig. 1, the schematic of the imaging setup contains three parts; a THz transceiver system to generate and detect the signal, system optics to guide the beam as per coupling requirements, and a flexible low-loss hollow metal-coated waveguide for propagating the THz beam. The THz beam exiting the FIR cell is collimated using a 61 cm focal length (fl) polymethylpentene (TPX) lens and then passed through a wire grid polarizer and a 50-50 Mylar beam splitter, and is finally focused to 0.68 mm using a 9 cm fl off-axis parabolic mirror (OAP1). Previously, we reported the transmission characteristics of low-loss () flexible THz waveguides that are small enough in diameter (1 to 2 mm) for endoscopic applications.10 To confine the THz radiation inside the tube, a highly (99%) reflective metal such as silver or gold was coated on the inner surface of the polycarbonate tube using a liquid phase chemical deposition process.11 To obtain maximum transmission through the waveguide, and to attain maximum coupling efficiency, the ratio of beam size and waveguide diameter was maintained as 0.77 by adjusting OAP1 parameters. Fig. 1Schematic of single-channel prototype terahertz (THz) endoscopic imaging setup (inset: spot size measurement, THz transmission, and reflection images of an aluminum plate with 0.25-mm hole). 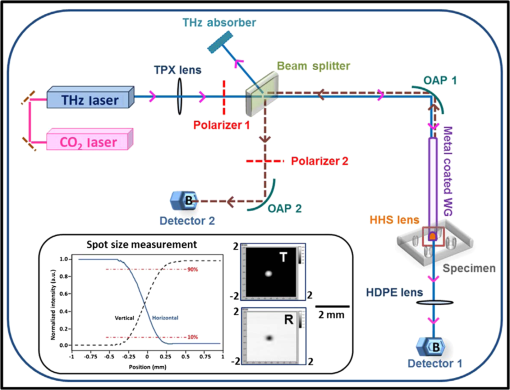 The waveguide assembly contains a 45-cm long flexible 4-mm inner diameter metal-coated waveguide to transport the THz radiation. The total transmission loss of a flexible waveguide increases as a function of bending angle and bend radius. For example, the transmission loss in a 2.5 deg bent waveguide, where a area must be scanned, increases from 1.62 to . In order to obtain a diffraction limited beam waist (0.28 mm), located behind the lens ( distance) that is free of spherical aberration and coma, the waveguide assembly uses a 5-mm diameter z-cut quartz hyperhemispherical lens at the output end. An automated XY scan stage was used to raster scan the sample in the imaging plane with a scanning resolution of 0.1 mm. The laser beam was optically modulated at 80 Hz frequency. For data acquisition, the signal from the detector is sent to a lock-in amplifier that uses the frequency of the optical chopper as a reference. The signal transmitted through the sample was collected by a liquid helium cooled silicon bolometer (detector 1) in the transmission arm, whereas the reflected signal remitted from the specimen was focused into another silicon bolometer (detector 2) in the reflection arm, using a 9 cm fl off-axis parabolic mirror (OAP2). THz images were processed using a Labview® program that synchronized the sample position in the imaging plane with the return signal from the lock-in amplifier. The inset of Fig. 1 shows the THz beam waist measurement, and two-dimensional THz transmission and reflection images of an aluminum plate with a hole. By fitting Gaussian spatial profile to the transmission curve obtained from the knife-edge measurement, a THz beam radius of 0.28 mm can be found. Copolarized and cross-polarized THz images were obtained by placing an appropriate analyzing polarizer in the reflection arm. Figure 2 shows the THz transmission image of a small leaf where the contrast is generated primarily by the transmittance difference due to water content in the veins, as well as the reflected THz image of a metal coin. These images were plotted in logarithmic dB scale and provide an evidence for the system’s high spatial resolution. Fig. 2(a and d) Digital photographs, (b) transmitted THz image of a leaf, and (c) reflected THz image of a metal coin. 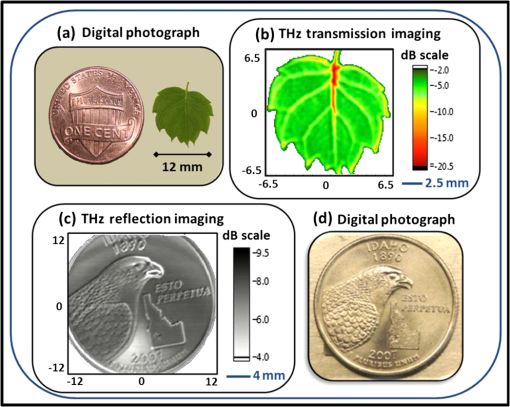 A recent ex vivo THz reflectance study showed an intrinsic contrast between normal and cancerous colonic tissues based on polarization sensitive detection.3 The results indicated that the cross-polarized imaging not only provides consistent relative reflectance difference values (7.3 to 8%) but also a parameter that is invariant with the subject/individual. Therefore, the aim of the current study is to show similar level of contrast using prototype endoscopic system. The colon specimens used in this study were obtained within 1 h after the standard surgical procedures performed at University of Massachusetts Memorial Medical Center, under an Institutional Review Board approved protocol. The thickness of the specimens varied between 4 and 5 mm, with lateral dimensions from 10 to 15 mm. The tissue specimens were fixed with 10% neutral-buffered formalin solution and were left refrigerated at 4°C for 96 h. For THz imaging, the tissue specimens were mounted in an aluminum sample holder with a aperture that was covered by a 1.5 mm z-cut quartz slide. To prevent tissue dehydration during the imaging procedure, the specimens were covered with saline (pH 7.4) soaked gauze. Figure 3 shows the digital photographs and cross-polarized THz images of normal versus cancerous human colonic tissues. In total, we measured six specimens (three cancerous and three normal) from three subjects. An intrinsic contrast was observed between cancerous C and normal N tissues. The cross-polarized reflectance varied between and for formalin fixed and and for fresh colon specimens. The cross-polarized reflectance from fresh normal samples was found to be between 0.38% and 0.41%, whereas for cancerous specimens it was between 0.44% and 0.46%. In the case of formalin fixed samples, the cross-polarized reflectance from normal and cancerous tissues varied from 0.85% to 0.88%, 0.92% to 0.96%, respectively. Analysis of the reflectivity data obtained from formalin fixed and fresh samples showed that cancerous tissue had higher reflectivity than normal tissue. An increased reflection from the cancerous region can possibly be attributed to the greater scattering (local refractive index mismatch) resulting from increased vasculature, lymphatic systems, and other structural changes in the diseased tissues. The normalized relative reflectance difference acquired in this study from fresh colonic tissues was found to be 7.73%, which is consistent with the values obtained in the previous THz colon studies.3 During formalin fixation, the water content (refractive index of 2.4 at 584 GHz) in the tissue will be replaced by formalin solution (with lower refractive index of 2) which results in lower reflectance values. The relative reflectance difference obtained in this study from fixed colonic tissues is 5.31%, which is consistent with previous formalin fixed studies.12 Therefore, the single-channel system developed in this study represents a significant step toward clinical endoscopic application of THz technology to aid cancer screening. Fig. 3Digital photograph, cross-polarized THz reflection images of normal N versus cancerous C human colonic formalin fixed (a and b) and fresh (c and d) tissue sets. 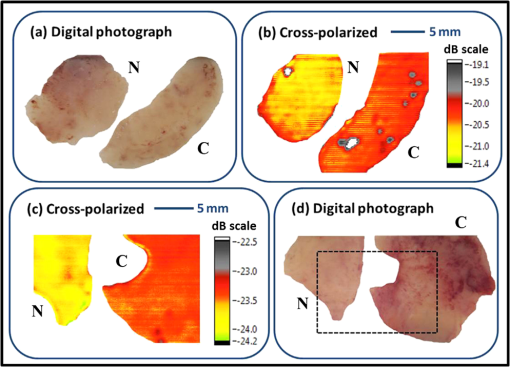 To the best of our knowledge, this is the first study to demonstrate a prototype CW THz endoscopic system for cancer detection. By implementing cross-polarized reflectance THz imaging, we were able to show an intrinsic contrast between normal and cancerous colonic tissues. The analysis indicates that the imaging system is capable of registering reflectance differences between normal and abnormal colons. The experimental demonstration confirms the potential and feasibility of the device in accessing THz reflectivity data from previously inaccessible organs, thereby significantly increasing the overall impact of THz imaging for biomedical detection/screening applications. A pilot study is underway to establish the sensitivity and specificity of the technique. To further apply the THz endoscopic technology to a clinical setting, the current transceiver system has to be replaced with a compact solid-state transceiver system with a large dynamic range and SNR. AcknowledgmentsThe authors would like to acknowledge Dr. Jayant Kumar and Dr. Thomas Goyette for their useful discussions. ReferencesW. L. Chanet al.,
“Imaging with terahertz radiation,”
Rep. Prog. Phys., 70
(8), 1325
–1379
(2007). http://dx.doi.org/10.1088/0034-4885/70/8/R02 RPPHAG 0034-4885 Google Scholar
P. Siegel,
“Terahertz technology in biology and medicine,”
IEEE Trans. Microwave Theory Tech., 52
(10), 2438
–2447
(2004). http://dx.doi.org/10.1109/TMTT.2004.835916 IETMAB 0018-9480 Google Scholar
P. Doradlaet al.,
“Detection of colon cancer by continuous-wave terahertz polarization imaging technique,”
J. Biomed. Opt., 18
(9), 090504
(2013). http://dx.doi.org/10.1117/1.JBO.18.9.090504 JBOPFO 1083-3668 Google Scholar
C. Josephet al.,
“Imaging of ex-vivo nonmelanoma skin cancers in optical and terahertz spectral regions,”
J. Biophotonics, 7
(5), 295
–303
(2012). http://dx.doi.org/10.1002/jbio.201200111 JBOIBX 1864-063X Google Scholar
J. Fletcheret al.,
“CT colonography and MR colonography: current status, research directions and comparison,”
Eur. Radiol., 10
(5), 786
–801
(2000). http://dx.doi.org/10.1007/s003300051006 EURAE3 1432-1084 Google Scholar
O. Schaeferet al.,
“Detection of recurrent rectal cancer with CT, MRI and PET/CT,”
Eur. Radiol., 17
(8), 2044
–2054
(2007). http://dx.doi.org/10.1007/s00330-007-0613-2 EURAE3 1432-1084 Google Scholar
Y. Jiet al.,
“A miniaturized fiber-coupled terahertz endoscope system,”
Opt. Express, 17
(19), 17082
–17087
(2009). http://dx.doi.org/10.1364/OE.17.017082 OPEXFF 1094-4087 Google Scholar
H. Chenet al.,
“Terahertz endoscope based on anti-resonant hollow-core waveguides,”
in Conf. on Lasers and Electro-Optics, CLEO,
(2011). Google Scholar
C. Chiuet al.,
“All-terahertz fiber-scanning near-field microscopy,”
Opt. Lett., 34
(7), 1084
–1086
(2009). http://dx.doi.org/10.1364/OL.34.001084 OPLEDP 0146-9592 Google Scholar
P. Doradlaet al.,
“Dual-frequency characterization of bending loss in hollow flexible terahertz waveguides,”
Proc. SPIE, 8985 898518
(2014). http://dx.doi.org/10.1117/12.2038596 PSISDG 0277-786X Google Scholar
P. Doradlaet al.,
“Characterization of bending loss in hollow flexible terahertz waveguides,”
Opt. Express, 20
(17), 19176
–19184
(2012). http://dx.doi.org/10.1364/OE.20.019176 OPEXFF 1094-4087 Google Scholar
P. Doradlaet al.,
“Continuous-wave terahertz reflection imaging of human colorectal tissue,”
Proc. SPIE, 8624 86240O
(2013). http://dx.doi.org/10.1117/12.2004385 PSISDG 0277-786X Google Scholar
|

