|
|
1.IntroductionLight-emitting diodes (LEDs) are known to possess advantageous attributes, such as compactness, long life, high efficiency, and high brightness. They have been widely applied in biomedical illumination under various scenarios1,2 and present as a viable replacement of xenon arc lamp in many forthcoming and critical applications.3–5 In this work, we have developed a foldable and flexible LED lighting module using white light LED chips to serve as auxiliary lighting for video-assisted thoracic surgery (VATS), which is widely adopted in thoracoscopy procedures.6 VATS allows the doctors to view the chest cavity by opening small incisions and is a form of minimal invasive surgery.7 The small wounds greatly facilitate shorter hospital stay, faster recoveries,8 and reduced risk of infection for patients, in comparison with traditional thoracotomy.9 However, the complex structures and limited operative space in the chest cavity may result in blinded or blocked regions that could compromise the quality of video imaging and thus the corresponding surgical procedures in thoracoscopy. As in most other endoscopies, the main lighting in current endoscopic VATS is usually the fiber bundle coupled light sources10,11 through laparoscope assembly. However, the fiber light guiding is hardly efficient and the solid angle of illumination (the lighting cone) is also restricted, usually within 40 deg and less than the field-of-view (FOV) of the endoscopy camera. Specifically, in angularly deviated regions from the distal end of the endoscope, the luminance is nonuniform and falls off quickly. Henceforth, the LED module used in this study was designed to remedy the problem as auxiliary background lighting source. Given the illuminance achieved (60,000 lux at 5 cm), it may also serve as a sufficient main lighting source. Specifically, the novel advantages and potentials of LED lighting for surgery include direct and highly efficiency illumination, greater flexibility in lighting configuration, and broader solid angle and better uniformity in overall illumination. 2.Methods2.1.Experimental SetupDuring the experiment (Fig. 1), a senior surgeon (one of the authors) removed the right lung of the test animal to secure a larger space for characterizing the illuminance under various parameters and conditions. The other incision was made 14 cm away to allow the insertion of the rigid endoscope into the chest. The whole surgical process was recorded digitally in HD format (720p). Fig. 1The schematic of the cross-sectional view of the illuminance testing setup. It consists of a foldable ring light source (with overheat protection), a photometer (TES1339, Taishi Co., Taipei, Taiwan), a rigid endoscope (Olympus, Tokyo, Japan), and the video imaging system. A live sow under general anesthesia is used as the experimental subject. The testing parameters used in the illuminance study are , , , , , , , and . 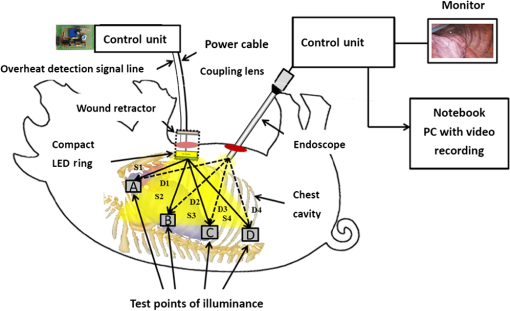 2.2.LED LightingThe LED chips were arrayed in a foldable ring configuration,12,13 with an inner diameter of 55 mm and an outer diameter of 65 mm. It was composed of surface mount white light LEDs (5050-A66, SURTEK CO., LTD, Hsin-Chu, Taiwan) of size .14,15 Transparent medical grade isolation tape (3M™ Transpore™ Clear Porous Plastic Hypo-Allergenic Surgical Tape) was used to secure the LED assembly. The foldable design of the ring assembly allowed its easy insertion through the wound retractor into the thoracic cavity.16,17 It can be attached to the rubber ring of the retractor inside the chest cavity, as shown in Fig. 2. The module was powered with overheat protection to prevent accidental burning of the LED chips or nearby tissues in direct contact, with the maximum temperature set at 40°C. The powering control module consisted of an AC to DC converter (A1-111612A, Jin-Hua Electronic Co., Taipei, Taiwan), a regulator (UA7815, Texas Instrument Corp., Dallas, Texas), an overheat detector, an alarm circuit, a buzzer (AX1203D, Advanced Acoustic Technology Corp., Taipei, Taiwan) and a 3 mm dip red light LED for displaying warning signal (W-Great Enterprise Co., Kaohsiung, Taiwan). When the voltage reached the preset value due to elevated temperature, the buzzer and the red light LEDs were activated to remind the operators. Fig. 2The foldable ring design of the LED module allows its easy introduction into the chest cavity through the wound retractor. (a) Illustrates the side view of the placement of the LED ring, which is attached to the bottom of the wound retractor. (b) The front view. The LED chips used in this work are three-chip (RGB) high brightness white light LEDs with two thermistors attached to monitor the local temperature in order to prevent potential burning of the neighboring tissues or premature burn-out of LED chips. The yellow shade surrounding the bottom part of the wound retractor in (b) emulates the lighting from the LED ring. Note that the parts of the LED ring module are marked by (1) overheat protector, (2) upper ring of wound retractor, (3) middle part of wound retractor, (4) LED ring (in yellow color), (5) bottom ring of wound retractor, (6) the light radiated from LED ring, and (7) thermistor. 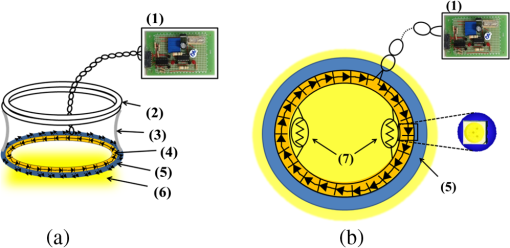 The color temperature of our LED module was determined to be 5800 K. As shown in Fig. 3(a), the horizontal and the vertical illumination angles were and , respectively. Note that the concept of full width at half maximum was used in determining the illumination angles from the luminous intensity distribution curve. Both angles were wider than the FOV (62 deg) of the rigid endoscope. For comparison, the illumination angles from the endoscope light source were approximately 45 deg [Fig. 3(b)], which were less than the FOV of the camera and would result in nonuniformity in video imaging, as also shown in Fig. 5. Fig. 3Luminous intensity distribution curves (LIDC) of (a) the compact LED ring and (b) the fiber bundle coupled endoscope light source (unit: cd/klm). The red solid line shows the LIDC measured in the vertical plane (0 to 180 deg) while the blue line shows the LIDC in the horizontal plane (90 to 270 deg). 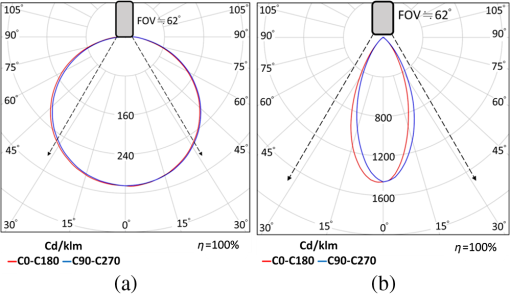 2.3.Animal TestA sow weighing 20 kg was used for this study. The animal experiment was reviewed and approved by the Animal Care and Ethical Committee of National Yang-Ming University. Overnight fasting for 12 h was imposed to avoid complications such as vomiting during the introduction of anesthesia and resuscitation and gastric distension. Free intake of water was allowed up to 4 to 6 h before the surgical procedure. The female hog was given long-term anesthesia with a combination of ketamine plus pentobarbital. After the premedication, the young sow was placed on the surgery table and in the left decubitus position. The surgeon stood facing the sow’s back, with the monitor positioned on the left-hand side of the surgical table. The assistants stood around the operator helping handling surgical instruments and measuring the illuminance data. Throughout the experiment, a high frequency electrosurgical device was used to cut tissues and coagulate bleeding. The surgeon removed a rib and the right lung to provide a larger space for the successive illumination measurements. When comparing the luminance of the compact LED lighting ring and the fiber bundle coupled light source, a 15-cm long white paper ruler was inserted into the sow’s chest cavity, along with the photo-detector of the lux meter and the LED ring light module. The paper ruler was attached to the chest wall. The photo detector was positioned to face the LED ring with the forceps. The LED ring was fixed inside the thoracic cavity with sutures, illuminating the central part of the cavity. In order to introduce the rigid endoscope into the sow’s chest cavity, another 1.5 cm port near the diaphragm and 14 cm away from the original incision was cut. As shown in Fig. 1, the illumination testing points were selected as A, B, C, and D, respectively. Point A was at apex and was set to be the initial point (corresponding to the 0th cm of the paper ruler), with B, C, and D being 5, 10, and 15 cm away from it, respectively. The distances between the test points and the center of the LED lighting ring were denoted as S1, S2, S3, and S4 (solid line arrows). The rigid endoscope was placed approximately 3 cm above point D, and the distances from the tip of the endoscope to the test points were denoted as D1, D2, D3, and D4, respectively (dashed arrows). As shown in Table 1, the illuminance of the LED lighting module and endoscope at the four measurement points inside the sow’s chest cavity were recorded and compared under various conditions. The measurements were repeated at each point with the on-off state of the light sources switched. Table 1The illumination characterization at the four test points in the living sow’s chest cavity.
The overall process took approximately 4 h and the illumination of our LED lighting module remained stable during the period. Upon completion of data recording, the subject was sacrificed by intravenous administration of potassium chloride. 3.ResultsRepeated measurements were conducted both with the lux meter and the video recording. Table 1 presented the measured illumination of the LED lighting module and endoscope at the four testing points, with the corresponding data plot shown in Fig. 4. The maximum illuminance was obtained atpoint B, which was the surgical site in this study. The illumination from the endoscope light source was relatively less since it was at a glancing angle relative to the photo-detector of the lux meter. The two images shown in Fig. 5 well elucidated the illumination inside the cavity. Figure 5(a) showed the image with only the fiber bundle coupled light source of the endoscope being activated. The highly directional output of the endoscope light source cast the dark region on the right part of Fig. 5(a). For comparison, more balanced lighting was achieved, as shown in Fig. 5(b), when both the LED lighting ring and the light source of the endoscope were activated. As also shown in Table 1, uniform and bright background illumination was achieved with the LED lighting ring alone. The uniformity in background illumination was attributed to the lighting design of the LED module, which exhibited a much larger solid angle of illumination. Nonetheless, the fiber coupled endoscope light source was still indispensable in illuminating the targeted area with very high luminosity. Fig. 4The corresponding illumination graph, as of Table 1, inside the chest cavity. Note that A, B, C, and D indicate the illuminance test points as shown in Fig. 1, with the distances between the LED ring and the lux meter in the parenthesis. 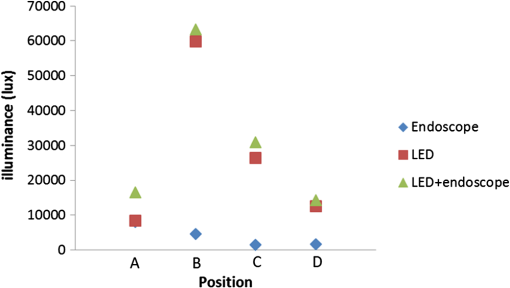 Fig. 5The images acquired inside the sow’s chest cavity under different illumination conditions. (a) The image showing only the endoscope light source was activated. (b) The image showing both the LED lighting module and the endoscope light source were activated. Note that the contrast is greatly improved due to the more uniform lighting from the LED module. 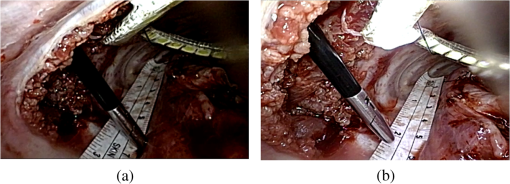 Note that the temperature of the LED lighting module was kept a bit above the body temperature of the sow and did not cause tissue burning or device overheat failure. The vital signs of the sow were stable during the surgery. There were no intraoperative complications (fever, bleeding, respiratory failure) and no conversion to open surgery. 4.Discussion and ConclusionThe applications of minimally invasive techniques have revolutionized the practice of surgery, including thoracic surgery. The advantages include small incisions, shorter hospital stay, and rapid recovery.7,18–20 However, the illumination conditions may be somewhat restricted under current practice and method. Briefly, the lighting cone from the endoscope may not be sufficiently uniform within the FOV of the corresponding camera [Fig. 3(b)], especially for a large operation space. Additionally, the video camera detects mostly back-scattered light, since there is no sideways illumination. The lack of side-illumination may thus limit the contrast obtained. Previous studies have demonstrated the feasibility in adopting white light LEDs to improve the luminance, such as attaching the LEDs on the goggle and retractor.21–23 We have further demonstrated the potentials of LEDs by implementing a foldable lighting ring, which could be inserted either through a wound retractor or a trocar, to show the feasibility of improving background lighting in the thoracic cavity through a porcine model. This study provides the first successful example in VATS on more uniformly illuminated background with LED lighting, by reducing the blinded area. The foldable LED ring light is projected to accommodate a broader range of applications in VATS and other minimal access surgeries. In summary, the apparent benefits in using LEDs come in two ways, direct and indirect. Directly, LEDs have shown better performance, as demonstrated through this study, and are usually far more cost effective to purchase and to maintain than halogen light sources with the same illumination. Additionally, LEDs are miniature in size and mechanically robust. They can be arrayed flexibly in ways that are not possible with traditional light sources. The relatively long lifetime, the user friendliness, the power consumption, and the ease of manufacturing of LEDs greatly surpass those of halogen ones, which would contribute tremendously toward cost saving and reduce the environmental impact in an indirect way. The comparison of LED and halogen light sources in medical applications is shown in Table 2. Table 2Performance and cost comparison of LED and halogen lamp.24
The potentials of LEDs also include fast switching electrically and the spectrally resolved illumination. The low thermal load and capacitance of LEDs allow very fast switching. This capacity can be appropriated for stroboscopic imaging. The very wide illumination spectrum, from IR to UV, of LEDs is also unparalleled. It can be used to reveal unique contrasts not easily attainable with other light sources. These potentials are becoming important subjects in the biomedical applications of photonics. AcknowledgmentsThe authors appreciate greatly the generous support of this study by National Science Council, Taiwan (NSC 101-2321-B-075-003) and Taipei Veterans General Hospital (VGHUST102-G7-2-1). ReferencesA. C. Leeet al.,
“Solid-state semiconductors are better alternatives to arc lamps for efficient and uniform illumination in minimal access surgery,”
Surg. Endosc., 23
(3), 518
–526
(2009). http://dx.doi.org/10.1007/s00464-008-9854-7 SUREEX 1432-2218 Google Scholar
P. Swainet al.,
“Development and testing of a tethered, independent camera for NOTES and single-site laparoscopic procedures,”
Surg. Endosc., 24
(8), 2013
–2021
(2010). http://dx.doi.org/10.1007/s00464-010-0897-1 SUREEX 1432-2218 Google Scholar
J. E. Benderet al.,
“Noninvasive monitoring of tissue hemoglobin using UV-VIS diffuse reflectance spectroscopy: a pilot study,”
Opt. Express, 17
(26), 23396
–23409
(2009). http://dx.doi.org/10.1364/OE.17.023396 OPEXFF 1094-4087 Google Scholar
F. Huet al.,
“Rapid determination of oxygen saturation and vascularity for cancer detection,”
PLoS One, 8
(12), e82977
(2013). http://dx.doi.org/10.1371/journal.pone.0082977 1932-6203 Google Scholar
W. Bisharaet al.,
“Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution,”
Opt. Express, 18
(11), 11181
–11191
(2010). http://dx.doi.org/10.1364/OE.18.011181 OPEXFF 1094-4087 Google Scholar
T. J. Kirbyet al.,
“Lobectomy—video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial,”
J. Thorac. Cardiovasc. Surg., 109
(5), 997
–1002
(1995). http://dx.doi.org/10.1016/S0022-5223(95)70326-8 JTCSAQ 0022-5223 Google Scholar
M. Guet al.,
“Cancer-cell microsurgery using nonlinear optical endomicroscopy,”
J. Biomed. Opt., 15
(5), 050502
(2010). http://dx.doi.org/10.1117/1.3502566 JBOPFO 1083-3668 Google Scholar
L. R. Kaiser,
“Video-assisted thoracic surgery. Current state of the art,”
Ann. Surg., 220
(6), 720
–734
(1994). http://dx.doi.org/10.1097/00000658-199412000-00003 ANSUA5 0003-4932 Google Scholar
B. A. Whitsonet al.,
“Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer,”
Ann. Thorac. Surg., 83
(6), 1965
–1970
(2007). http://dx.doi.org/10.1016/j.athoracsur.2007.01.049 ATHSAK 0003-4975 Google Scholar
N. T. Clancyet al.,
“Development and evaluation of a light-emitting diode endoscopic light source,”
Proc. SPIE, 8214 82140R
(2012). http://dx.doi.org/10.1117/12.909331 PSISDG 0277-786X Google Scholar
P. Swainet al.,
“Wireless capsule endoscopy of the small bowel: development, testing, and first human trials,”
Proc. SPIE, 4158 19
–23
(2001). http://dx.doi.org/10.1117/12.413789 PSISDG 0277-786X Google Scholar
W.-D. Jenget al.,
“Design of illumination system in ring field capsule endoscope,”
Proc. SPIE, 7893 78930E
(2011). http://dx.doi.org/10.1117/12.874535 PSISDG 0277-786X Google Scholar
J. Donget al.,
“Optical design of color light-emitting diode ring light for machine vision inspection,”
Opt. Eng., 50
(4), 043001
(2011). http://dx.doi.org/10.1117/1.3567053 OPEGAR 0091-3286 Google Scholar
M. Mylonakiet al.,
“Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding,”
Gut, 52
(8), 1122
–1126
(2003). http://dx.doi.org/10.1136/gut.52.8.1122 GUTTAK 0017-5749 Google Scholar
I. Morenoet al.,
“Designing light-emitting diode arrays for uniform near-field irradiance,”
Appl. Opt., 45
(10), 2265
–2272
(2006). http://dx.doi.org/10.1364/AO.45.002265 APOPAI 0003-6935 Google Scholar
E. D. Biewengaet al.,
“An innovative wound retractor/protector for prosthetic urologic surgery,”
Curr. Urol., 6
(4), 205
–208
(2012). http://dx.doi.org/10.1159/000343540 CUOUEQ 0963-0643 Google Scholar
K. P. Chenget al.,
“ALEXIS O‐Ring wound retractor vs conventional wound protection for the prevention of surgical site infections in colorectal resections (1),”
Colorectal Dis., 14
(6), e346
–e351
(2012). http://dx.doi.org/10.1111/codi.2012.14.issue-6 1462-8910 Google Scholar
D. L. IngramK. F. Legge,
“Variations in deep body temperature in the young unrestrained pig over the 24 hour period,”
J. Physiol., 210
(4), 989
–998
(1970). JPHYA7 0022-3751 Google Scholar
A. K. AyedR. Raghunathan,
“Thoracoscopy versus open lung biopsy in the diagnosis of interstitial lung disease: a randomized controlled trial,”
J. R. Coll. Surg. Edinb., 45
(3), 159
–163
(2000). JRCSAC 0035-8835 Google Scholar
L. Santambrogioet al.,
“Video thoracoscopy versus thoracotomy for diagnosis of the indeterminate solitary pulmonary nodule,”
Ann. Thorac. Surg., 59
(4), 868
–871
(1995). http://dx.doi.org/10.1016/0003-4975(94)00952-4 ATHSAK 0003-4975 Google Scholar
D. A. Walleret al.,
“Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax,”
Ann. Thorac. Surg., 58
(2), 372
–476
(1994). http://dx.doi.org/10.1016/0003-4975(94)92210-1 ATHSAK 0003-4975 Google Scholar
J. Shimadaet al.,
“Medical lighting composed of LEDs arrays for surgical operation,”
Proc. SPIE, 4278 165
–172
(2001). http://dx.doi.org/10.1117/12.426847 PSISDG 0277-786X Google Scholar
J. Shimadaet al.,
“Surgical retractor with RGB-WHITE LEDs,”
Proc. SPIE, 6910 69100T
(2008). http://dx.doi.org/10.1117/12.761979 PSISDG 0277-786X Google Scholar
S. Talbot,
“Efficiency, output, and costs in outdoor LED lighting,”
(2013) http://blog.metroledlighting.com/soffit-lighting/led-lighting-effective-soffit-lighting/ April ). 2013). Google Scholar
BiographyMing-Kuan Lu received his master’s degree in optoelectronics from National Central University in 1997. He has been working for the Research Institute of Chung-Hwa Telecom since then. He is currently pursuing his PhD degree at the Institute of Biophotonics of National Yang-Ming University. Feng-Chen Chang is a second-year master’s student in the Institute of Biophotonics of National Yang-Ming University. Wen-Zhe Wang received his master’s degree from the Institute of Biophotonics of National Yang-Ming University in 2013. He is currently working for Innolux Corporation as an engineer. Chih-Cheng Hsieh is a senior thoracic surgeon. He graduated from the School of Medicine of National Yang-Ming University in 1992. He is currently a surgeon at the division of thoracic surgery of Taipei Veterans General Hospital. Fu-Jen Kao received his PhD degree in physics from Cornell University in 1993. He pioneered the development of multiphoton microscopy in Taiwan. In 2004, he moved to National Yang-Ming University and helped to establish the Institute of Biophotonics, the first of its kind in Taiwan. His research focuses on optical microscopy, photonic physics, and medical photonics. He is currently the vice president of the Physical Society in Taiwan. |

