|
|
|
The translation of tissue-engineered products into a clinical setting or a commercial market requires cost effective and robust monitoring of tissue constructs during development both in vitro and in vivo.1 Traditional destructive methods (e.g., assays and mechanical testing) are a clear impediment to such translation as dynamic or time-lapse measurements–important for long-term cell/tissue studies, are difficult to perform using such methods due to their inefficiency in time and cost. Thus, there is a clear need for nondestructive methods that allow time-lapse evaluation of engineered tissue constructs during development, both in vitro and in vivo, without sacrificing the sample or animal. Engineering of vascular grafts is an important area of research aimed at enabling vascular reconstruction in patients with atherosclerosis, surgical excision, and trauma-related vascular problems. Such grafts can serve as an alternative when autologous saphenous vein or mammary artery is not available or of inadequate length.2 A percutaneous technique capable of time-lapse characterization of vascular grafts in vivo without having to sacrifice the experimental animal would be extremely useful. A bimodal imaging catheter3 can enable such an investigation. Here, we report results from a pilot study aimed at evaluating ex vivo vascular grafts using two intravascular compatible modalities, namely time-resolved fluorescence spectroscopy (TRFS) and ultrasound backscatter microscopy (UBM). TRFS allows detection of compositional changes in tissue such as alteration in collagen, elastin, and cellular content, whereas UBM allows visualization of structure and morphology of arterial vessels. Our prior work has shown that TRFS and UBM can be integrated into a compact rotational catheter3 for characterization of atherosclerotic lesions.4 The overall goal of this study is to test whether these modalities can complement each other in inferring compositional and structural information from explanted vascular grafts. The results from this study will help define the framework for future in vivo vessel graft characterization studies using a catheter-based imaging technique.3 Vascular grafts can be composed of synthetic materials or derived from processed native tissue or cell seeded scaffolds. We studied Hemashield®, a synthetic graft (Dacron®) impregnated with collagen, and CorMatrix®, a porcine small intestinal submucosal extracellular matrix (ECM) graft, to assess the morphological and compositional differences between a synthetic and biopolymer graft using TRFS and UBM. 1.MethodsVascular patch materials (CorMatrix® or Hemashield®) were implanted as a patch in a porcine carotid arteriotomy model. A 2-cm longitudinal arteriotomy was made on the anterior surface of the right carotid artery and repaired using a patch (either CorMatrix® or Hemashield®) sutured in place with running suture of 6-0 Prolene® (Ethicon, Inc., Somerville, New Jersey). Hemostasis was achieved using manual pressure, additional sutures, and a topical hemostatic agent (Surgicel®, Ethicon, Inc.). Carotid explants from three pigs (one with Hemashield® and two with CorMatrix® vascular grafts) were available for this pilot study. The vessels ( to 2 cm segments) were harvested (90 days postimplantation) and incised longitudinally to expose the luminal surface for TRFS and UBM measurements ( postharvest). TRFS and UBM measurements of a given tissue region of interest (1-mm diameter for TRFS) were performed using a prototype system5 developed in our laboratory. Point TRFS measurements (excitation: 337 nm, , 800-ps pulse width, emission: 360 to 500 nm, 5-nm steps) were performed in triplicates for two different luminal surface locations in regions containing the graft and regions of normal nongrafted vessel (identified by the presence of sutures). Fluorescence decay values (average lifetime, and second-order Laguerre coefficient, LC-2) were estimated using a constrained least-square deconvolution with Laguerre expansion technique.6 UBM (40 MHz, single-element transducer, resolution—, axial; , lateral) imaging scans were performed across regions containing the graft as well as regions without the graft for comparison. UBM data were analyzed as described in our previous work.7 Histologic sections (formalin fixed) were obtained from regions of the sample with and without grafts and stained with hematoxylin and eosin (for visualization of morphology), Verhoeff van Gieson (VVG, for the visualizing elastic fibers), and Masson’s trichrome (for visualizing the collagen content in intima/media layers). Statistical significance between measurements was determined by student’s -test (). 2.Results and DiscussionThe sample shown in Fig. 1 depicts the TRFS and UBM results from synthetic vascular graft material (Hemashield®). The spatial location of the graft is observed in the UBM cross-sectional image [arrow C, Fig. 1(d)] and confirmed in histology. The region between the internal and external elastic laminas [arrow D, Fig. 1(f)] appears hypoechoic in a normal vessel due to lower collagen content and higher elastin content. The graft appeared to not integrate well with the surrounding tissue as demonstrated by the hypoechoic regions seen in the UBM image corresponding to lack of collagen fibers in and around the graft. These regions are observed as collagen poor areas in the graft in histology [Fig. 1(e)]. The histologic results also show that the intimal/medial surface above the graft is composed primarily of collagen [arrow A, Fig. 1], and lacks elastic fibers and the external elastic lamina when compared with normal tissue [Fig. 1(e), arrow B and Fig. 1(g), arrow D]. These features are also be observed by UBM as loss of visible layered structure over the grafted region [arrow A, Fig. 1(d)] and discontinued external elastic lamina [arrow B, Fig. 1(d)]. TRFS results show narrowing of the fluorescence emission spectra and slight blue-shifted peak [Fig. 1(a)] for the grafted tissue region when compared with the normal tissue region. These changes in fluorescence properties can be attributed to the increase in collagen content and lack of elastin fibers over the grafted region. Collagen is known to have a narrower, blue-shifted emission spectra and higher value when compared with elastin.8 Consequently, the values are higher () over the grafted region for shorter wavelengths (, 395 to 410 nm band) when compared with normal tissue (, 395 to 410 nm band). The trend reverses for higher wavelengths owing to higher fluorescence signal contribution from cellular components and elastin in normal tissue. LC-2 values reflect the changes in fluorescence decay dynamics. These values (465 to 500 nm band) were higher () for the grafted region () as compared with the normal regions (). LC-2 values had an opposite trend for shorter wavelengths but were not significantly different between grafted and normal regions. Nonetheless, the change in composition in the vessel from the graft is made evident by TRFS results. Integrated backscatter coefficients (IBC) correlate to tissue stiffness, and hence increase in collagen content will cause an increase in these coefficient values ( for normal regions and for grafted regions, Fig. 1). Fig. 1Pig carotid sample (1) with Hemashield® graft. TRFS results: (a) fluorescence spectra, (b) , and (c) LC-2 values from normal and grafted tissue areas. Standard deviation (SD) is shown as shaded areas. UBM image of vessel showing (d) grafted (arrow C) and (f) normal area with corresponding registered Verhoeff van Gieson (VVG)-stained histology section in (e) and (g), respectively. 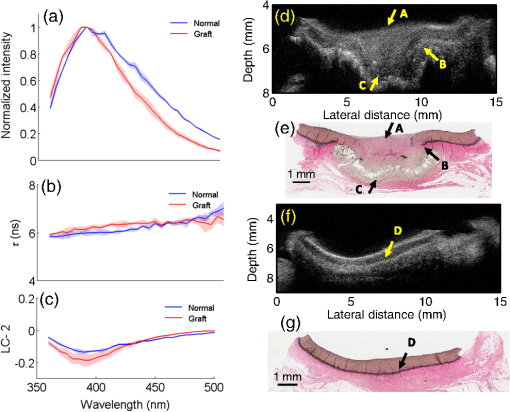 Figure 2 depicts the results from the porcine small intestinal submucosal ECM-based graft (CorMatrix®) that appears to integrate more completely with the surrounding tissue although, based on histology, the lack of elastin fibers is still evident [arrow A, Fig. 2(e)]. The normal tissue regions [Fig. 2(g)] have relatively higher elastin content in the medial layer that generates a stark change in chemical composition (elastin) between the normal and grafted regions. The high elastin content in normal vessel is characteristic of large elastic arteries such as the proximal portions of the carotid artery. Higher elastin content appears to contribute to a large spectral shift in peaks between normal and grafted regions [Fig. 2(a)]. The relatively lower elastin content in the normal regions surrounding the graft may be because of graft-induced remodeling. The values at shorter wavelengths (370 to 380 nm band) are higher () over the grafted region () when compared with normal tissue (). LC-2 values (410 to 500 nm band) were higher () for the grafted region () when compared with the normal regions (). IBC values were higher for the grafted regions () when compared with the normal regions (). Fig. 2Pig carotid sample (2) with CorMatrix® graft. TRFS results: (a) fluorescence spectra, (b) , and (c) LC-2 values from normal and grafted tissue areas. SD is shown as shaded areas. UBM image of vessel showing (d) grafted (arrow C) and (f) normal area with corresponding registered VVG-stained histology section in (e) and (g), respectively. 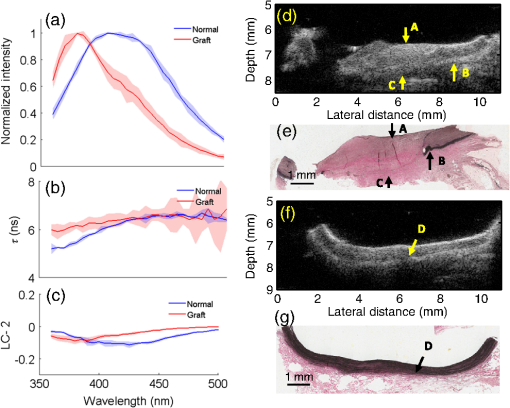 Figure 3 depicts the results from another CorMatrix® vascular graft. Similar to the previous samples, loss of elastic fibers in the grafted regions is evident in histology [Fig. 3(e)]. UBM cross-sectional image allows visualizing the presence of the graft [arrow C, Fig. 3(d)] and also the loss of the external elastic lamina [arrow B, Fig. 3(d)]. This sample, however, showed low elastin content (significantly fewer elastic fibers in media) in the normal tissue areas as well [Fig. 3(g)]. TRFS results between normal and grafted regions were not significantly different unlike the sample shown in Fig. 2. The trends in spectra, , and LC-2 values, however, were similar to the results from the sample in Fig. 2. The values (395 to 410 nm) from the grafted region () were higher () than normal tissue (). The LC-2 values (440 to 460 nm) were higher () for grafted regions () when compared with normal regions (). IBC values for normal and grafted regions were and , respectively. Fig. 3Pig carotid sample (3) with CorMatrix® graft. TRFS results: (a) fluorescence spectra, (b) , and (c) LC-2 values from normal and grafted tissue areas. SD is shown as shaded areas. UBM image of vessel showing (d) grafted (arrow C) and (f) normal area with corresponding registered VVG-stained histology section in (e) and (g), respectively. 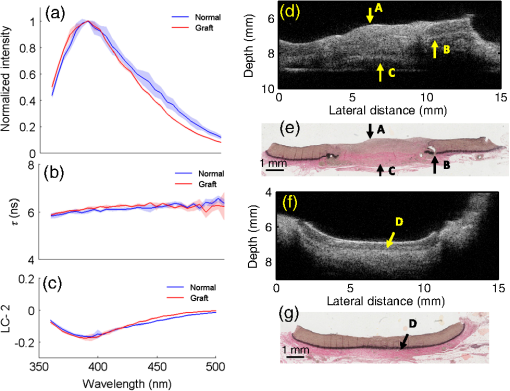 These results show the ability of TRFS to track compositional changes in grafted vessels (collagen and elastin contents) and UBM to image graft integration with surrounding tissue along with related morphological changes (loss of elastic lamina and intimal/medial thickening over the grafted region). Interestingly, despite variations in the compositional features between the two CorMatrix® vascular grafts (Figs. 2 and 3), as confirmed by histology and depicted by TRFS, current data demonstrate that these grafts integrate better with the surrounding tissue when compared with the Hemashield® graft (Fig. 1). This integration can be nondestructively observed using UBM. 3.ConclusionsResults from this pilot study demonstrate that TRFS and UBM together allow for nondestructive evaluation of vascular tissue graft composition and morphology, respectively. UBM allows visualizing the spatial location of the graft and its integration with surrounding tissue. It also enables visualizing the intimal/medial thickening and loss of the external elastic lamina over the grafted region. These observations are complemented with compositional information about collagen and elastin contents via changes in fluorescence spectra, , and LC-2 values. This work lays the foundation for the future use of a bimodal catheter combining both techniques for nondestructive evaluation of vascular grafts in vivo. ReferencesM. L. MatherS. P. MorganJ. A. Crowe,
“Meeting the needs of monitoring in tissue engineering,”
Regener. Med., 2
(2), 145
–160
(2007). http://dx.doi.org/10.2217/rme.2007.2.issue-2 RMEECZ 1746-0751 Google Scholar
M. R. Hoeniget al.,
“Tissue-engineered blood vessels: alternative to autologous grafts?,”
Arterioscler., Thromb., Vasc. Biol., 25
(6), 1128
–1134
(2005). http://dx.doi.org/10.1161/01.ATV.0000158996.03867.72 ATVBFA 1079-5642 Google Scholar
D. Maet al.,
“Rotational multispectral fluorescence lifetime imaging and intravascular ultrasound: bimodal system for intravascular applications,”
J. Biomed. Opt., 19
(6), 066004
(2014). http://dx.doi.org/10.1117/1.JBO.19.6.066004 JBOPFO 1083-3668 Google Scholar
Y. Sunet al.,
“Multimodal characterization of compositional, structural and functional features of human atherosclerotic plaques,”
Biomed. Opt. Express, 2
(8), 2288
–2298
(2011). http://dx.doi.org/10.1364/BOE.2.002288 BOEICL 2156-7085 Google Scholar
Y. Sunet al.,
“Development of a dual-modal tissue diagnostic system combining time-resolved fluorescence spectroscopy and ultrasonic backscatter microscopy,”
Rev. Sci. Instrum., 80
(6), 065104
(2009). http://dx.doi.org/10.1063/1.3142478 RSINAK 0034-6748 Google Scholar
J. Liuet al.,
“A novel method for fast and robust estimation of fluorescence decay dynamics using constrained least-squares deconvolution with Laguerre expansion,”
Phys. Med. Biol., 57
(4), 843
–865
(2012). http://dx.doi.org/10.1088/0031-9155/57/4/843 PHMBA7 0031-9155 Google Scholar
H. Fatakdawalaet al.,
“Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques,”
Biomed. Opt. Express, 4
(9), 1724
–1741
(2013). http://dx.doi.org/10.1364/BOE.4.001724 BOEICL 2156-7085 Google Scholar
M. Y. BerezinS. Achilefu,
“Fluorescence lifetime measurements and biological imaging,”
Chem. Rev., 110
(5), 2641
–2684
(2010). http://dx.doi.org/10.1021/cr900343z CHREAY 0009-2665 Google Scholar
|

