|
|
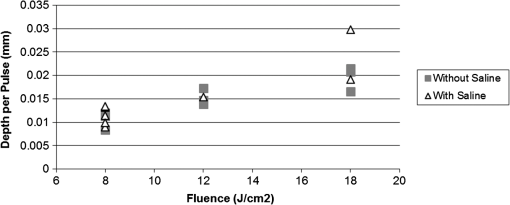
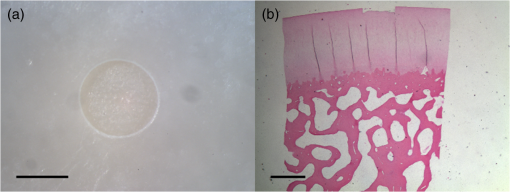
1.IntroductionArticular cartilage injury of the knee may be caused by traumatic mechanical destruction or progressive mechanical degeneration. These articular lesions are frequently associated with joint pain, diminished function, and development of osteoarthritis if left untreated.1–4 Self-repair of cartilage in the knee joint is very limited, as there are no blood vessels within the tissue and repair mechanisms rely on blood from the underlying subchondral bone. Various treatment options are currently available for cartilage defects. One potential treatment is microfracture surgery, a bone marrow stimulation technique. The procedure stimulates fibrocartilaginous repair tissue to grow into the defect and cover the underlying bone.1,5 The procedure is used in conjunction with arthroscopy and is minimally invasive. The surgeon first removes any chondral flaps, clearing up the region before penetrating the subchondral bone with an awl.1–3,5 Microholes are created in this fashion to induce bleeding from the bone marrow, leading to the formation of fibrocartilaginous repair tissue from the released stem cells.2 These microholes are typically 1.5 mm in diameter, 2 to 4 mm deep, and 3 to 4 mm apart from each other.3,6,7 The number of holes needed depends on the size of the lesion, which could range from 24 to , as reported in an evaluation of the technique.8 The patients are then required to undergo a rehabilitation program to optimize the results from the surgery. In a review of the outcomes of microfracture surgery for traumatic chondral defects, 95% of the 72 patients studied were found to have experienced improved function and less pain in an average 11-year follow-up.9 Although this technique is being used with increased frequency, the procedure is limited by its reliance on surgical skill. An arthroscope is used to visualize different areas of the knee and may require several incisions. When creating the microholes, the surgeon must manually advance the awl.3 Other potential drawbacks include the creation of loose bony particles within the joint and bone fracturing in the surrounding area resulting in tissue necrosis.10 These side effects are major weaknesses due to reliance on a manual surgical tool, supporting the need to develop improved techniques. In a novel study, use of drilling with cooled irrigation was proposed as an alternative to traditional microfracture techniques, showing a reduction in osteocyte necrosis.11 Similarly, as proposed by this study, there may be a promising application using lasers to create the microholes. There are many advantages in using laser ablation to bore holes for the application of microfracture surgery. Appropriate selection of wavelength and dosimetry can produce char-free ablation. Noncontact lasers allow the laser to ablate tissue without serving as a thermal scalpel, vaporizing the tissue without direct contact of a heated tip. Risk of tissue fragments breaking off is reduced compared to surgical drills, which may also fracture and break at the tip as well. Use of lasers offers precise control of crater shape and size as well. The purpose of this study is to present and assess a new application for currently available laser technology. The potential use of laser ablation as an alternative way to create microholes is evaluated by examining crater depth and peripheral thermal damage. The lasers evaluated in this study, femtosecond (fs) lasers and erbium:YAG, were chosen for their potentially precise ablation and minimal thermal injury to tissue. The appearance of thermal damage was evaluated with digital photography using a dissecting microscope and depth of ablation was measured with conventional histology. 2.Materials and Methods2.1.Sample PreparationTissue from bovine femoral condyles obtained from a local abattoir was extracted from the same region of the condyles using a saw and precisely sectioned to ensure similar cartilage thicknesses and bone composition between different specimens. All samples () were maintained in normal saline solution upon extraction and throughout the experiment. 2.2.Laser SystemsThree different lasers were used for this study. A clinical erbium:YAG laser () used primarily for cosmetic dermatology applications (Ultrafine, Coherent, Santa Clara, California, USA) was used with fluences of 8, 12, and , a spot diameter of 2 mm, and a pulse repetition rate of 1 Hz. The first fs laser was a titanium:sapphire fs laser system (Legend, Coherent) combined with an optical parametric amplifier (Opera, Coherent). The Legend is an all-solid-state fs laser based on passive mode locking and chirped pulse amplification. The output laser beam from the fs laser was used to pump the optical parametric amplifier system. The output laser beam used in the experiment had a wavelength of 1700 nm, repetition rate of 5 kHz, and laser energy of up to . The second fs laser was a clinical ophthalmic laser system used for refractive eye surgery (IntraLase fs laser, Abbott Medical Optics, Santa Ana, California, USA). This laser operated at a wavelength of 1053 nm, with a repetition rate of 30 kHz, and pulse energy of . 2.3.Experimental SetupThe bovine samples were placed in a Petri dish and kept moist with normal saline throughout the experiments. For the erbium:YAG laser studies, the handpiece of the device was secured and remained fixed in position during ablation. A total of 40 to 100 pulses were used for fluences of 8, 12, and based on the changes in the acoustic sound of the ablation heard as the laser ablated through the cartilage and into the bone. This audible change is characteristic of the transition from cartilage to bone and was observed in pilot experiments. For example, at , the change in the popping sound occurred after , so a total of 60 pulses was used to ensure that both the cartilage and bone layers were ablated. An additional parameter of 100 pulses was then used for the 8 and fluences for easier comparison between the two. In addition, each ablation was done twice, once dry and once with the addition of intermittent saline drops in between pulses to evaluate the effect on the extent of thermal damage. The total ablation time was 40 to 100 seconds per sample, depending on the number of pulses delivered. For the Legend titanium:sapphire fs system, the sample was placed on a computer-controlled three axis-translational stage, moving at a speed of 20 mm per second. The fs laser beam was focused onto the specimen and a square hole of was created by scanning line-by-line movement of the stage. A layer separation of 9 or was used. The power used was , and the output of the laser was measured using an optical power meter before each experiment. In preliminary trials, pulse energies ranging from 20 to were tested to determine the impact of pulse energies. With the present device and setup, pulse energies of yielded clean ablation craters with no visible carbonization and was used for the experiment. The total time required to ablate a region of interest of 1 mm depth varied from 5 to 6 h per sample depending on the cartilage layer thickness. For the IntraLase fs laser, the spot size, spot separation, laser energy, and firing pattern were controlled by the computer. The laser spots were programmed to be fired in an expanding spiral pattern to form a hole 1 mm in diameter. As the laser is usually used to create cuts or flaps at various depths in the tissue, the settings were not ideally suited for the purposes of this experiment. The settings of the laser allowed only a depth of tissue to be ablated at a time. Hence, layer by layer of tissue had to be ablated serially. A depth of was achieved in this fashion. Only one sample was attempted to demonstrate feasibility. The time required to ablate layer was and the whole ablation sequence lasted for . 2.4.Histological PreparationAfter ablation, the samples were fixed in 10% formalin solution for 48 h and then kept in phosphate buffer solution. The ablation target sites were digitally photographed using a dissecting microscope. Samples were decalcified using a hydrochloric acid decalcifying agent (RDO Rapid Decalcifier, Apex Engineering Corp., Aurora, Illinois) and checked daily until soft enough for histologic sectioning. The samples were then embedded into paraffin wax, sectioned into sections and stained with conventional hematoxylin and eosin. 3.Results3.1.Erbium:YAG AblationHard tissue ablation with erbium:YAG lasers at different parameters was compared for carbonization and crater depth. Figure 1 presents a montage of the ablation craters for the three different parameters. Specimens irradiated in combination with saline application showed less carbonization than those without; at the same time, visual geometry of the lesion had not changed. Gross thermal injury to the tissue surface was assessed by comparing the degree of carbonization, as seen in the dissecting microscope images. Figure 2 is a montage of histological sections with appropriate scale bars indicated. Depth of ablation was analyzed by using the sections along the equator of the ablation craters. Hydration with saline during irradiation did not impact the depth of ablation. For the specimens treated with a fluence of , the cartilage layer was completely removed within the crater, and only a modest amount of bone tissue removal was observed. For the 12- and specimen protocols, significant bone tissue was removed. Fig. 1Dissecting microscope images of ablation sites after treatment with three different fluences. (a), (b), and (c) are erbium:YAG ablation with saline hydration and (d), (e), and (f) are without saline hydration. (a) and (d) were treated with , (b) and (e) were treated with , and (c) and (f) were treated with . The scale bars in each image indicate 0.5 mm. 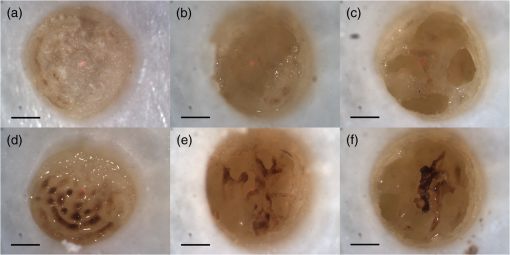 Fig. 2Histology images of the ablation craters obtained with three different fluences. (a), (b), and (c) are erbium:YAG ablation with saline hydration and (d), (e), and (f) are without saline hydration. (a) and (d) were treated with , (b) and (e) were treated with , and (c) and (f) were treated with . The scale bars in each image indicate 1 mm. 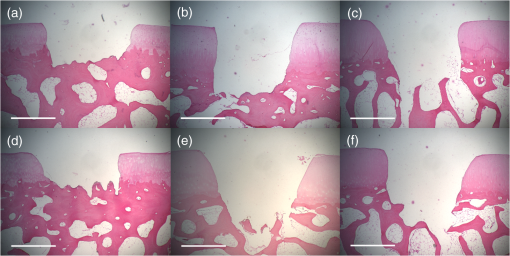 Figure 3 shows a dependence of the ablation depth per pulse from fluence. As expected, the depth per pulse increased with higher fluence. Values were consistent for both with and without saline, except for one sample in the parameter, which may be an outlier. 3.2.Femtosecond AblationSamples irradiated using the Legend titanium:sapphire fs laser were also analyzed for thermal injury and depth of ablation. Figure 4 is a montage of high-power images and histological sections for two of the samples, ablated at with layer separation of . Very little tissue carbonization was observed and crater dimensions were extremely precise. Fig. 4Dissecting microscope and histology images of two samples treated with the Legend titanium:sapphire femtosecond laser. (a) and (b) are dissecting microscope images and (c) and (d) are matching histology images. The scale bars in the images indicate 0.5 mm. 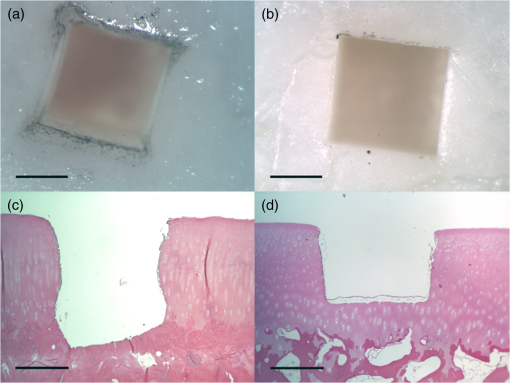 One sample successfully ablated cartilage and bone, shown in Figs. 4(a) and 4(c), while the other Legend fs treated samples only ablated through cartilage, an example is shown in Figs. 4(b) and 4(d). Figure 5 shows a graph of the maximum depth achieved in all fs laser treated samples. Fig. 5Maximum depth achieved in all the Legend femtosecond treated samples and depth of the AMO IntraLase femtosecond sample. The average depth of the Legend titanium:sapphire femtosecond laser treated samples is shown (AVG). 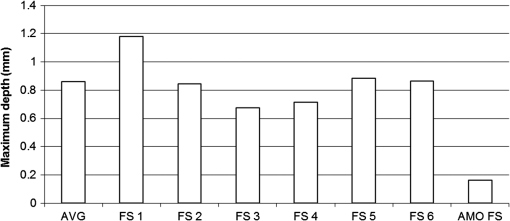 Tissue specimens irradiated using the IntraLase fs laser also showed no noticeable carbonization, indicating very little peripheral thermal injury. The depth of ablation was found to be , exactly as programmed by the system. Figure 6 shows images of the IntraLase fs laser ablated sample. With the limitations of this system, creation of craters with clinically meaningful depth (through to the bone) was not possible. 4.DiscussionThis study demonstrated that the erbium:YAG laser, Legend, and IntraLase fs laser systems are capable of articular cartilage and bone ablation, and may be possible approaches to creating microfracture holes. A simple comparison of both laser systems is shown in Table 1. By utilizing the most effective parameters (12 and ), rapid and efficient cartilage and bone tissue removal with little apparent carbonization was achieved using the erbium:YAG laser. With the fs lasers, precise ablation of cartilage with very little carbonization was possible, although a clinically significant amount of bone removal (to the bone marrow) was difficult to achieve with the technology used in this study. Even if not as precise as the fs lasers, ablation using the erbium:YAG laser yielded fast and reproducible results. This mid-infrared laser was also less expensive compared to the fs laser systems. In contrast, the fs laser systems were technically more complex and, with current technology, took significantly more time for tissue removal. The precision and lack of thermal damage within the crater of fs ablation was, however, superior to the rough edges and ablation craters created by the erbium:YAG. Table 1Comparison of the erbium:YAG and femtosecond laser systems.
4.1.Use of Erbium:YAG Laser in BoneHard tissue ablation with erbium:YAG lasers is characterized as thermo-mechanical and the amount of thermal injury relates to whether conditions for thermal confinement are met.12 The absorption coefficient of water peaks at wavelength, allowing the erbium:YAG light to be strongly absorbed by the main components of cartilage and bone.12 In this study, articular cartilage was successfully removed using fluences of 8, 12, and , while significant bone ablation was possible at fluences of 12 and . The application of erbium:YAG lasers to ablate bone has been well documented in many other medical applications, such as in stapes (middle ear) surgery and dentin and enamel ablation (dentistry). However, although hard and soft tissue ablation by erbium:YAG has been studied extensively,12–14 this is a specific evaluation for the proposed future clinical application. The laser is reported to be highly advantageous for ablating the stapes, as there are few negative effects on the inner ear afterward.15 Similarly, with dental ablation, several studies have yielded results favoring the use of the laser in traditional surgical techniques, citing histological evidence that tissue healing is improved with the use of lasers rather than surgical drills or burrs.16 These applications of lasers to ablate hard tissue in other areas of the body inspire the present study to create the holes needed in orthopedic microfracture surgery. No significant differences in ablation depths were observed between samples treated with and without saline hydration, but as expected, much less carbonization was observed in samples treated with saline. These findings mirror investigations using the laser with spray in dental ablation, which demonstrated that ablation of bone with spray resulted in relatively clean edges without thermal damage while more charring and peripheral cracks were observed in dry ablation.17–19 Our results are in accord with the observations of others, as there was reduced charring and carbonization on surfaces with saline drops, although the ablation depth/rate was not noticeably different. The craters were observed to be irregular in shape, being more of a parabolic cone shape as opposed to the almost perfect squares and circles that were produced by the fs systems. The irregular craters should not present any problems to the microfracture procedure, as the holes created with surgical awls are similarly irregular in nature. Irregular and rough edges are also frequently observed in dental studies.17 One major limitation of most high-power erbium:YAG lasers is the need for mirror-based, bulky articulated arm delivery systems. The particular laser used in this study would be difficult to adapt to an arthroscopic setting and also difficult to use to generate patterned ablation. The ablation would also have to be done in air and not under water. Nevertheless, the main advantage of this laser is its speed. It would require only 100 s for 100 pulses to remove an adequate amount of tissue, whereas the same could not be done with the present fs laser system. Several groups have investigated the use of flexible fiber optic systems to translate the laser light.20–24 The use of delivering light via silica glass fibers may be a promising solution for the minimally invasive surgical procedure. 4.2.Use of Femtosecond Laser in BoneFemtosecond laser ablation of biological tissues is known to be plasma-mediated and demonstrates a different cutting mechanism than longer pulse lasers. The absorption process in this regime is largely independent of material properties.25 High precision and lack of thermal injury has been shown in many clinical fields, most elegantly in photorefractive surgery.26 Using 1700-nm fs laser pulses, subsurface ablation was demonstrated in human sclera with no damage on the surface.27 Femtosecond laser ablation in bone has been shown to have reduced side effects using irradiation near threshold conditions,28 accelerated bone healing,29 and only a few cell layers of disrupted cellular membrane integrity.25 The IntraLase fs laser has previously been shown to ablate bone (the stapes) efficiently with minimal thermal and negligible mechanical damage.30 As expected, the fs laser systems used in this study created precise ablation craters with smooth surfaces. Using the Legend titanium:sapphire fs laser, significant amounts of articular cartilage were removed, ranging from 0.7 to 1.2 mm in ablated tissue depth. There was little carbonization and visible thermal damage using powers around 300 mW. In one sample, the laser was able to ablate through the layer of cartilage into bone. Using the IntraLase fs laser, a precise circular crater was created, although a significant depth was not possible due to the limitations of this repurposed ophthalmic laser system. The system was originally designed for creating cuts and flaps; to create holes, the tissue was ablated layer at a time, an inefficient, time-consuming procedure. The major limitation in using the present fs systems was ablation time. Systems with higher repetition rates than the ones used in this study may be needed to achieve greater ablation depths within a more reasonable amount of time. Using the current system, it took to create a rectangular hole 1 mm in diameter and in depth. In order to create a hole in depth, an estimated 20 h of continuous ablation would be required. This is entirely impractical; however, the construction of a system with a faster repetition rate may lead to a more reasonable time frame. For example, if an fs laser operates at 5 MHz like the commercially available Ziemer FEMTO LDV™ Crystal Line (Ziemer Group AG, Port, Switzerland), the time needed to create a rectangular hole of 4 mm can be reduced to 1.2 min per hole. This technology operates at a repetition rate 1000 times greater than the translational staged Legend titanium:sapphire system used in this study and 166.67 times faster than the IntraLase fs laser used. One major challenge with respect to this technology may be the difficulty to bore a deep-enough hole (2 to 4 mm in bone) to allow bleeding from the bone marrow. The use of fs lasers to ablate soft tissue is widely explored, but studies in its use in mass ablation of hard tissue are more limited. However, in agreement with other studies, higher pulse energies and faster repetition rates using fs lasers allow for hard tissue ablation within a reasonable time frame.31 The clinical goal is to be able to ablate holes into the bone deep enough to reach the bone marrow and allow blood and stem cells to flow out. A fundamental challenge with respect to the application of this technology is the vascular component, whether or not bleeding will be allowed. In a laser-assisted apicectomy procedure, erbium:YAG ablation was shown to produce a wet incision (some bleeding) for soft tissue and an enhanced healing response in bone tissue.16 Compared to conventional lasers, the use of ultrafast lasers for hard tissue ablation shows more of a lack of hemostasis.32 Laser ablation in this fashion is expected to allow the blood to flow into the area of the lesion. Future studies should include analysis of the vascular component and potential animal studies to show further feasibility of the application. 5.ConclusionBased on the results, bone ablation for the purposes of microfracture is feasible. Erbium:YAG ablation is relatively fast and removes mass while causing some carbonization and thermal damage to the surrounding tissue. Use of saline drops during this procedure reduces carbonization just as spray cooling helps reduce thermal damage in dental tissue. Femtosecond ablation is currently limited by the stage movement, repetition rates, and pulse energies of present systems, but ablation of bone tissue appears possible. With additional studies and further development, there is a potential for a future system capable of operating at a more reasonable time frame for mass ablation. The use of lasers to create holes allows for the possibility of creating different patterns by varying spot sizes or crater sizes. The geometry of the holes may also differ by changing various parameters. This can allow the alignment of holes near blood supplies or across the stress distribution in the region of interest. It is possible to alter surface features to promote adherence of clot and, thereby, increase the efficacy of the procedure. This study is novel in its examination of the use of lasers to create microfracture holes in the knee. It aims to be the first in its paradigm to change the way the microfracture surgery is done. ReferencesR. J. H. Custerset al.,
“Articular cartilage degeneration following the treatment of focal cartilage defects with ceramic metal implants and compared with microfracture,”
J. Bone Joint Surg. Am., 91
(4), 900
–910
(2009). http://dx.doi.org/10.2106/JBJS.H.00668 JBJSA3 1058-2436 Google Scholar
S. E. Domayeret al.,
“MRI monitoring of cartilage repair in the knee: a review,”
Semin. Musculoskelet. Radiol., 12
(4), 302
–317
(2008). http://dx.doi.org/10.1055/s-0028-1100638 1089-7860 Google Scholar
J. R. SteadmanW. G. RodkeyJ. J. Rodrigo,
“Microfracture: surgical technique and rehabilitation to treat chondral defects,”
Clin. Orthop. Relat. Res., 391
(Suppl), S362
–S369
(2001). http://dx.doi.org/10.1097/00003086-200110001-00033 CORTBR 0009-921X Google Scholar
J. S. TemenoffA. G. Mikos,
“Review: tissue engineering for regeneration of articular cartilage,”
Biomaterials, 21
(5), 431
–440
(2000). http://dx.doi.org/10.1016/S0142-9612(99)00213-6 BIMADU 0142-9612 Google Scholar
A. J. Detterlineet al.,
“Treatment options for articular cartilage defects of the knee,”
Orthop. Nurs., 24
(5), 361
–366
(2005). Google Scholar
M. Asiket al.,
“The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results,”
Arthroscopy, 24
(11), 1214
–1220
(2008). http://dx.doi.org/10.1016/j.arthro.2008.06.015 ARTHE3 0749-8063 Google Scholar
A. GobbiL. BathanL. Boldrini,
“Primary repair combined with bone marrow stimulation in acute anterior cruciate ligament lesions: results in a group of athletes,”
Am. J. Sports Med., 37
(3), 571
–578
(2009). http://dx.doi.org/10.1177/0363546508327141 AJSMDO 0363-5465 Google Scholar
K. Mithoeferet al.,
“High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique,”
Am. J. Sports Med., 34
(9), 1413
–1418
(2006). http://dx.doi.org/10.1177/0363546506288240 AJSMDO 0363-5465 Google Scholar
J. R. Steadmanet al.,
“Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up,”
Arthroscopy, 19
(5), 477
–484
(2003). http://dx.doi.org/10.1053/jars.2003.50112 ARTHE3 0749-8063 Google Scholar
C. D. MurawskiJ. G. Kennedy,
“Operative treatment of osteochondral lesions of the talus,”
J. Bone Joint Surg. Am., 95
(11), 1045
–1054
(2013). http://dx.doi.org/10.2106/JBJS.L.00773 JBJSA3 1058-2436 Google Scholar
H. Chenet al.,
“Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair,”
J. Orthop. Res., 27
(11), 1432
–1438
(2009). http://dx.doi.org/10.1002/jor.20905 JOREDR 0736-0266 Google Scholar
S. Stübinger,
“Advances in bone surgery: the Er:YAG laser in oral surgery and implant dentistry,”
Clin. Cosmet. Investig. Dent., 2010
(2), 47
–62
(2010). http://dx.doi.org/10.2147/CCIDE.S8352 1179-1357 Google Scholar
M. R. Dickinsonet al.,
“Studies of Er-YAG laser interactions with soft tissue,”
Lasers Med. Sci., 6
(2), 125
–131
(1991). http://dx.doi.org/10.1007/BF02032539 LMSCEZ 1435-604X Google Scholar
R. HibstR. Kaufmann,
“Effects of laser parameters on pulsed Er-YAG laser skin ablation,”
Lasers Med. Sci., 6
(4), 391
–397
(1991). http://dx.doi.org/10.1007/BF02042461 LMSCEZ 1435-604X Google Scholar
M. R. Marcheseet al.,
“One-shot versus Er:YAG laser stapedotomy: is the outcome the same?,”
Eur. Arch. Otorhinolaryngol., 268
(3), 351
–356
(2011). http://dx.doi.org/10.1007/s00405-010-1399-4 0937-4477 Google Scholar
A. ReyhanianS. ParkerJ. Moshonov,
“The use of the erbium yttrium aluminium garnet (2,940 nm) in a laser-assisted apicectomy procedure,”
Br. Dent. J., 205
(6), 319
–323
(2008). http://dx.doi.org/10.1038/sj.bdj.2008.804 BDJOAJ 0007-0610 Google Scholar
T. Harashimaet al.,
“Morphological comparative study on ablation of dental hard tissues at cavity preparation by Er:YAG and Er,Cr:YSGG lasers,”
Photomed. Laser Surg., 23
(1), 52
–55
(2005). http://dx.doi.org/10.1089/pho.2005.23.52 PLDHA8 1549-5418 Google Scholar
M. Hossainet al.,
“Ablation depths and morphological changes in human enamel and dentin after Er:YAG laser irradiation with or without water mist,”
J. Clin. Laser Med. Surg., 17
(3), 105
–109
(1999). JCLSEO Google Scholar
H. W. KangI. RizoiuA. J. Welch,
“Hard tissue ablation with a spray-assisted mid-IR laser,”
Phys. Med. Biol., 52
(24), 7243
–7259
(2007). http://dx.doi.org/10.1088/0031-9155/52/24/004 PHMBA7 0031-9155 Google Scholar
A. Urichet al.,
“Delivery of high energy Er: YAG pulsed laser light at 2.94 μm through a silica hollow core photonic crystal fibre,”
Opt. Express, 20
(6), 6677
–6684
(2012). http://dx.doi.org/10.1364/OE.20.006677 OPEXFF 1094-4087 Google Scholar
A. Urichet al.,
“Flexible delivery of Er: YAG radiation at 2.94 μm with negative curvature silica glass fibers: a new solution for minimally invasive surgical procedures,”
Biomed. Opt. Express, 4
(2), 193
–205
(2013). http://dx.doi.org/10.1364/BOE.4.000193 BOEICL 2156-7085 Google Scholar
J. S. SangheraL. B. ShawI. D. Aggarwal,
“Applications of chalcogenide glass optical fibers,”
C. R. Chim., 5
(12), 873
–883
(2002). http://dx.doi.org/10.1016/S1631-0748(02)01450-9 CRCOCR 1631-0748 Google Scholar
N. J. Scottet al.,
“Mid-IR germanium oxide fibers for contact erbium laser tissue ablation in endoscopic surgery,”
IEEE J. Sel. Topics Quantum Electron., 13
(6), 1709
–1714
(2007). http://dx.doi.org/10.1109/JSTQE.2007.910557 IJSQEN 1077-260X Google Scholar
N. M. Friedet al.,
“Transmission of Q-switched erbium:YSGG () and erbium:YAG () laser radiation through germanium oxide and sapphire optical fibres at high pulse energies,”
Lasers Med. Sci., 19
(3), 155
–160
(2004). http://dx.doi.org/10.1007/s10103-004-0316-8 LMSCEZ 1435-604X Google Scholar
B. Girardet al.,
“Effects of femtosecond laser irradiation on osseous tissues,”
Lasers Surg. Med., 39
(3), 273
–285
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
H. K. Soonget al.,
“Femtosecond laser-assisted lamellar keratoplasty,”
Arq. Bras. Oftalmol., 71
(4), 601
–606
(2008). http://dx.doi.org/10.1590/S0004-27492008000400028 AQBOAP 0004-2749 Google Scholar
Z. S. Sackset al.,
“Femtosecond subsurface photodisruption in scattering human tissues using long infrared wavelengths,”
Proc. SPIE, 4241 98
–111
(2001). http://dx.doi.org/10.1117/12.431510 PSISDG 0277-786X Google Scholar
G. NicolodelliRde. F. LizarelliV. S. Bagnato,
“Influence of effective number of pulses on the morphological structure of teeth and bovine femur after femtosecond laser ablation,”
J. Biomed. Opt., 17
(4), 048001
(2012). http://dx.doi.org/10.1117/1.JBO.17.4.048001 JBOPFO 1083-3668 Google Scholar
D. D. Loet al.,
“Femtosecond plasma mediated laser ablation has advantages over mechanical osteotomy of cranial bone,”
Lasers Surg. Med., 44
(10), 805
–814
(2012). http://dx.doi.org/10.1002/lsm.v44.10 LSMEDI 0196-8092 Google Scholar
R. G. McCaugheyet al.,
“Femtosecond laser ablation of the stapes,”
J. Biomed. Opt., 14
(2), 024040
(2009). http://dx.doi.org/10.1117/1.3120490 JBOPFO 1083-3668 Google Scholar
Y. LiuM. Niemz,
“Ablation of femural bone with femtosecond laser pulses—a feasibility study,”
Lasers Med. Sci., 22
(3), 171
–174
(2007). http://dx.doi.org/10.1007/s10103-006-0424-8 LMSCEZ 1435-604X Google Scholar
C. L. Hoyet al.,
“Clinical ultrafast laser surgery: recent advances and future directions,”
IEEE J. Sel. Topics Quantum Electron., 20
(5), 1
–14
(2014). http://dx.doi.org/10.1109/JSTQE.2013.2287098 IJSQEN 1077-260X Google Scholar
BiographyErica Su is a research specialist at the Beckman Laser Institute at the University of California, Irvine. She received her BS degree in biomedical engineering from the University of California, Irvine. Her current research interests include optical coherence tomography, 3D airway reconstruction, and laser technology. Hui Sun got his PhD degree from University Heidelberg, Germany. He did his postdoc training at the University of California, Irvine, USA. Right now he is a full professor at the Academy of Opto-Electronics, Chinese Academy of Science, Beijing, China. Brian J. F. Wong, MD, is professor of otolaryngology—head and neck surgery, biomedical engineering, and surgery at the Beckman Laser Institute, UC Irvine. He received his undergraduate degree from the University of Southern California, and his medical degree from Johns Hopkins. His interests are in optical coherence tomography, tissue reshaping, and airway modeling. Clinically, his focus is on rhinoplasty and nasal airway surgery. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||