|
|
1.IntroductionHealthcare associated infection (HAI) affects approximately 10% of patients admitted to the hospital, and is responsible for over 5000 deaths in the UK annually (Improving Patient Care by Reducing the Risk of Hospital Acquired Infection: A Progress Report, National Audit Office 2004). HAI can be caused by airborne and environmental bacterial contamination which can be transmitted to surgical site wounds either directly from the environment or indirectly via vectors such as healthcare workers’ hands.1,2 A 405-nm high-intensity narrow spectrum (HINS) light has antimicrobial activity against HAI-related bacterial pathogens including methicillin-resistant Staphylococcus aureus3 and, due to the use of visible light wavelengths, this does not pose the same health concerns as those associated with using ultraviolet light. This 405-nm light technology has already been used to develop an environmental decontamination system for the disinfection of air and environmental surfaces, which has been successfully clinically evaluated within isolation rooms at Glasgow Royal Infirmary.4 Infection rates following orthopedic arthroplasty surgery are as high as 4%, while the infection rates are even higher after revision surgery,5 and the environment and surgical devices have been highlighted as sources of bacterial contamination.6 The bactericidal properties of 405-nm light suggest that this may aid in reducing the incidence of infections that arise from environmental contamination during arthroplasty surgery. The toxic effect of 405-nm light on bacterial cells is not replicated to the same extent in mammalian cells, and this has been successfully demonstrated in previous studies.7,8 If 405-nm light technology was to be promoted for localized disinfection during arthroplasty surgery, the bone would likely be exposed to 405-nm light during the course of surgery and any inhibitory effects on the function of osteoblasts may affect the integration of the orthopedic implant into the bone postsurgery. It is therefore essential to consider the duration of an orthopedic surgical procedure and establish the effects of 405-nm light on osteoblast function during this potential exposure. Discussion with surgeons has revealed that the duration of routine arthroplasty surgeries was between 1 and 2 h, although a complex revision may take up to 4 h. Previous studies on fibroblasts for wound healing have shown that 405-nm light had no significant effect on cell viability when exposed to a dose of ( for 1 h), while a substantial decline in cell viability was observed at a dose of ( for 1 h).7 It has also been found that exposing osteoblasts to 405-nm light with a dose of ( for 3 h) had a detrimental effect on cell viability.9 Hence, the aim of this study was to establish whether the adverse effects of 405-nm light seen on mammalian cells were dose dependent, to determine a damage threshold of exposure of osteoblasts to 405-nm light, and to find out whether doses below the damage threshold can exert a bactericidal effect on clinically relevant pathogenic bacteria. Demonstration of these key parameters would be an essential step toward assessing whether this novel technology could be developed and applied for localized decontamination of the patient environment during arthroplasty surgeries. 2.Methods and Materials2.1.Mammalian Cell StudyImmortalized osteoblast (OST 5) cells isolated by SV40 transfection of neonatal rat calvarial osteoblasts were chosen for use in the experiments. The cells were cultured as monolayers using Dulbecco’s modified Eagle’s medium (DMEM) containing 10% v/v fetal calf serum in an atmosphere of 5% in air at 37°C. The 405-nm light system used in this study consisted of an array of nine 405-nm light-emitting diodes (LEDs) (GE Illumination), with a full-width half-maximum of 20 nm. The LEDs were arranged in a grid pattern (), and attached to a heat sink for thermal management, thus preventing sample heating during light exposure. The heat sink was supported by two pillars above a molded base designed to fit a multiplate in order to fix the position of the treatment plates directly below the LEDs. The light source was set at a height of 8 cm above the multiwell plate. The middle 4 wells in a 24-well plate and the middle 16 wells in a 96-well plate were used for cell exposures. Cells were seeded at a seeding density of in a 24-well plate and in a 96-well plate and left in the incubator overnight at 37°C. To investigate the dose-dependent effects of 405-nm light on the osteoblasts, the samples were exposed to light in 1 ml Dulbecco’s phosphate-buffered saline (DPBS), inside an incubator at 37°C and 5% , at a low dose of and a high dose of using varying irradiance/exposure regimes (Table 1). Dose () is calculated according to the equation: where is the dose (energy density) in , is the irradiance (power density) in , and is the time in seconds.Table 1Irradiance levels and exposure times used to apply low dose (18 J/cm2) and high dose (54 J/cm2) 405-nm light to osteoblasts.
Unexposed controls were treated in the same way. After the stipulated exposure time, the DPBS solution was removed, 1 ml of DMEM was added to the wells, and the cells were incubated for 48 or 72 h before assessing the cell population using crystal violet staining and Lowry protein assay.10 To establish a damage threshold for osteoblast cells, samples were exposed to 405-nm light at an irradiance of in 1 ml DPBS for increasing time periods (60, 90, 120, and 150 min corresponding to doses of 18, 27, 36, and ) inside an incubator at 37°C and 5% . Unexposed controls were treated in the same way. After the stipulated exposure time, the DPBS was removed, 1 ml DMEM was added to wells, and the cells were incubated for 48 or 72 h before carrying out the following assays. To measure cell viability, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was carried out as described by Ho et al.11 Cell function was measured using the alkaline phosphatase (ALP) assay as described by McDonald et al.7 The bromodeoxyuridine (BrdU) assay was carried out to measure the cell proliferation rate postlight exposure using QIA 58 BrdU cell proliferation assay kit (Calbiochem—Merckmillipore) as described by the manufacturer. Live/dead staining of the cells was carried out using acridine orange (AO; live cells stain green) and propidium iodide (PI; dead cells stain red). The cells were stained with phalloidin-fluorescein isothiocyanate (FITC) (green stain for actin; Sigma-Aldrich Company Ltd., UK)/4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (blue nuclear stain; Life technologies, Paisley, Scotland, UK) to study the cytoskeletal actin structures of cells. For microscopy, cells were seeded on glass coverslips (13-mm diameter) at and viewed under a Zeiss AxioImager Z1 fluorescent microscope using a wet lens (). 2.2.Bacterial StudyThe bacteria used in this study were S. aureus NCTC 4135, Escherichia coli NCTC 9001, Pseudomonas aeruginosa LMG 9009, Klebsiella pneumoniae NCTC 09633, Staphylococcus epidermidis LMG 10474, and Acinetobacter baumannii LMG 1041 (NCTC, National Collection of Type Cultures, Collindale, UK; LMG, Laboratorium voor Microbiologie, Universiteit Gent, Belgium). Bacteria were cultured in nutrient broth (Oxoid, UK) at 37°C for 18 h under rotary conditions (120 rpm). Broths were centrifuged at 4300 rpm for 10 min, and the cell pellet was resuspended in PBS. The cell suspension was then diluted in PBS to obtain a concentration of colony forming units (CFU)/ml. Samples () were then spread onto 55 mm agar plates, with approximately 150 to 300 CFU/plate, and seeded agar plates were then exposed to 405-nm light at an irradiance of for increasing time periods (15, 30, 60, 90, and 120 min corresponding to doses of 4.5, 9, 18, 27, and ). Postexposure, plates were incubated at 37°C for 24 h before enumeration of the viable CFU/plate. Results are reported as % surviving CFU/plate as compared to nonexposed control samples. 3.Results and DiscussionData in Fig. 1 show that exposure of cells to 405-nm light using different irradiance/exposure period regimes caused no significant difference in the crystal violet staining or protein concentration between the control and the treated samples after 48- and 72-h posttreatment periods. In contrast, a significant difference was evident between the control and the 405 nm treated samples exposed to higher doses of irrespective of how the dose was administered. These results confirm that the effect of 405-nm light on mammalian cells is dose dependent. Fig. 1Crystal violet staining and protein content of osteoblasts exposed to different doses of 405-nm light. (a) Crystal violet absorbance (% control), (b) protein concentration (% control) of rat osteoblasts exposed to 405-nm light at a low dose of and (c) crystal violet absorbance (% control), (d) protein concentration (% control) of rat osteoblasts exposed to a high dose of . Doses were achieved using different irradiance/exposure time regimes, and were followed by either 48 or 72-h posttreatment periods. Results are means of . *Statistically different using unpaired Student’s t-test comparing control and light treated samples, . 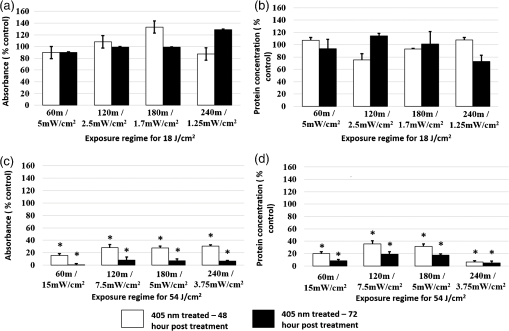 Data in Fig. 2 show that cells exposed to doses of 18, 27, and ( for 60, 90, and 120 min, respectively) exhibited no significant effect on the MTT reduction, the ALP activity or the cell proliferation rate as measured by the BrdU method. In contrast, cells exposed to doses above ( for more than 120 min) showed a significant decrease in all the parameters of cell function. Fig. 2MTT reduction, ALP activity, and cell proliferation in osteoblasts exposed to different doses of 405-nm light. (a) and (b) MTT reduction (, ), (c) and (d) ALP activity (, ), and (e) and (f) BrdU absorbance (, ) of rat osteoblasts exposed to 405-nm light doses of 18, 27, 36, and ( for 60, 90, 120, and 150 min, respectively) followed by 48- (left) and 72-h (right) posttreatment period. *Statistically different, using unpaired Student’s t-test comparing control and light treated samples, . 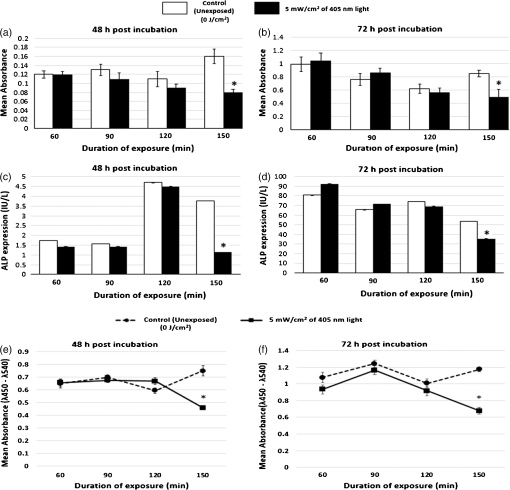 AO and PI dyes were used in this study to differentiate live cells, apoptotic and necrotic cells and study the viability of osteoblasts after 48- and 72-h postlight exposure periods. All live nucleated cells fluoresce green, early apoptotic cells fluoresce bright green, late apoptotic cells fluoresce orange, and all dead nucleated cells fluoresce red. After both 48- and 72-h culture postexposure to light, more apoptotic and dead cells were observed following 150 min () exposure compared to 120 min () exposure to 405-nm light (Fig. 3), confirming the findings with MTT. Staining of the actin with phalloidin-FITC showed that after exposure to light, the actin formed a ring around the cell membrane, and more cells rearranged themselves into a circular shape, when compared with the samples exposed to for both 48- and 72-h posttreatment period (Fig. 4). Accumulation of actin around the outer-cell perimeter is indicative of membranes being strengthened to prevent leakage of the cell contents and is a sign of apoptosis. At 48 h in culture postexposure to light, the cells show early apoptosis [Fig. 3(b)], but this damage appears to be repaired at 72 h in culture postexposure to light [Fig. 3(f)]. Exposure to 405-nm light may indeed cause sublethal damage to the cells, and further studies such as assessment of deoxyribose nucleic acid (DNA) integrity will be required. Fig. 3The cell viability of osteoblast cells after exposure to of 405-nm light. [(a) and (c) Control ( for 120 and 150 min, respectively for a 48-h postincubation period), (e) and (g) control ( for 120 and 150 min, respectively for a 72-h postincubation period). (b) and (d) Exposure for 120 min () and 150 min (), respectively, for a 48-h posttreatment period and (f) and (h) exposure for 120 min () and 150 min (), respectively, for a 72-h posttreatment period).] Acridine orange stains live cells green, early and late apoptotic cells bright green and orange, respectively, and propidium iodide stains dead cells red. 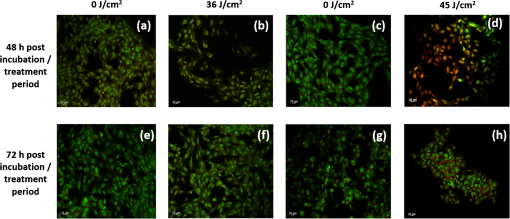 Fig. 4The cell morphology of osteoblast cells after exposure to of 405-nm light. [(a) and (c) Control ( for 120 and 150 min, respectively, for a 48-h postincubation period), (e) and (g) control ( for 120 and 150 min, respectively, for a 72-h postincubation period). (b) and (d) exposure for 120 min () and 150 min (), respectively, for a 48-h posttreatment period and (f) and (h) exposure for 120 min () and 150 min (), respectively, for a 72-h posttreatment period).] Phalloidin-FITC stains the cytoskeleton green and DAPI stains the nucleus blue. 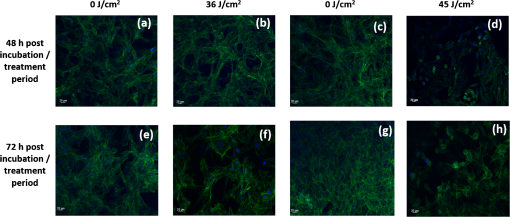 The mammalian cell studies suggest that cell exposures to 405-nm HINS light for up to a dose of cause no observable effects on the cell viability, function, proliferation rate, and morphology using the techniques employed in this study. When investigating the comparative effects of 405-nm light exposure on bacterial cells, results demonstrate that doses up to , which were found to have no impact on the mammalian cells, induce significant bactericidal effects. Results in Fig. 5 highlight the inactivation kinetics of a range of Gram-positive (S. aureus, S. epidermidis) and Gram-negative (P. aeruginosa, A. baumannii, E. coli, K. pneumoniae) bacteria. The susceptibility of the organisms varied with A. baumannii proving most susceptible with kill achieved after exposure to (). Significant inactivation of all organisms was achieved by exposure to and between 99.5% to 100% inactivation of all species was shown after application of . A previously published study reported the antibacterial effects of 405-nm light against these clinically relevant organisms, demonstrating their susceptibility at population densities of up to CFU/ml in liquid suspension;3 however, the present study uses light doses and surface-seeded bacterial contaminants at population densities ( CFU/plate) that are more relevant to practical clinical environmental decontamination applications,4 as has been demonstrated in a previous work.9 The present study confirms, by direct comparison, the selective detrimental effect that light of this wavelength can have on infection-causing organisms in comparison to osteoblasts. Fig. 5Survival of a range of surface seeded clinically relevant bacterial pathogens exposed to 405-nm HINS light at doses ranging from 4.5 to . Results are means of . *Statistically different, using unpaired Student’s t-test comparing control and light treated samples, . 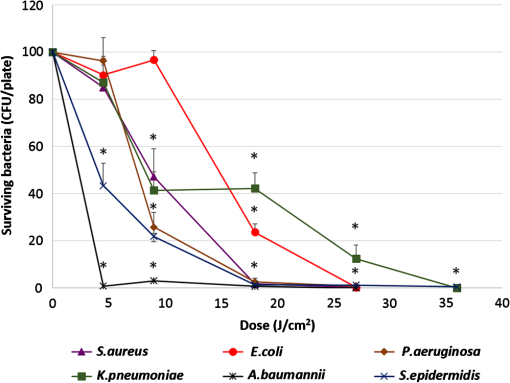 The antibacterial effect of 405-nm light is not thought to be mediated through direct DNA crosslinking, unlike that of UV light.12 Instead, the 405 nm violet-blue light inactivation effect is considered to be caused by the excitation of intracellular photosensitive porphyrin molecules, which results in the production of reactive oxygen species (ROS), inducing oxidative damage and, consequently, cell death.3,13–15 Indeed, previous studies have detected endogenous porphyrins within S. aureus,15 P. aeruginosa,14 and A. baumannii,16 and implicated these violet-blue light sensitive molecules in the inactivation mechanism. In addition, a study by Lipovsky et al.15 compared the sensitivity of two strains of S. aureus and found that the more light-sensitive strain contained 10-fold more endogenous porphyrins than the more resilient strain. Further evidence of the involvement of porphyrins in the inactivation process was provided by Nitzan et al., who demonstrated that bacterial susceptibility to violet-blue light inactivation can be increased when exogenous -aminolevulinic acid (ALA), the precursor of the porphyrin biosynthesis pathway, is added in order to amplify the levels of endogenous porphyrins within the bacterial cells.15–18 Since ROS are considered to cause widespread oxidative damage to bacterial cells and the mechanism of action is not site specific, as is the case with many antibiotics, this explains not only the broad spectrum antimicrobial efficacy of 405-nm light but also why highly antibiotic resistant species such as Acinetobacter baumanii show no greater resistance to 405-nm light than nonantibiotic resistant bacteria. Bacterial cells have also been shown to have much greater sensitivity than mammalian cells to violet-blue light in the region of 405 nm.9,14 Low levels of antioxidant defence enzymes in certain bacteria have been implicated as a possible reason for this,19 and as such are more susceptible to ROS mediated oxidative damage. In contrast, most mammalian cells are well equipped to survive in high oxygen conditions, and contain ample cytoprotective mechanisms such as superoxide dismutase, catalase, and glutathione peroxidise.19 These findings thus highlight the potential for development of 405-nm light for inactivation of bacterial contaminants around surgical sites. Exposure to 405-nm light has also been proven to cause no adverse effects on wound healing,7 thus providing further evidence for potential clinical application of this disinfection technology. Importantly, the dose-dependent effects of 405-nm light on osteoblast cells suggest that the dose of , which may be achieved by applying different irradiances for varying time periods (e.g., for 1 h, for 2 h, for 3 h, and for 4 h), does not cause detectable damage to mammalian cells, regardless of how the dose is applied. These findings are significant, and should provide a basis for the development of light sources which could be applied practically within operating theaters at levels appropriate for the typical operating times relevant to arthroplasty surgery. Further safety advantages of light of this wavelength relate to the photon energy of 405-nm light. The depth of penetration of visible light into tissue increases with wavelength20 and the penetrability of 405-nm light, which although greater than that of UV light, is low and will not penetrate deeply into bone; particularly when compared to light of a longer wavelength, e.g., 690-nm laser light.21 Also, the 405-nm intensities suggested for use during surgical exposures would be low; therefore, theater personnel are unlikely to require protection from these visible violet-blue light wavelengths. This is unlike UV light which, due to the high photon energy and associated detrimental health effects, is normally only used in the absence of people. There have been limited attempts to utilize UV light during surgical procedures but these have required operating staff to wear protective clothing during exposure.22,23 4.SummaryA 405-nm HINS light has proven to have strong antimicrobial effects against a variety of medically significant bacteria at a dose level of . Conversely, mammalian cell studies found that the osteoblasts appear healthy when exposed to doses up to ; at higher doses, there is a negative impact on cell viability, function, and proliferation rate. The results obtained from the application of low dose and high dose light using a range of irradiance/exposure time regimes have demonstrated that the effects of 405-nm light on osteoblast cells are dose dependent. This study has established a dose-dependent level at which there is a differential sensitivity to the effects of 405-nm light between osteoblasts and bacteria; exposure of mammalian cells up to a dose of does not cause any observable effect on osteoblast viability. At this dose, the 405-nm light is still bactericidal indicating that there is potential to develop the technology for decontamination of the environment around patients during arthroplasty surgery. AcknowledgmentsP.R. is supported by a DTC studentship from the Engineering and Physical Sciences Research Council. The authors are grateful for the help of Mrs. Katie Henderson with mammalian cell culture. ReferencesM. AlbrechtR. GauthierD. Leaper,
“Forced-air warming: a source of airborne contamination in the operating room?,”
Orthop. Rev. (Pavia), 1
(2), 85
–89
(2009). http://dx.doi.org/10.4081/or.2009.e28 2035-8237 Google Scholar
R. W. Loftuset al.,
“Hand contamination of anesthesia providers is an important risk factor for intraoperative bacterial transmission,”
Anesth. Analg., 112
(1), 98
–105
(2011). http://dx.doi.org/10.1213/ANE.0b013e3181e7ce18 AACRAT 0003-2999 Google Scholar
M. Macleanet al.,
“Inactivation of bacterial pathogens following exposure to light from a 405-nm LED array,”
Appl. Environ. Microbiol., 75
(7), 1932
–1937
(2009). http://dx.doi.org/10.1128/AEM.01892-08 AEMIDF 0099-2240 Google Scholar
M. Macleanet al.,
“Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light,”
J. Hosp. Infect., 76
(3), 247
–251
(2010). http://dx.doi.org/10.1016/j.jhin.2010.07.010 JHINDS 0195-6701 Google Scholar
H. HamiltonJ. Jamieson,
“Deep infection in total hip arthroplasty,”
Can. J. Surg., 51
(2), 111
–117
(2008). CJSUAX 0008-428X Google Scholar
N. Daviset al.,
“Intraoperative bacterial contamination in operations for joint replacement,”
J. Bone Joint Surg. Br., 81
(5), 886
–889
(1999). http://dx.doi.org/10.1302/0301-620X.81B5.9545 JBSUAK 0301-620X Google Scholar
R. McDonaldet al.,
“Effect of 405 nm high-intensity narrow-spectrum light on fibroblast populated collagen lattices: an in vitro model of wound healing,”
J. Biomed. Opt., 16
(4), 048003
(2011). http://dx.doi.org/10.1117/1.3561903 JBOPFO 1083-3668 Google Scholar
S. Smithet al.,
“Exposure of 3T3 mouse fibroblasts and collagen to high intensity blue light,”
in IFMBE Proc. 13th Int. Conf. on Biomedical Engineering,
1352
–1355
(2009). Google Scholar
R. McDonaldet al.,
“405 nm light exposure of osteoblasts and inactivation of bacterial isolates from arthroplasty patients: potential for new disinfection applications?,”
Eur. Cell Mater., 25 204
–214
(2013). ECMUBB 1473-2262 Google Scholar
O. H. Lowryet al.,
“Protein measurement with the folin phenol reagent,”
J. Biol. Chem., 193
(1), 265
–275
(1951). JBCHA3 0021-9258 Google Scholar
G. Hoet al.,
“Low-level laser therapy on tissue-engineered skin substitutes: effect on the proliferation rate of 3T3 mouse fibroblast cells,”
Proc. SPIE, 5610 124
–134
(2004). http://dx.doi.org/10.1117/12.584384 PSISDG 0277-786X Google Scholar
K. Ogumaet al.,
“Determination of pyrimidine dimmers in Escherichia coli and Cryptosporidium parvum during UV light inactivation, photoreactivation, and dark repair,”
Appl. Environ. Microbiol., 67
(10), 4630
–4637
(2001). http://dx.doi.org/10.1128/AEM.67.10.4630-4637.2001 AEMIDF 0099-2240 Google Scholar
R. Lubartet al.,
“A possible mechanism for the bactericidal effect of visible light,”
Laser Ther., 20
(1), 17
–22
(2011). http://dx.doi.org/10.5978/islsm.20.17 LATHE5 0898-5901 Google Scholar
T. Daiet al.,
“Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action,”
Antimicrob. Agents Chemother., 57
(3), 1238
–1245
(2013). http://dx.doi.org/10.1128/AAC.01652-12 0066-4804 Google Scholar
A. Lipovskyet al.,
“Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing,”
Lasers Surg. Med., 42
(6), 467
–472
(2010). http://dx.doi.org/10.1002/lsm.v42:6 LSMEDI 0196-8092 Google Scholar
Y. Zhanget al.,
“Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections,”
J. Infect. Dis., 209
(12), 1963
–1971
(2014). http://dx.doi.org/10.1093/infdis/jit842 JIDIAQ 0022-1899 Google Scholar
Y. NitzanM. Kauffman,
“Endogenous porphyrin production in bacteria by -aminolaevulinic acid and subsequent bacterial photoeradication,”
Lasers Med. Sci., 14
(4), 269
–277
(1999). http://dx.doi.org/10.1007/s101030050094 LMSCEZ 1435-604X Google Scholar
Y. Nitzanet al.,
“ALA induced photodynamic effects on Gram positive and negative bacteria,”
Photochem. Photobiol. Sci., 3
(5), 430
–435
(2004). http://dx.doi.org/10.1039/b315633h PPSHCB 1474-905X Google Scholar
B. HalliwellJ. M. C. Gutteridge,
“Antioxidant defences,”
Free Radicals in Biology and Medicine, 105
–245 Oxford University Press, Oxford
(2001). Google Scholar
E. SternbergD. Dolphin,
“Porphyrin-based photosensitizers for use in photodynamic therapy,”
Tetrahedron, 54
(17), 4151
–4202
(1998). http://dx.doi.org/10.1016/S0040-4020(98)00015-5 TETRAB 0040-4020 Google Scholar
S. K. BislandS. Burch,
“Photodynamic therapy of diseased bone,”
Photodiagn. Photodyn. Therapy, 3
(3), 147
–155
(2006). http://dx.doi.org/10.1016/S1572-1000(06)00036-6 PPTHBF 1572-1000 Google Scholar
P. E. GosdenA. P. MacGowanG. C. Bannister,
“Importance of air quality and related factors in the prevention of infection in orthopaedic implant surgery,”
J. Hosp. Infect., 39
(3), 173
–180
(1998). http://dx.doi.org/10.1016/S0195-6701(98)90255-9 JHINDS 0195-6701 Google Scholar
G. J. S. TaylorG. C. BannisterJ. P. Leeming,
“Wound disinfection with ultraviolet radiation,”
J. Hosp. Infect., 30
(2), 85
–93
(1995). http://dx.doi.org/10.1016/0195-6701(95)90148-5 JHINDS 0195-6701 Google Scholar
|
|||||||||||||||||||||||||||||||||

