|
|
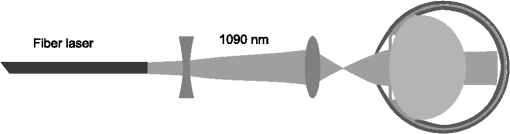
1.IntroductionVerhoeff and Bell1 summarized observations indicating an association between cataracts and occupational exposure to infrared radiation (IRR). A high prevalence of cataract was observed in glass blowers, steel puddlers, and foundry workers.2,3 Two action mechanisms for IRR cataract formation were proposed and have been reviewed by Vos and Van Norren.4 Goldmann suggested that cataract results from heat produced by nonspecific absorption of IRR in the iris and transfer of the heat to the lens.5 However, Vogt hypothesized that cataract is associated with direct exposure to IRR.6 Wolbarsht7–10 reported the cataract formation after IRR exposure to the lens. This was based on experimental in vivo exposures of rabbits to approximately on the lens only with a CW Nd:YAG (1064 nm) laser and irradiances ranging between 1.4 and . Wolbarsht also found that exposure of in vitro rabbit lenses to a 1064-nm laser and a broadband IRR heat lamp, respectively, induced less electrophoretic mobility of lens crystallines and the appearance of large molecular aggregates. Pitts and coworkers11,12 claimed a threshold dose for in vivo exposure to a low irradiance IRR of . This was based on in vivo exposure of rabbits to wide band IRR derived from a Xenon arc source, 715 to 1400 nm (mainly below 1100 nm) and irradiances ranging between 2 and . The reciprocal relationship between irradiance and exposure duration observed by Wolbarsht7–10 and Pitts and coworkers11,12 indicates a photochemical effect. The finding that steel and glass workers developed cataracts after daily 8 h exposures to 80– IRR for 10 to 15 years3 is compatible with the photochemical effect of IRR, but could also be explained by a slightly increased rate of protein denaturation due to a low temperature increase. Okuno derived temperature rise in human ocular tissues exposed to blackbody radiators based on theoretical calculations.13 Using the same model, Okuno found, assuming a 2-mm pupil, that an exposure to 1090-nm near IRR induces an asymptote temperature increase of 1.3°C at the anterior surface of the cornea, 1.6°C at the anterior surface of the lens, and 0.6°C at the posterior surface of the lens and concluded that a near IRR up to 1200-nm absorption in the iris will cause a higher temperature increase at the anterior surface of the lens than of the cornea due to absorption in the iris.14 Based on Goldmann’s15 and Wolbarsht’s9 findings and Scott’s heat transport model,16 Vos and Van Norren17 calculated a threshold temperature rise of 5°C in the lens and stated that an irradiance of would increase the temperature of the anterior segment of the eye not more than the threshold temperature rise. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) sets the exposure limit based on thermal injury and emphasizes that the temperature increase induced by exposure to near IRR depends on the environmental temperature.18,19 In 1960, Langley et al. observed the appearance of anterior subcapsular dots at 24 h after exposure of the iris to for 30 s () to broadband IRR.20 Unfortunately, the spectral irradiance was poorly defined. In 1983, McCally et al. observed corneal damage 48 h after exposure to IRR derived from a laser.21 We observed an approximate 16 h delay between in vivo 1090-nm IRR exposure to for 8 s and light scattering increase.22 A potential photochemical effect of near IRR in the lens as proposed by Wolbarsht7–10 and Pitts et al.11,12 would require attention when establishing safety guidelines for near IRR exposure to the eye.23 Current safety guidelines for protection of the ocular lens from near IRR-induced cataract consider a thermal damage mechanism.18 Further research is required to exclude an additional photochemical mechanism, which potentially could cause accumulated damage from exposure to near IRR over a long time at low irradiance. Such information is increasingly important considering the rapidly increasing use of near IRR in remote control and sensing. Therefore, we decided to aim for confirmation/rejection of irradiance exposure time reciprocity using a quantitative cataract measurement combined with temperature monitoring. Such an investigation would require exposure times in the interval seconds-hours for single exposures. For comparison to Wolbarsht’s findings,7–10 the irradiance in such experiment should be in a range similar to that used by Wolbarsht.7–10 In a previous study,22 we found that an in vivo exposure to 1090-nm IRR on the cornea required a minimum exposure time of 8 s () to induce light scattering in the lens. The purpose of the present study was to determine the temperature time evolution in the eye during an in vivo exposure to 1090-nm IRR for 8 s, and the heat diffusion associated with the exposure. 2.Materials and Methods2.1.AnimalsSix-week-old albino Sprague-Dawley female rats were used. Animals were kept and treated according to the association for research in vision and ophthalmology statement for the use of animals in ophthalmic and vision research. Ethical permission was obtained by Uppsala Djurförsöksetiska Nämnd (C 29/10). 2.2.Radiation SourceThe infrared radiation was generated with a single-mode CW fiber laser, emitting at a wavelength of 1090 nm (Model SP-10C-0011, SPI Lasers, Hampshire, UK) with a maximum output power of 10 W. The laser beam was first expanded with a negative lens, focal length 50 mm, projecting the beam on a second positive lens, focal length 50 mm (Fig. 1). The distance between the lenses was approximately 340 mm. The second lens focused the beam in front of the eye aiming for a 2-mm spot size on the cornea with a vergence of 400 D (22 deg planar angle of divergence, focal point corneal plane ). The position of the negative lens was adjusted to obtain the desired vergence and spot size on the cornea while measuring the three-dimensional distribution of the beam with a 0.3 mm pinhole in front of the detector (3A-SH, Ophir Optics, North Andover, Massachusetts, USA). Finally, the center of the corneal plane in space was indicated with the cross over between two diode laser beams. The anterior surface of the cornea under exposure was centered on the cross over between two diode laser (TIM-201-5D/650, New Taipei City, Taiwan) beams. The optical axis of the eye under exposure was adjusted to coincide with the beam optics. The beam propagation through the eye was calculated based on Hughes schematic rat eye model24 and resulted in a close to collimated beam between lens and retina. The power incident on the cornea was measured with a thermopile radiometer (L40(150)A-SH-V2, Ophir Optics, North Andover, Massachusetts, USA) calibrated by the manufacturer. The spatial irradiance profile of the beam incident on the cornea was measured by moving a pinhole (0.3 mm) through the beam. The spatial intensity distribution on the cornea was found to be pseudo-flat top. 2.3.Temperature MeasurementTemperature was measured with thermocouples (HYP0, OMEGA, Stamford, Connecticut) connected to an amplifier with an integrated analog-digital converter (TC-08, OMEGA, Stamford, Connecticut). The thermocouples with an external diameter of 0.2 mm and a length of 25 mm and LabVIEW (National Instruments, Austin, Texas) were used for acquisition, processing, and storage of measurement data. In one experiment, the temperature was measured in five channels from five thermocouples placed in the exposed eye; externally at the limbus, in the vitreous just behind lens aiming outside the field of the exposure, and on the outer sclera next to the optic nerve, respectively, and in the contralateral not exposed eye; at the limbus and on the outer sclera next to the optic nerve, respectively (Fig. 2). Fig. 2Schematic position of temperature sensors and beam propagation in the eye during infrared radiation exposure, and evolution of temperature in the eye measured, induced by in vivo exposure to 1090-nm IRR, for 8 s. Continuous lines are measurements at the limbus. Dotted lines are measurements behind the lens. Dashed and dotted lines are measurements at the outer sclera close to the optic nerve. Thick lines are the average best fit among animals. Thin lines around the thick lines are the average upper and lower 95% confidence intervals (CIs) for the predicted temperature as a function of time for animals (). (a) Evolution of temperature at the limbus, behind the lens and at the outer sclera close to the optic nerve during IRR exposure. (b) Evolution of temperature at the limbus and at the outer sclera close to the optic nerve during IRR exposure. 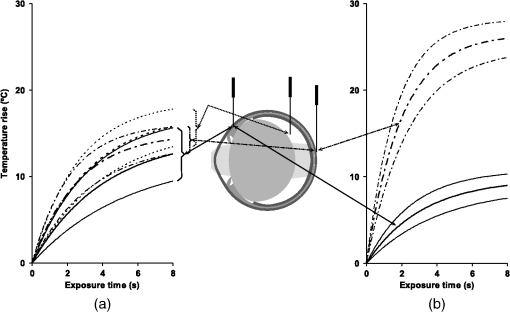 In a subsequent experiment, two thermocouples were externally placed at the limbus and on the sclera next to the optic nerve on both eyes, respectively (Fig. 2). 2.4.Experimental ProcedureThe animals were anesthetized with ketamine plus xylazine intraperitoneally, 10 min before exposure. The pupils of both eyes were dilated with tropicamide, . Five minutes after pupillary dilation, the animals were unilaterally exposed to IRR for 8 s at 1090 nm resulting in a radiant exposure of . The temperature was recorded from the start of the exposure until the temperature descended back to the baseline (within 5 min after exposure). Immediately after the temperature recording finished, the rats were sacrificed and both lenses were extracted by a posterior approach for light scattering measurements. The intensity of forward light scattering in the lens was measured as described elsewhere.25 3.Results3.1.Initial Ocular TemperaturesThe room temperature was 22°C and the initial temperature at the limbus, behind the lens, and at the sclera close to the optic nerve was , , and , respectively. 3.2.Laser-Induced Temperature IncreaseThe in vivo exposure to 1090-nm IRR for 8 s was found to cause a temperature elevation in the exposed eye. The temperature rise recorded at the end of the exposure, , was shown in Table 1. No temperature change was measured in the contralateral eyes. Table 1Parameters for temperature increase as a function of exposure time.
Note. Exp. no. 1: Thermocouples placed at the limbus, behind the lens and on the sclera close to the optic nerve.Exp. no. 2: Thermocouples placed at the limbus and on the sclera close to the optic nerve. The temperature rise as a function of time of exposure was fitted to a nonlinear model for each eye (Appendix A) [Eq. (1)]. Then, the average and the standard deviation for the maximum temperature rise, , and the rate constant, , respectively, among animals were calculated. Finally, a 95% confidence interval (CI) for the mean, and the mean was estimated (Table 1). The confidence limits for each of the parameters were introduced in the model [Eq. (1)], not considering the error term. The dependence of temperature on exposure time, estimated as the average temperature, and upper and lower extreme temperatures estimated from the upper and lower confidence limits for the mean of the parameters (Table 1) was provided in Fig. 2. The increase time, , appears longer at the limbus than behind the lens and close to the optic nerve on the outer sclera (Table 1). An analysis of variance according to the model in Appendix B showed a difference between the limbus and the sclera close to the optic nerve in the asymptote temperature (, ) and a difference of the asymptote temperature between locations among experiments (, ). Further, a subsequent analysis of variance according to the model in Appendix B verified that there is a difference of the rate constants between locations (, ), but no difference between locations among experiments (, ). After the rats were sacrificed, it was noticed in the first experiment that most of the lenses were found to be damaged by the thermocouples placed in the vitreous, whereas in the second experiment none of the lenses were damaged. 3.3.Post-Exposure Temperature DecreaseAfter the end of the 1090-nm IRR exposure, the temperature in the exposed eye decreased with an initial fast phase and a later slow phase (Fig. 3). We believe that the initial fast phase reflects heat loss through conduction in the thermocouple, partly to the tissue and partly to air. For estimation of the rate constant for temperature decrease after exposure, the data were fitted to a nonlinear model for each eye (Appendix C) [Eq. (3)]. Then a 95% CI for the mean slow-phase rate constant, , was estimated (Table 2). 4.DiscussionThe present study aimed to elucidate the ocular temperature evolution in the rat eye during an 8 s exposure to of 1090 nm delivered on the cornea, confined within 2 mm, and the decrease of temperature after the end of IRR exposure. The current experiment was designed to elucidate the temperature increase at 8 s exposure to threshold irradiance of 1090-nm IRR for cataract induction22 with the long-term intention to investigate irradiance exposure time reciprocity below a threshold temperature for cataract induction. The rat model was chosen since it is possible to generate independent data of sufficient sample size to allow appropriate precision while considering costs. It is realized that the close proximity between retina and posterior surface of the lens in the rat eye will lead to thermal conduction from the retina to the lens. However, this is not a problem if the irradiance-induced temperature is kept below the threshold for thermal cataracts, which is intended in future experiments. The exposure time in the current experiment was chosen to be close to the threshold exposure time for induction of light scattering in the lens at the current irradiance at the eye.22 The presently used optical setup was designed to result in a close to collimated beam through the optics of the eye by focusing the beam in front of the eye (Fig. 1). For this reason, the distance between the focal point and the eye at exposure is critical for the irradiance at the corneal plane. This was carefully considered when positioning the eye for exposure, but may have contributed to variations in the measurements. It was carefully checked that the spot of the laser beam was confined within the pupil with a margin. Therefore, it can be ruled out that absorption in the lens was partly due to heat conduction of the energy absorbed in the iris. Table 2Rate constants estimated for temperature decrease as a function of time after end of exposure.
Note. Exp. no. 1: Thermocouples placed at the limbus, behind the lens and on the sclera close to the optic nerve.Exp. no. 2: Thermocouples placed at the limbus and on the sclera close to the optic nerve. Temperature measurement inside the eye is challenging. The purpose of the present temperature measurements was to estimate the temperature increase at different locations in the eye for threshold irradiance for cataract induction with an 8 s exposure,22 the current local threshold temperature. The long-term plan is to use this information to limit temperature increase at 1090-nm near IRR exposure below the current threshold temperature for investigation of irradiance exposure time reciprocity. In the present experiment, temperature was measured with needle thermocouples. Therefore, it is possible that the measured temperatures are somewhat underestimated due to heat conduction in the needle thermocouples. Further, although we aimed at keeping the thermocouple behind the lens outside the field of the exposure, it cannot be excluded that in some animals the thermocouple was in the field of the exposure. An optical fiber temperature sensor would have been preferable to limit heat conduction in the sensor, but was not available to us for the experiment. Another interesting sensor that was recently used for temperature measurements in the eye is thermochromic liquid crystals.26 However, we did not have access to the optical instrumentation required for temperature sensing with liquid crystals. Okuno found that the threshold irradiance of 900-nm IRR steeply increases with decreasing exposure time up to 1 min based on modeling of the human eye and a critical temperature increase for cataract induction of 5°C.14 We previously showed that the currently used irradiance and 8 s exposure time is the threshold for cataract induction,22 which was shown to cause a 10°C temperature rise (Fig. 2). The currently chosen irradiance is on the order of seven times higher than the irradiance used by Wolbarsht7–10 and 50 times higher than the irradiance used by Pitts et al.,11,12 and is anticipated to thermally damage the retina and the optic nerve. However, the radiant exposure in the current experiment is almost identical to the threshold radiant exposure found by Wolbarsht7–10 and half of that found by Pitts et al.11,12 in their experiments indicating reciprocity. The baseline temperatures at the locations measured are consistent with room temperature conditions. At lower ambient temperature conditions, a higher axial gradient of temperature, therefore, a higher threshold radiance is expected. Inversely, at a higher ambient temperature, a lower threshold radiance is expected. For our future intended experiments on irradiance exposure time reciprocity, slight species differences in the baseline ocular surface temperature do not matter. The finding that the estimated asymptote temperature, , as well as the recorded temperature rise, , was higher at the limbus when the temperature was measured with a thermocouple behind the lens than without (Fig. 2, Table 1) is probably explained by absorption of energy in the thermocouple behind the lens. It is also possible that heat dissipated with leaking liquid at the eye puncture for the thermocouple behind the lens. Both explanations are compatible with the observation that the asymptote temperature, as well as the recorded temperature rise at the outer sclera close to the optic nerve, was lower with the thermocouple behind the lens than without (Table 1). The observation that the rate constant estimated for increase of temperature at the outer sclera close to the optic nerve was on the order of 1.5 times higher than the rate constant estimated for the limbus in both experiments (Table 1) is probably due to heat transfer out from the retina into choroidal blood circulation and concurrently less heat transfer at the eye-air interface close to the limbus. The rate constants estimated for increase of temperature (Table 1) were on the order of 10 times higher than the rate constants estimated for decrease of temperature (Table 2). This asymmetry is probably explained by less heat conduction off the surface of the eye during the temperature increase than heat conduction within the eye after the end of the exposure. The estimated rate constants for exposure to 1090-nm IRR indicate that close to the maximum temperature increase is reached at the end of an 8 s exposure. The finding that there was no increased light scattering in the lens after the exposure implicates that the lens temperature rise due to the exposure ( for 8 s) (Fig. 2) does not cause immediate denaturation of lens proteins. In reality, the temperature increase in the lens was probably higher than the 10°C recorded at the limbus since there was a temperature increase on the outer sclera close to the optic nerve of approximately 26°C (Table 1). The temperature increase at the sclera close to the optic nerve is consistent with retinal thermal damage and reflects that, as long as the iris is not exposed, the main absorption of 1090 nm in the rat eye occurs in the retina. The lack of increased light scattering after the exposure is consistent with our previous finding that there is a 16-h latency before the onset of light scattering after in vivo exposure at the equivalent exposure conditions.22 In the current experiment, no attempt was made to measure light scattering at the delayed interval since the information is available22 and it was considered unethical to keep the animal after the thermocouples had been inserted at different locations in the eye. It cannot be excluded that the sensor behind the lens was in the beam and the sensor at the sclera close to the optic nerve was in the beam. Therefore, it is possible that some of the temperature increase recorded behind the lens and at the sclera close to the optic nerve, respectively, is due to absorption in the sensors, although for the sensor at the sclera close to the optic nerve a large fraction of the intensity should have been attenuated in front of the sensor. In the previous experiments indicating reciprocity,7–12 no temperature recordings were reported. The temperature increase induced in the anterior segment was estimated to be at least 10°C (Fig. 2, Table 1), which is two times the threshold temperature rise for cataract induction claimed by Vos and Van Norren.17 Despite a higher irradiance that rendered a significant temperature increase and a radiant exposure above the threshold dose reported by Wolbarsht,7–10 we did not observe an immediate increase in light scattering. 5.ConclusionExposure to 1090-nm IRR for 8 s, centered on the cornea, within the dilated pupil, with a close to collimated beam between the lens and the retina, induces a temperature increase of about 10°C at the limbus and about 25°C close to the retina. The previous observation of delayed cataract development after exposure with the equivalent parameters22 most probably reflects a delayed biological expression of thermal molecular events. AppendicesAppendix AThe temperature change at laser exposure was modelled with Newton’s law of heating, implying that the temperature rise (°C) increases exponentially with time (s) as determined by a rate constant (), and the asymptote maximum temperature rise (°C). Measurement variation was considered by adding an error term, [Eq. (1)]. Appendix BModel for Analysis of VarianceAn experimental measurement, , is the sum of the population mean, , a term for the variation among experimental groups, (, 2), a term for the variation among locations, (, 2), a term for the interaction between experimental groups and locations, and a term for the variation among animals, including measurement error, [Eq. (2)]. Appendix CThe temperature decrease after laser exposure was modelled with Newton’s law of cooling. For each location of the thermocouple, the difference of time between the baseline temperature and end of exposure temperature, (°C), is the sum of a decrease with time, (s), determined by an initial fast-phase rate constant, () and a fraction, , of the initial temperature difference between the baseline and end of exposure, , and a later slow phase determined by the rate constant, () and the remaining fraction, , of the initial temperature difference between the baseline and end of exposure, and a measurement error, [Eq. (3)]. AcknowledgmentsThe current study was supported by Carmen och Bertil Regnérs fond för forskning, Gun och Bertil Stohnes Stiftelse, Karin Sandqvists Stiftelse, Svenska Läkaresällskapet Resebidrag, Konung Gustav V:s och Drottning Victorias Frimurarstiftelse, Uppsala Läns Landsting’s Research grants (ALF), Ögonfonden, Stiftelsen Sigurd och Elsa Goljes Minne. ReferencesF. H. VerhoeffL. BellC. B. Walker,
“The pathological effects of radiant energy on the eye: an experimental investigation, with a systematic review of the literature,”
Proc. Am. Acad. Art Sci., 51
(13), 627
–818
(1916). PAAAAV 0065-6836 Google Scholar
E. LydahlB. Philipson,
“Infrared radiation and cataract ii - epidemiologic investigation of glass workers,”
Acta Ophthalmol., 62
(S166), IV1
–IV29
(1984). AOPSAP 0065-1451 Google Scholar
E. Lydahl,
“Ocular exposure to infrared radiation in the Swedish iron and steel industry,”
Health Phys., 46
(3), 529
–536
(1984). http://dx.doi.org/10.1097/00004032-198403000-00003 HLTPAO 0017-9078 Google Scholar
J. J. VosD. Van Norren,
“Thermal cataract, from furnaces to lasers,”
Clin. Exp. Optom., 87
(6), 372
–376
(2004). http://dx.doi.org/10.1111/cxo.2004.87.issue-6 0816-4622 Google Scholar
H. Goldmann,
“Experimental investigation on the genesis of heat cataract,”
Arch. Ophthalmol., 130 93
–179
(1933). AROPAW 0003-9950 Google Scholar
A. Vogt,
“Augenschadigungen durch die strahlende energie,”
Klin. Monatsbl. Augenheilkd., 85 321
–344
(1930). Google Scholar
M. L. Wolbarshtet al.,
“The origin of cataracts in the lens from infrared laser radiation: annual progress report,”
(1977). Google Scholar
M. L. Wolbarsht,
“Safe ocular levels for IR occupational exposure: final report,”
(1978). Google Scholar
M. L. Wolbarsht,
“Ocular effects of non-ionizing radiation,”
Proc. SPIE, 229 121
–142
(1980). http://dx.doi.org/10.1117/12.958797 PSISDG 0277-786X Google Scholar
M. L. Wolbarsht,
“Cataract from infrared lasers: evidence for photochemical mechanisms,”
Lasers Light Ophthalmol., 4
(2), 91
–96
(1991). Google Scholar
D. G. Pittset al.,
“Determination of ocular threshold levels for infrared radiation cataractogenesis,”
(1980). Google Scholar
D. G. PittsA. P. Cullen,
“Determination of infrared radiation levels for acute ocular cataractogenesis,”
Albrecht. Von Graefes. Arch. Klin. Exp. Ophthalmol., 217
(4), 285
–297
(1981). http://dx.doi.org/10.1007/BF00429289 AKOGAO 0065-6100 Google Scholar
T. Okuno,
“Thermal effect of infrar-red radiation on the eye: a study based on a model,”
Ann. Occup. Hyg., 35
(1), 1
–12
(1991). http://dx.doi.org/10.1093/annhyg/35.1.1 AOHYA3 0003-4878 Google Scholar
T. Okuno,
“Thermal effect of visible light and infra-red radiation (i.r.-A, i.r.-B and i.r.-C) on the eye: a study of infrared cataract based on a model,”
Ann. Occup. Hyg., 38
(4), 351
–359
(1994). AOHYA3 0003-4878 Google Scholar
H. Goldmann,
“Kritische und experimentelle Untersuchungen über den sogenannten Ultrarotstar der Kaninchen und den Feuerstar,”
Albrecht. Von Graefes. Arch. Ophthalmol., 125
(3), 313
–402
(1930). http://dx.doi.org/10.1007/BF01853199 GACODL 0721-832X Google Scholar
J. A. Scott,
“A finite element model of heat transport in the human eye,”
Phys. Med. Biol., 33
(2), 227
–241
(1988). http://dx.doi.org/10.1088/0031-9155/33/2/003 PHMBA7 0031-9155 Google Scholar
J. J. VosD. Van Norren,
“Weighing the relative significance of three heat dissipation mechanisms to produce cataract,”
Lasers Light Ophthalmol., 6
(2), 107
–114
(1994). LLOPED Google Scholar
“ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation,”
Health Phys., 105
(4), 74
–91
(2013). HLTPAO 0017-9078 Google Scholar
“ICNIRP guidelines on limits of exposure to laser radiation of wavelengths between 180 and 1000 um,”
Health Phys., 105
(3), 271
–295
(2013). HLTPAO 0017-9078 Google Scholar
R. K. LangleyC. B. MortimerC. C. Mc,
“The experimental production of cataracts by exposure to heat and light,”
A.M.A. Arch. Ophthalmol, 63
(3), 473
–488
(1960). http://dx.doi.org/10.1001/archopht.1960.00950020475006 AAOPAF 0096-6339 Google Scholar
R. L. McCallyet al.,
“Stromal damage in rabbit corneas exposed to CO2 laser radiation,”
Exp. Eye Res., 37
(6), 543
–550
(1983). http://dx.doi.org/10.1016/0014-4835(83)90130-6 EXERA6 0014-4835 Google Scholar
Z. Yuet al.,
“1090 nm infrared radiation at close to threshold dose induces cataract with a time delay,”
Acta Ophthalmol.,
(2014). http://dx.doi.org/10.1111/aos.12508 ACOPAT 0001-639X Google Scholar
D. H. Sliney,
“Need for experimental research study of infrared cataract,”
(2006). Google Scholar
A. Hughes,
“A schematic eye for the rat,”
Vision Res., 19
(5), 569
–588
(1979). http://dx.doi.org/10.1016/0042-6989(79)90143-3 VISRAM 0042-6989 Google Scholar
P. G. SöderbergE. ChenB. Lindström,
“An objective and rapid method for the determination of light dissemination in the lens,”
Acta Ophthalmol., 68
(1), 44
–52
(1990). http://dx.doi.org/10.1111/j.1755-3768.1990.tb01648.x ACOPAT 0001-639X Google Scholar
C. R. SmithD. R. SabatinoT. J. Praisner,
“Temperature sensing with thermochromic liquid crystals,”
Exp. Fluids, 30
(2), 190
–201
(2001). http://dx.doi.org/10.1007/s003480000154 EXFLDU 0723-4864 Google Scholar
BiographyKarl Schulmeister is a consultant and expert on laser product safety and in safety-relevant effects of exposure of both the eye and the skin. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||