|
|
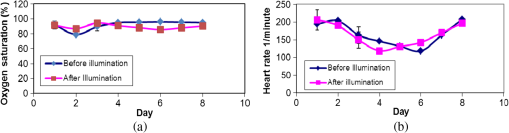
1.IntroductionPhotodynamic therapy (PDT) has been used in the last three decades as a clinical technique for the treatment of several diseases, including cancer, rheumatoid arthritis, age-related macular degeneration, skin disease, and arteriosclerosis.1–11 One important application of PDT is its application for antibacterial treatments involving the killing of bacteria by reactive oxygen species generated in the presence of a photosensitizer and light.12–14 Examples include inactivation of the bacteria in skin and wound infections and reduction in the density of nosocomial multiresistant infections.15–18 The major advantage of antibacterial PDT is that acquiring resistance is not simple and the current resistance mechanisms exerted by bacteria against the common commercially available antibiotics do not protect against the mode of action of PDT.19 As with PDT, usage of light to biostimulate cells is a common approach in low-level laser therapy (LLLT).20–24 We introduce a newly developed implantable pill that aims to perform in-body phototherapy, LLLT, blue light for sterilization, controlled injection of chemicals, and communication with an external control box including the transmission of images from inside the patient’s body. We present the pill as well as its preliminary experimental validation. The term PDT from a medical point of view can mean a large number of diverse effects on various organs and tissues of a living organism. In this paper, we conducted experiments having practical importance in the treatment of epilepsy (illumination of sinus—carotid region), cancer of internal organs (laser ablation of liver tissues), and phlegmon (blue light therapy). Note that the usage of a wireless capsule25,26 for endoscopy as a diagnostic device and for multipurpose robotic systems has been demonstrated before in Ref. 27, while, in general, swallowable-capsule technology28 is very useful for gastrointestinal endoscopy.29 In Sec. 2, we present the developed implantable pill. In Sec. 3, we show the preliminary experimental results. This paper is concluded in Sec. 4. 2.Implantable PillOur proposal is to use photostimulation and phototherapy from inside the body. An important difference of this approach is that laser radiation can be delivered to specific internal organ or tissue in vivo, without any loss of its coherence and polarization.30,31 This fact is very significant for the imaging and shaping of beams for more efficient illumination. We will do this with an implantable pill. Currently, all those medical procedures are done externally. Thus, we have developed a special pill that can be implanted into the body during medical surgery and can then monitor the region of the wound by a camera located inside it as well as apply PDT or LLLT using its light sources. Four light emitting diodes (LEDs) as sources were inserted into the pill: at blue (450 nm) for sterilization and at green (518 nm), at red (650 nm), and at infrared (IR) (850 nm) for PDT. Images of the two different types of pills that were fabricated may be seen in Fig. 1. A control card is connected to the pill and communication is done through Bluetooth with an external computer. The camera sends its images at a frequency of 2.4 GHz to an external receiver/transmitter unit which is also connected to a computer. Fig. 1Two types of the fabricated pills. The pill in the left figure contains a camera and four light emitting diodes at blue for sterilization, at green, at red, and at IR. The camera and the light sources are controlled through the control card via Bluetooth. The camera sends its images to an external transmitter/receiver at a frequency of 2.4 GHz. The pill in the right figure contains same parts excluding the camera and is packaged to one piece. 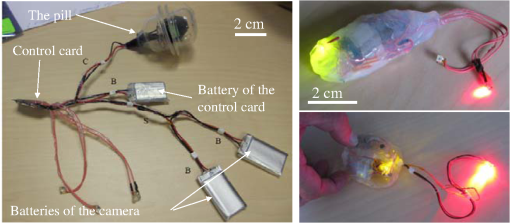 Note that the reason we used cables in the current paper and did not use wireless as in Ref. 26 is because our implantable module is now more complicated than that in Ref. 26. Our current pill includes several units. The implantation of the control unit and power sources is expected to be subcutaneous for minimization of surgical intervention, but the camera and light sources units are supposed to be adjusted to the necessary organ of the body. Our clinical trials performed with the implantable pill were performed both in rabbits as well as in dogs. In Fig. 2, we present the implantation experiments. In Fig. 2(a), we show our wireless implant. We performed sterilization before implantation with 70% ethanol for 15 min. Figures 2(b)–2(h) are related to implantation in rabbits. In Fig. 2(b), we show the electrical energy connection by hypodermic port. In Fig. 2(c), one may see the injection of the electrical hypodermic port as part of the implantation process. In Figs. 2(d) and 2(e), one may see the injection of lidocaine through the hypodermic port. The electrical hypodermic port is used. The light in the rabbit is turned on. In Fig. 2(f), we repeat the dissection of a wound after 14 days of experiment. The illumination is on and an infusion catheter is present. In Fig. 2(g), one may see a pulsoximeter applied on the rabbit’s ear. In Fig. 2(h), one may see the implanted light turned on inside the rabbit. The energy is supplied through the hypodermic port. In Fig. 2(i), we show our implantation experiment performed in dogs. Fig. 2The implantation experiments. (a) Wireless implant. (b)–(h) Implantation in rabbits: (b) electrical energy connection by a hypodermic port. (c) Injection of the hypodermic port as part of the implantation process. Electrical hypodermic port is applied. (d) and (e) Injection of lidocaine through the hypodermic port, electrical hypodermic port is used. Light in the rabbit is turned on. (f) Repeated dissection of a wound after 14 days of experiment. Illumination is on, infusion catheter is present. (g) Pulsoximeter applied on rabbit’s ear. (h) Implanted light in rabbit. Energy is supplied through the hypodermic port. (i) Implantation experiments in dogs. 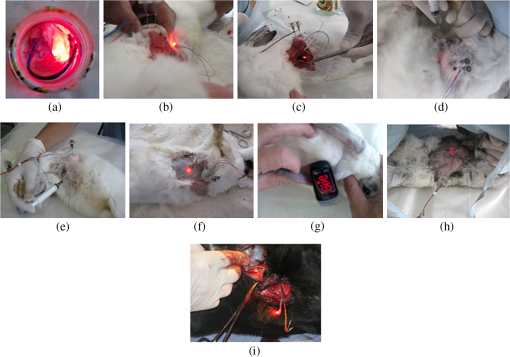 3.Experimental ResultsIn Figs. 3(a) and 3(b), we present preliminary clinical experiential results where the given developed pill was implanted into a dog close to sinus carotid zone and used to demonstrate that an internal illumination of 650 nm, having a 2700 mcd luminous intensity can control the arterial pressure and the heart beat rate. This may be also very applicable for controlling blood pressure and heart rate parameters in humans. In Fig. 3, arterial pressure versus time is presented. One can see that in the case of illumination of the sinus carotid region, systolic and diastolic pressures are larger, in comparison with the case when illumination was stopped. The red arrow in Figs. 3(a) and 3(b) designates the timing when the illumination was stopped. One may see how the blood pressure is reduced and the heart rate indicator is increased right after the illumination is stopped. Thus, in-body illumination may increase the healing rate of a wound. Fig. 3(a) and (b) Experimental demonstration showing that through photostimulation of the “sinus carotid zone” one may cause reduction in arterial pressure and an increase in the heart rate (can be good for heart diseases where one needs to control the blood pressure and the heart rate). The red arrow designated the timing when the illumination was stopped. 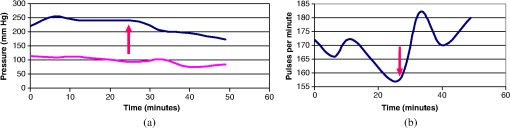 Figure 4 presents another application for the in-body implantable pill. Here, we injected nanoparticles (NPs) into a liver and applied phototherapy from inside the body. The illuminated gold NPs had surface plasmon resonance at the illumination wavelength (900 nm). Counting the overall hemorrhage fields’ area was done by sectioning and counting the “hemorrhaged” squares using an Olympus microscope (Olympus Corporation, Shinjuku, Tokyo, Japan). After histology analysis of the liver, we saw that in the ablated liver with the NP, the hemorrhage is observed to be 1.5 to 2 times denser in comparison with the reference case (laser illuminated liver without NP). Fig. 4Illumination from inside the body (900-nm wavelength, power 3 W focused to diameter ) of liver with implanted gold nanoparticles (NPs) having absorption resonance at the illumination wavelength. After histology analysis we saw that in the ablated liver with the NP, the hemorrhage is observed to be 1.5 to 2 times denser in comparison with the reference case (laser illuminated liver without NP). (a) The experiment. (b) Microscope image for laser illumination without NP. (c) Microscope image for laser illumination with NP. 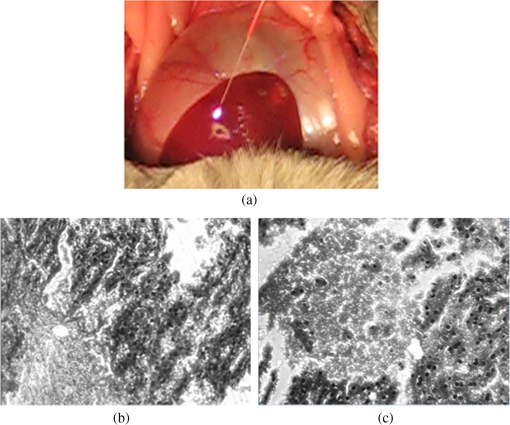 The next set of experiments was performed on rabbits. In Table 1, we present the experimentally extracted results for the oxymetry and the heart rate when the implantable pill was illuminating the sinus carotid. In this experiment, illumination was applied during 8 days (20 min daily), and the organism reaction on illumination was estimated by heart rate and oximetry. Measurements were carried out directly before and after illuminations. The goal of this experiment was to see the change of the influence of same illumination on organism during the postsurgery period. Table 1(a) Oxymetry data for illumination on sinus carotid. (b) Heart rate data for illumination on sinus carotid.
For illustration purposes, the obtained results as presented in Table 1, are plotted as curves in Figs. 5(a) and 5(b), respectively. Three days after implantation, the illumination of sinus carotid resulted in the reduction of the heart rate and in an increase of oxygen saturation in blood. The difference of heart rate reached 20% and the difference in oxygen saturation was 10%. This fact can be explained as a positive influence of light on postoperative stress. From days 4 to 8, we have observed the opposite reaction, with both differences reaching the value of 10%. This fact can be explained as the reaction of a nonstressed organism. Our next experiment included the investigation of the liver healing after heat ablation. Illumination was applied for 20 min in a daily manner. After the experiment was finished, histology probes were taken. The histology results are described in Table 2. The typical way for estimation of the liver tissue regeneration level is to count the multinuclear cells (hepatocytes having more than one nucleus). Quantities of three types of cells were counted: one nuclear cell, two nuclear cells, and degenerative hepatocites cells. All were counted for healthy liver (norm), for preablated liver without illumination (ref.), and for preablated liver with illumination (exp). The notation of “” designates the standard deviation, i.e., the error of the performed measurements. Table 2Histology results for liver regeneration.
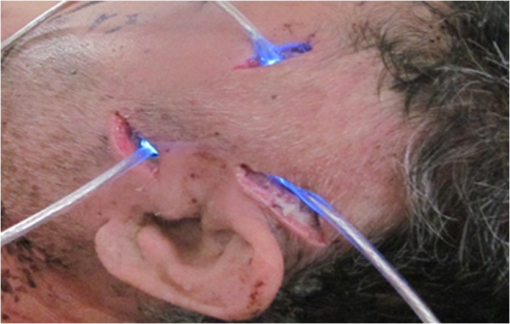
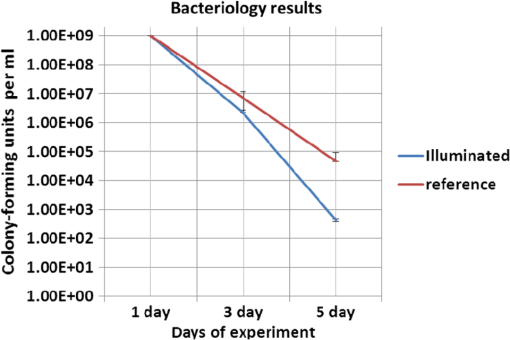
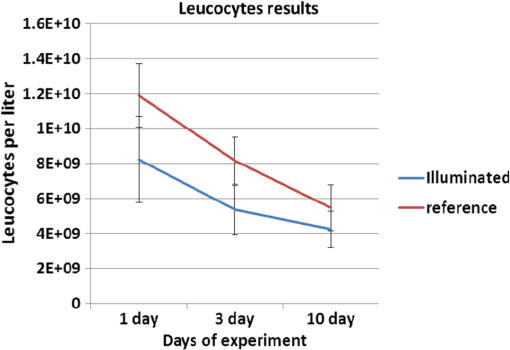
Colored hematoxylin-eosin images taken at a magnification of under the microscope can be seen in Fig. 6 for reference [Fig. 6(a)] and for illuminated cells [Fig. 6(b)]. Fig. 6(a). Reference photo obtained after 2 weeks. Colored: hematoxylin-eosin. Microscope magnification of . (b). Experimental results photo obtained after 2 weeks. Colored: hematoxylin-eosin. Microscope magnification . 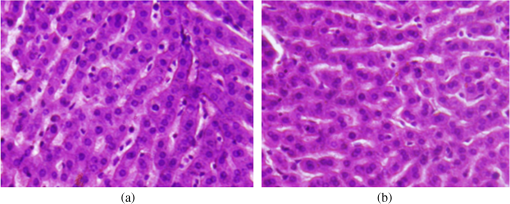 Histological studies of the material obtained from four rabbits with burn defects in the liver showed the following results: In the control experiments, in which a surface burn of the liver alone was performed; in 1-week observation hepatocytes which showed necrotic and necrobiotic changes, insular necrosis of hepatocytes, marked edema, hyperemia, and sites of hemorrhages. In comparison with normal tissue, the quantities of one and two nuclear hepatocytes and degenerative cells were increased (see Table 2). After a daily contact application of red light radiation (20 min a day), marked edema, hyperemia, decondensation of liver tissue were observed; necrotic and necrobiotic changes were less expressed. In comparison with control experiments, the quantity of one nuclear hepatocytes cells was increased, while the quantities of two nuclear hepatocytes and degenerative cells were not changed. In another experiment with a 2-week observation period, in reference experiments we observed islets of infiltration of netrophiles, lymphocytes, hyperemia of small veins, hepatocytes necrosis sites, decondensation of liver tissue, and marked hemorrhage. The quantity of degenerative cells was decreased and the quantity of nuclear hepatocytes cells was not changed. In basic experiments, the same picture was observed; however, tissue was less decondensated and the structure of hepatocytes was more preserved, while infiltration of lymphocytes was more pronounced in portal tracts. The quantity of two nuclear cells in the illuminated region was larger than at the nonilluminated region. The quantity of degenerative cells was decreased. The summary of the abovementioned results are shown in Fig. 7, where Fig. 7(a) is the chart for the quantity of the degenerative cells, and Figs. 7(b) and 7(c) show the quantities of one and two nuclear hepatocytes cells, respectively. Fig. 7(a). Degenerative cells’ quantity. (b). One nuclear hepatocites cells’ quantity. (c). Two nuclear hepatocites cells’ quantity. 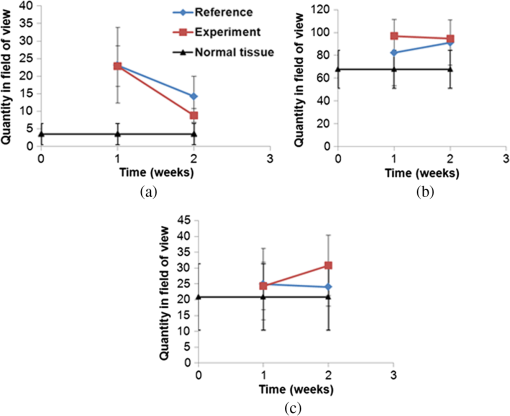 Thus, as a summary of this experimental section, basic experiments showed an improvement of the regenerative capacity of liver tissue during 2 weeks of illumination as compared to reference experiments. The compensatory reaction of hepatocytes to trauma is accompanied by an increase in the quantity of one and two nuclear hepatocytes cells in both the references as well as the experimental samples. With illumination, the quantity of degenerative cells was decreased after a period of 2 weeks. Our last experiment offers a new approach to deal with a medical condition known as phlegmon of the maxillofacial area. To date, a common treatment for such an infection can take up to 2 weeks and it includes surgery in which the infected area is removed followed by antibiotic care.32,33 The recovery process after such a procedure can take a few weeks during which the infection can spread to the brain. We have decided to investigate the effect that light therapy has on the recovery process after such a treatment. In order to do so, we conducted a clinical experiment which included 26 patients that were diagnosed as suffering from phlegmon of the maxillofacial area. All patients underwent a surgery to remove the infected area. A control group of 15 patients received antibiotic treatments while a group of 11 patients received both antibiotic treatments and three sessions of light irradiation therapy in which an implantable device based on our technology was installed inside their head area. One of the patients participating in our experimental trials is presented in Fig. 8. For this experiment, only blue light at 450 nm was used. Each session included irradiation of 20 min with a 450 nm, 1 candela luminous intensity LED (Kingbright AA3535QB24Z1S, New Taipei City, Taiwan) that was inserted with a catheter. Thus for the experiment of Fig. 8, we dismantled our pill and used only portions of it. The reason for illumination using only blue light LEDs without our pill is due to the medical compatibility of materials that our pill contains. As of now, we have only biocompatibility (for experiments on animals), but not full medical compatibility. The interval between irradiations was 3 days. The reason for choosing a wavelength of 450 nm is that while it is considered safe to humans, it has been shown that irradiating bacteria with similar wavelengths has a negative effect on their ability to prosper.34 The first irradiation session was carried out right after the surgery and after each session samples were taken for testing the leukocyte levels and number of bacterial colonies. The results of this experiment are shown in Fig. 9. As can be seen, the number of bacterial colonies decreased faster among the group of patients that received irradiation therapy as compared with the control group. Hence, it can be assumed that combining light therapy with antibiotic treatment shortens the recovery process. Note that the chart in Fig. 9 is logarithmic and the decrease in the bacteriology measurement in the case of irradiation was orders of magnitude. In addition, leukocyte levels decreased more slowly among patients that received irradiation therapy as compared with the control group (see Fig. 10). This result can be explained by the fact that light irradiation therapy has a positive effect on the immune system. It has been shown that visible light irradiation (400 to 700 nm) led to a significant increase in the number of lymphocytes.35 In light of these findings, one may conclude that the irradiation therapy led to a more effective response of the immune system which manifested itself in the continuous formation of leukocytes. These preliminary results demonstrate the positive effect that light irradiation therapy has when combining it with antibiotic care as part of a novel medical treatment for phlegmon of the maxillofacial area. 4.ConclusionsWe have introduced an implantable pill that has several functionalities, including having light sources at different wavelengths capable of performing phototherapy treatments. In addition to low laser light treatment, it includes UV light for sterilization and has the capability for controlled injection of chemicals as well as the capability of transmission of images from inside the patient’s body to an external box via wireless communication. We presented several preliminary clinical results performed with dogs, rabbits, and humans, and showed the biomedical potential of the proposed implantable pill. In this experiment, we showed how illuminating the wound from inside assists in its healing, how illumination can control the blood pressure, the average oxygen saturation, and the heart rate. Specific experiments with rabbit’s liver cells were performed. We presented preliminary clinical results demonstrating the positive effect that light irradiation therapy has when combining it with antibiotic care as part of a medical treatment for phlegmon of the maxillofacial area. We also showed how the illumination related treatment can be accompanied with NPs for further affecting a given tissue. ReferencesY. ChangaC. Yu,
“Successful treatment of oral verrucous hyperplasia with photodynamic therapy combined with cryotherapy—report of 3 cases,”
Photodiagn. Photodyn. Ther., 11
(2), 127
–129
(2014). http://dx.doi.org/10.1016/j.pdpdt.2014.02.003 PPTHBF 1572-1000 Google Scholar
M. Alvarezaet al.,
“Photodynamic properties and photoinactivation of Candida albicans mediated by brominated derivatives of triarylmethane and phenothiazinium dyes,”
Photodiagn. Photodyn. Ther., 11
(2), 148
–155
(2014). http://dx.doi.org/10.1016/j.pdpdt.2014.03.005 PPTHBF 1572-1000 Google Scholar
F. van Leeuwen-van Zaaneet al.,
“The effect of fluence rate on the acute response of vessel diameter and red blood cell velocity during topical 5-aminolevulinicacid photodynamic therapy,”
Photodiagn. Photodyn. Ther., 11
(2), 71
–81
(2014). http://dx.doi.org/10.1016/j.pdpdt.2014.03.012 PPTHBF 1572-1000 Google Scholar
V. G. MaidannikI. V. Maidannik, Handbook of Modern Medicines, AST, Moscow
(2005). Google Scholar
O. FeuersteinN. PersmanI. E. Weiss,
“Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in-vitro study,”
Photochem. Photobiol., 80
(3), 412
–415
(2004). http://dx.doi.org/10.1562/2004-06-13-RA-196.1 PHCBAP 0031-8655 Google Scholar
H. Baaret al.,
“Clinical aspects of photodynamic therapy,”
Sci. Prog., 85
(2), 131
–150
(2002). http://dx.doi.org/10.3184/003685002783238825 SCPRAY 0036-8504 Google Scholar
J. S. GuffeyJ. Wilborn,
“In vitro bactericidal effects of 405 nm and 470 nm blue light,”
Photomed. Laser Surg., 24
(6), 684
–688
(2006). http://dx.doi.org/10.1089/pho.2006.24.684 PLDHA8 1549-5418 Google Scholar
C. Hopper,
“Photodynamic therapy: a clinical reality in the treatment of cancer,”
Lancet Oncol., 1
(4), 212
–219
(2000). http://dx.doi.org/10.1016/S1470-2045(00)00166-2 LOANBN 1470-2045 Google Scholar
Y. B. Kogan,
“Nonlinear photodynamic therapy: method of pulsed oxygen depletion,”
Photochem. Photobiol. Sci., 4
(11), 903
–906
(2005). http://dx.doi.org/10.1039/b500345h PPSHCB 1474-905X Google Scholar
T. Maisch,
“Anti-microbial photodynamic therapy: useful in the future,”
Lasers Med. Sci., 22
(2), 83
–91
(2007). http://dx.doi.org/10.1007/s10103-006-0409-7 LMSCEZ 1435-604X Google Scholar
M. Wainwright,
“Photodynamic antimicrobial chemotherapy (PACT),”
Antimicrob. Chemother., 42
(1), 13
–28
(1998). http://dx.doi.org/10.1093/jac/42.1.13 JACHDX 0305-7453 Google Scholar
X. FuY. FangM. Yao,
“Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection,”
Biomed. Res. Int., 2013 159157
(2013). http://dx.doi.org/10.1155/2013/159157 BRIIDT 2314-6141 Google Scholar
A. Hanakovaaet al.,
“The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin,”
Microbiol. Res., 169
(2–3), 163
–170
(2014). http://dx.doi.org/10.1016/j.micres.2013.07.005 MCRSEJ Google Scholar
T. Maischet al.,
“Antibacterial photodynamic therapy,”
Hautarzt, 56
(11), 1048
–1055
(2005). http://dx.doi.org/10.1007/s00105-005-0977-7 HAUTAW 1432-1173 Google Scholar
X. Wanget al.,
“Cellular and molecular mechanisms of photodynamic hypericin therapy for nasopharyngeal carcinoma cells,”
J. Pharmacol. Exp. Ther., 334
(3), 847
–853
(2010). http://dx.doi.org/10.1124/jpet.110.168856 JPETAB 0022-3565 Google Scholar
R. Fink-Pucheset al.,
“Primary clinical response and long-term follow-up of solar keratoses treated with topically applied 5-aminolevulinic acid and irradiation by different wave bands of light,”
J. Photochem. Photobiol. B, 41
(1–2), 145
–151
(1997). http://dx.doi.org/10.1016/S1011-1344(97)00096-1 JPPBEG 1011-1344 Google Scholar
E. W. Jeffeset al.,
“Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid,”
Arch. Dermatol., 133
(6), 727
–732
(1997). http://dx.doi.org/10.1001/archderm.1997.03890420065007 ARDEAC 0003-987X Google Scholar
U. Hohenleutneret al.,
“Treatment of pediatric hemangiomas with the flashlamp-pumped pulsed dye laser,”
Hautarzt, 47
(3), 183
–189
(1996). http://dx.doi.org/10.1007/s001050050400 HAUTAW 1432-1173 Google Scholar
A. Lipovskyet al.,
“Sensitivity of Staphylococcus aureus strains to broadband visible light,”
Photochem. Photobiol., 85
(1), 255
–260
(2009). http://dx.doi.org/10.1111/php.2009.85.issue-1 PHCBAP 0031-8655 Google Scholar
M. L. de Moraes Maiaet al.,
“Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: a systematic review,”
J. Appl. Oral Sci., 20
(6), 594
–602
(2012). http://dx.doi.org/10.1590/S1678-77572012000600002 JAOSBM 1678-7757 Google Scholar
J. Hashmiet al.,
“Role of low-level laser therapy in neurorehabilitation,”
J. Phys. Med. Rehabil., 2
(12), S292
–S305
(2010). http://dx.doi.org/10.1016/j.pmrj.2010.10.013 Google Scholar
A. Stergioulaset al.,
“Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy,”
Am. J. Sports Med., 36
(5), 881
–887
(2008). http://dx.doi.org/10.1177/0363546507312165 AJSMDO 0363-5465 Google Scholar
M. R. Hamblins,
“Mechanism of low level light therapy,”
Photobiol. Sci. Online, 6140
(1), 614001
(2006). Google Scholar
N. Ben Dovet al.,
“Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro,”
Biochim. Biophys. Acta, 1448
(3), 372
–380
(1999). http://dx.doi.org/10.1016/S0167-4889(98)00147-5 BBACAQ 0006-3002 Google Scholar
A. Wanget al.,
“Wireless capsule endoscopy,”
J. Gastrointest. Endosc., 78
(6), 805
–815
(2013). Google Scholar
P. Swain,
“Wireless capsule endoscopy,”
Gut, 52
(4), iv48
–iv50
(2003). Google Scholar
A. Mogliaet al.,
“Wireless capsule endoscopy: from diagnostic devices to multipurpose robotic systems,”
Biomed. Microdev., 9
(2), 235
–243
(2007). http://dx.doi.org/10.1007/s10544-006-9025-3 BMICFC 1387-2176 Google Scholar
D. Panescu,
“An imaging pill for gastrointestinal endoscopy,”
IEEE Eng. Med. Biol. Mag., 24
(4), 12
–14
(2005). http://dx.doi.org/10.1109/MEMB.2005.1463383 IEMBDE 0739-5175 Google Scholar
C. McCaffreyet al.,
“Swallowable-capsule technology,”
IEEE Pervasive Comput., 7
(1), 23
–29
(2008). http://dx.doi.org/10.1109/MPRV.2008.17 1536-1268 Google Scholar
D. Fixleret al.,
“Determination of coherence length in biological tissues,”
Lasers Surg. Med., 43
(4), 339
–343
(2011). http://dx.doi.org/10.1002/lsm.v43.4 LSMEDI 0196-8092 Google Scholar
D. Fixleret al.,
“Depolarization of light in biological tissues,”
Opt. Lasers Eng., 50
(6), 850
–854
(2012). http://dx.doi.org/10.1016/j.optlaseng.2012.01.011 OLENDN 0143-8166 Google Scholar
J. Hochfilze,
“Phlegmon of the neck and osteomyelitis of the mandible following,”
Arch Otolaryngol., 12
(2), 175
–177
(1930). Google Scholar
C. TownsendD. Sabiston,
“Sabiston textbook of surgery board review,”
Colon and Rectum, 19th ed.Elsevier(2012). Google Scholar
L. Murdochet al.,
“Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in liquid suspensions and on exposed surfaces,”
Sci. World J., 2012 137805
(2012). http://dx.doi.org/10.1100/2012/137805 Google Scholar
J. Roberts,
“Light and immunomodulation,”
Ann. N. Y. Acad. Sci., 917
(1), 435
–445
(2000). http://dx.doi.org/10.1111/nyas.2000.917.issue-1 ANYAA9 0077-8923 Google Scholar
BiographyVictor Sheinman received his MD degree in the field of stomatology in 1963, PhD degree in the field of maxillofacial medicine in 1970, and a higher doctoral degree (Doctor of Sciences) in the field of maxillofacial medicine in 1989. He was the chief of the Academic Department of Maxillofacial Medicine. He is an author of more than 100 publications, two monographs, one surgeon atlas, and 20 patents. Now, he is a scientific researcher at Bar-Ilan University. Arkady Rudnitsky received his MSc degree in the field of system engineering at Samara State Aerospace University in 1995 and, in addition, a PhD degree in the field of electro-optics at Tel-Aviv University in 2012. He is currently the electro-optics lab engineer in the Engineering Department of Bar-Ilan University. His research interests are all-optical computational devices, novel application of nano-mechanical devices in optics and biomedical optics and engineering. Rakhmanbek Toichuev received his MD degree in the field of stomatology in 1970. He worked in a regional children’s hospital from 1972 to 1995. In 1989, he received a PhD degree in the field of pediatric surgery. Since 1994, he has been the director of the Institute of Medical Problems of the Academy of Science of Kyrgyzstan. He has more than 250 publications and about 20 patents. Abdyrakhman Eshiev received his MD degree in the field of stomatology in 1984, PhD degree in the maxillofacial medicine at 2004, and a higher doctoral degree (Doctor of Sciences) at 2011. Now, he is a professor at the Medical Department of Osh State University. He has more than 100 publications and about 30 patents. Svetlana Abdullaeva received her MD degree in the field of stomatology. She is a resident physician in the Maxillofacial Department of the Regional Hospital of Osh, Kyrgistan. In addition, she is involved in scientific research for a PhD degree in the topic of using blue light in the treatment of abscesses of the maxillofacial region. Talantbek Egemkulov received his MD degree in the field of stomatology in 2010. He is a resident physician in the Maxillofacial Department of the Regional Hospital of Osh, Kyrgistan. In addition, he is involved in scientific research for a PhD degree. He has 6 publications. Zeev Zalevsky received his BSc and direct PhD degrees in electrical engineering from Tel-Aviv University in 1993 and 1996, respectively. He is currently a full professor in the Faculty of Engineering in Bar-Ilan University, Israel. His major fields of research are optical super-resolution, biomedical optics, nanophotonics, and electro-optical devices. He published more than 350 journal papers, 190 conference proceeding papers, 315 international presentations, 32 issued patents, 9 authored and edited books, and 27 book chapters. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||