|
|
1.IntroductionThe current standard of care for patients harboring glioblastoma multiform involves as complete surgical resection as possible, combined with temozolomide chemotherapy and radiotherapy. Despite these aggressive therapeutic approaches, mean patient survival is currently a modest 14 months.1,2 Several gene therapy approaches have moved to clinical trials with the goal of delivering gene-based agents into the remaining tumor cells to prevent recurrences which inevitably reappear.3 Among several cancer gene therapy approaches currently being developed are insertion of wild type suppressor genes to correct primary genetic defects and suicide gene therapy. The tumor suppressor gene, phosphatase and tensin homolog (PTEN), has been studied in detail in many previous publications.4–6 PTEN is mutated or missing in glioblastoma, endometrium, prostate, head and neck, and colon tumors. Because of this high frequency of abnormalities and its important function as a tumor suppressor gene the transfection of wild type PTEN into malignant cells makes this approach a good candidate for cancer gene therapy. Pro-drug activating gene therapy (suicide gene therapy) is one other of the strategies employed for tumor reduction. Among these pro-drug activating genes are the transduction of the Escherichia coli cytosine deaminase (CD) gene into tumor cells and administration of 5-fluorocytosine, 5-FC. Expression of this gene within the target cell produces an enzyme that converts the nontoxic pro-drug, 5-FC, to the toxic metabolite, 5-fluorouracil (5-FU), resulting in cell death.7–9 In general, both in preclinical and clinical trials, viral vectors have been employed as gene carriers due to their efficiency as delivery agents. Viral vectors consist of a gene-carrying core and an outer envelope or surface proteins that mediate release of the capsid core into the cytoplasm from the cellular endosome. It is these two structurally and functionally distinctive components which account for their efficiency. Despite these advantages, viral vectors have significant safety issues, such as unwanted immune responses, secondary oncogenesis, and transfection of untargeted cells. In contrast, nonviral vectors are safer due to their low immunogenic response, are easier to produce in large scale manufacture than viral ones and possess the capacity to carry large gene payloads. Nonviral cationic polymers’ vectors interact with negatively charged DNA through electrostatic interactions leading to polyplexes. Protamine is a cationic small protein and acts as a nuclear protein that helps DNA packaging in sperm cells and as a transfection accelerator in gene delivery.10 Unfortunately, protamine/DNA polyplexes demonstrated relatively low transfection efficiency.11 Among many barriers in nonviral gene delivery, cytosolic release (endosomal escape) and dissociation of nucleic acids from the carriers once arrived at their intracellular targets are crucial. The problem of endosomal entrapment of DNA polyplexes can be circumvented by the technique of photochemical internalization (PCI).12–14 PCI is a technique which utilizes the photochemical properties of photodynamic therapy (PDT) for the enhanced delivery of macromolecules such as DNA plasmids into the cell cytosol. These macromolecules lack the ability to naturally permeate intracellular barriers such as the plasma and endosomal membranes. The concept of PCI is based on using photosensitizers, which will eventually localize in the endosome membranes encapsulating the gene polyplex. Following light application, the photosensitzer reacts with oxygen causing endosome membrane rupture, releasing the trapped polyplex into the cell cytoplasm, where, in contrast to being degraded by lysosomal hydrolases, it can exert its full biological activity. PCI-enhanced gene transfection, with nonviral gene carriers, has been demonstrated for wild type suppressor genes such as P53 and PTEN.15–17 PCI has also been shown to enhance the viral transfection of the herpes simplex virus thymidine kinase gene18 and the nonviral transfection of the CD gene into gliomas cells.19 In the PCI experiments previously reported, either the PTEN or CD DNA plasmid-protomine sulfate (PrS-DNA) polyplexes were delivered as “naked” polyplexes (cores).17,19 Although this functioned well in vitro, PrS-DNA polyplexes will be degraded in the circulation in vivo if systemically administered and during cellular internalization. In the present study reported here, polyamine PrS-DNA polyplexes were shelled and encapsulated in a nanoparticle (NP) with an acid degradable polyketal (PK) outer layer.20 Therefore, the polyplex core and acid-degradable PK shell synthetically mimic the roles of the viral capsid and envelope that carries and releases the gene, respectively. A simplified representation of PCI-mediated gene transfection employing shelled polyplex cores is shown in Fig. 1. The virus mimicking NPs are taken up by cells through endocytosis and are sequestered in intracellular endosomes. The PK shell hydrolyzes at the slightly acidic endosomal pH and releases the polyamine PS-DNA core polyplex which will be released from the endosome following light treatment, the PCI effect. Finally, DNA dissociated from the polyamine-DNA core is localized into the nucleus and is incorporated into the cell’s genome. The aim of the present research was to evaluate the effects of PCI-mediated suppressor and suicide gene transfection on glioma cells, comparing transfection efficiency between cores alone and NP-shelled cores. Fig. 1Cartoon representation of photochemical internalization (PCI)-mediated gene transfection. (1) Plasmid DNA complexed with protamine sulphate forms PrS-DNA polyplex core. (2) The core is encapsulated by surface-initiated photopolymerization in acid degradable shelled nanoparticles (NPs). (3) Cell membranes are loaded with an amphiphilic photosensitizer. (4) NP binds to the plasma membrane and is taken into the cell together with the photosensitizer by endocytosis. (5) The photosensitizer and the shelled polyplex colocalize in the endosome, with the photosensitizer localized in the membrane, the polyplex in the lumen. The shell hydrolyzes at the endosomal pH and releases the PrS-DNA core polyplex. (6) Light exposure leads to photoinduced rupture of the endosome. (7) The sequestered polyplex is released into the cytosol and the plasmid DNA disassociates from its carrier. (8) The plasmid DNA enters and is incorporated in the nucleus. 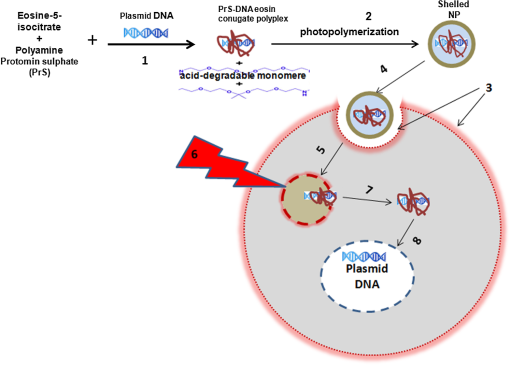 2.Materials and Methods2.1.Cell Lines and Plasmids2.1.1.Cell linesThe human U87 glioma cell line was obtained from the American Type Culture Collection (Manassas, Virginia). The U251 cell line was kindly supplied by Dr. Yi Hong Zhou. Both glioma lines have been demonstrated to have a mutated PTEN gene caused by sequence insertions or deletions. The tumor cells were grown as monolayers in DMEM medium (Invitrogen Corp., Carlsbad, California) with 10% heat-inactivated fetal bovine serum, 50-mM HEPES buffer (pH 7.4), penicillin (), and streptomycin () at 37°C and 5% . 2.1.2.PlasmidsThe plasmid coding for both the PTEN gene (PTEN Addgene plasmid 13039, submitted by Dr. Alonzo Ross, University of Massachusetts) and the CD gene was purchased from Addgene (Cambridge, Massachusetts). The plasmids were transformed in E. coli strain of DH5alpha as previously described.17 Plasmids were isolated with QIAGEN MidiKit in the ratio of one column per tube of bacteria pellet according to the manufacturer’s instructions. Plasmid DNA concentrations were measured with a spectrophotometer (Nanodrop 1000 Thermo Scientific, UCI DNA MicroArray Facility, Irvine, California). 2.2.Gene Carriers-Branched Polyethylenimine (bPEI), Protamine Sulfate (PS)Branched polyethylenimine (bPEI), MW 25,000, (Sigma Aldrich, St. Louis, Missouri) was used in comparative toxicity studies. bPEI/DNA polyplexes were formed as follows. of bPEI (Sigma Aldrich) was dispersed in of deionized (DI) water and of pDNA was dispersed in of DI water at pH of 8. pDNA was first added into microtubes along with of Hank’s Buffer (without calcium) and bPEI was drop-wise added. Resulting polyplexes were vortexed for at least 10 s and left undisturbed at room temperature for 15 min before use. An N/P ratio of the bPEI/DNA polyplexes of was used in these experiments. 2.3.Preparation of Polyamine Core and PK Core-Shell NPs2.3.1.CoresThe polyamine/DNA polyplexes were prepared as previously described.20 Briefly, protamine sulfate/DNA polyplexes (PrS-DNA core) were formed as follows. of pDNA was dispersed in of DI water (Nuclease-free water; Fisher Scientific, Waltham, Massachusetts) of protamine sulfate (Sigma Aldrich) was dissolved in of DI water. pDNA in water was drop-wise added to protamine sulfate solution while vortexing. The resulting polyplex was left undisturbed for 30 min and refrigerated prior to use. The size and surface charge of the prepared polyplex cores were characterized by dynamic light scattering and zeta-potential analysis (Malvern Instruments, Malvern, United Kingdom). The size of the PrS-DNA polyplexes were 64 nm and the zeta-potential was . An N/P ratio of the PS/DNA polyplexes of was used in all experiments. 2.3.2.NanoparticlesThe PK core-shell NPs were prepared as previously reported.20 Briefly, PrS-eosin conjugates were prepared by reacting protomine sulfate (PrS ) with eosin-5-isothiocyanate () in 1 mL of 100-mM sodium bicarbonate buffer (pH 8.5) for 2 h at room temperature. After unreacted eosin-5-isothiocyanate was removed using a PD 10 desalting column (GE Healthcare, Piscataway, New Jersey), PTEN or CD-encoding plasmid DNA ( in DI water) was gently mixed with eosin-conjugated PrS, forming eosin-PrS/DNA polyplexes. After 30 min, 10 mg of ascorbic acid in of DI water was added along with aminoketal methacrylamide monomers21 at a molecular ratio to DNA phosphates of 500 (). In addition, 20% (w/w) ketal diacrylamide cross-linker to aminoketal methacrylamide monomer in of 100-mM sodium bicarbonate buffer (pH 8.5) was added resulting in a cross-linked PK shell. Polymerization was performed by halogen lamp light irradiation of the mixture on ice with moderate stirring and was halted by adding DI water. Unreacted monomers, crosslinkers, and ascorbic acid were removed by centrifugal filtration () at 4500 rpm and 4°C for 40 min. The size and surface charge of the polyamine/PK core-shell NPs ( DNA/mL) in DI water was 180 nm and , respectively, as measured using a Malvern Zetasizer Nano ZS (for details see Ref. 20). 2.3.3.PDT and PTEN Polyplex Toxicity of U251 MonolayersThe 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS, Promega, Madison, Wisconsin), was used to determine cell viability after PDT and exposure to PTEN polyplexes. For PDT, 8 wells (in 96-well flat-bottomed plates) for each set of treatment conditions were seeded with U251 cells at a density of 5000 cells per well and incubated for 24 h prior to experimentation. Cells were only plated into every fourth well in the 12-well rows to reduce spill over light from the treated to the nontreated cultures. was then added to the cell culture for an additional 18 h, followed by a triple wash. The cells were incubated for 4 h in fresh medium. Light treatment was administered with 670-nm light from a diode laser (Intense, North Brunswick, New Jersey) at radiant exposures of 0, 0.5, 0.75, or at through an opaque mask that only admitted light to a single 8-well column at a time. Controls consisted of cultures that contained photosensitizer but were not exposed to light (dark controls). To determine the direct toxicity of the gene carrier used here, 5000 U251 cells/well in a 96-well plate were incubated in media () containing PrS-PTEN polyplexes at various concentrations for 18 h, followed by replacement with fresh media. Following either light treatment, or exposure to PrS-PTEN, incubation was continued for 48 h, at which point the culture medium was replaced with fresh clear buffer containing MTS and was incubated for a further 2 h. The optical density was read using an ELx800uv Universal Microplate Reader (BIO-TEK Instruments, Inc., Winooski, Vermont). 2.4.PCI-Mediated Gene TransfectionU251 cells were cultured in 35-mm dishes at 100,000 cells per well and allowed to grow overnight, followed by PCI transfection. of the photosensitizer and PrS-PTEN polyplexes either as cores or NP were added to the cell cultures for 18 h, followed by a triple wash. The cells were incubated for 4 h in fresh medium to allow some of the photosensitizer to leach from the cell membrane. Light treatment at various fluence levels at a fluence rate of was administered with a 670-nm light from a diode laser (Intense, North Brunswick, New Jersey). Light was coupled into a diameter optical fiber containing a microlens at the output end. Following irradiation, cells were washed with phosphate buffered saline, harvested by trypsinization, resuspended in MEM supplemented with serum, counted, and plated into 60-mm dishes per experimental group at 100 to 200 cells per dish. Cells were allowed to grow for 11 to 14 days where upon they were stained with 0.5% crystal violet in 95% ethanol. Colonies containing more than 50 cells were scored as survivors. The number of colonies was normalized to a control group consisting of cells incubated in photosensitizer and PrS-PTEN polyplexes but receiving no light treatment (dark control). All experiments were performed in quintuplicate and results are a combination of at least four separate experiments. 2.5.Multitumor Spheroid Generation and TransfectionSpheroids were formed by a modification of the centrifugation method as previously described.22,23 Briefly, 2500 U87 cells in of culture medium per well were aliquoted into the wells of ultralow attachment surface 96-well round-bottomed plates (Corning Inc., Corning, New York). The plates were centrifuged at 1000 g for 10 min. Immediately following centrifugation, the tumor cells formed into a disk shape. The plates were maintained at 37°C in a 7.5% incubator for 48 h to allow them to take on the usual three-dimensional (3-D) spheroid form. Spheroids were formed in every fourth well in each of the 12-well rows to allow several light treatment fluence levels to be performed on the same plate. of the photosensitizer and the PTEN or CD DNA polyplexes were added to the cell cultures for 18 h, followed by a triple wash. The spheroids were incubated for 4 h in fresh medium and then received light treatment as previously described. Controls consisted of cultures that contained PrS-DNA polyplexes, but were not exposed to light (dark controls). Determination of spheroid size was carried out by averaging two measured perpendicular diameters of each spheroid using a microscope with a calibrated eyepiece micrometer and their volume was calculated mathematically assuming a perfect sphere. Typically, 8-16 spheroids were followed in three individual trials for up to twenty-one days of incubation. Culture medium was changed every third day. 2.6.Statistical AnalysisAll data were analyzed and graphed using Microsoft Excel. The arithmetic mean and standard error were used throughout to calculate averages and errors. Statistical significances were calculated using the Student’s -test. Two values were considered distinct when their -values were below 0.05. 3.ResultsSince PCI is optimal with a radiant exposure that allows 70% to 80% of cell or spheroid survival, -mediated PDT at increasing light doses was carried out for both monolayer cultures and spheroids. Radiant exposures of proved highly, toxic killing more than 80% of the U251 cells in monolayers [Fig. 2(a)]. Radiant exposures of 0.5 to , therefore, seemed optimal for PCI-mediated transfection on monolayers. The direct toxicity of the PTEN/PrS polyplex proved to be nonsignificant compared to control cultures at the concentrations tested [Fig. 2(b)]. Fig. 2Photodynamic therapy (PDT) and toxicity control cultures for U251 monolayers and U87 spheroids. Cell survival for U251 monolayers (a) -mediated PDT at increasing fluence levels, and (b) toxicity of PTEN PrS polyplexes. MTS assay 48 h following light treatment. Each data point represents cell survival as a percent of controls. (c) AlPcS2a-mediated PDT at increasing fluence levels of U87 spheroids, and (d) increasing concentrations of 5-FC in the absence of cytosine deaminase (CD) gene. Multicellular glioma spheroid average volume growth measured as a percentage of control spheroids following 21 days in culture. Each data point represents the mean of triplicate experiments with 8 spheroids. Error bars denote standard errors. 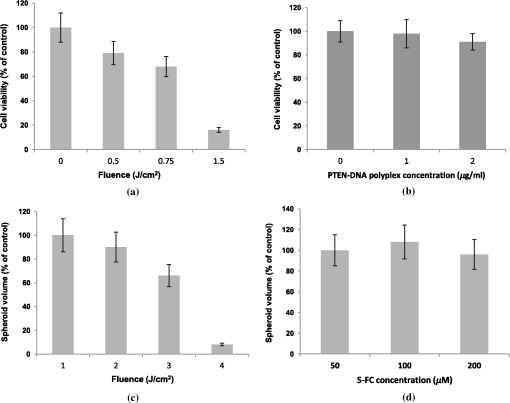 Cells in spheroids are known to be more resistant to PDT compared with monolayers. Radiant exposures of reduced U87 spheroid growth to less than 20% of controls [Fig. 2(c)]. Radiant exposures of 1.5 to seemed optimal and were subsequently used in all spheroid experiments. The direct toxicity of 5-FC was also examined in the absence of the CD gene. As can be seen from Fig. 2(d), 5-FC concentrations of up to gave no significant U87 spheroid growth inhibition. 3.1.Toxicity of bPEI, PrS/DNA (Core) Shelled Core (NP)The direct toxicity of the gene carriers used, PrS/DNA (cores) and shelled core (NP), was evaluated on U87 spheroids. The spheroids were incubated in media containing either CD gene core polyplexes or shelled CD cores (CD-NP) for 18 h. The pro-drug 5-FC was not present in the culture medium. Some of the spheroids were exposed to the gene carrier, branched polyethylenimine (bPEI), as a comparative control. The spheroids were followed for 2 weeks to determine their growth. Compared to nontreated controls, neither the cores nor the NP demonstrated significant toxicity even up to concentration (Fig. 3). In contrast, bPEI-CD polyplexes had a much greater toxicity at the higher concentrations tested. Fig. 3Toxicity of gene carriers on U87 spheroids. Spheroids were incubated with bPEI, CD gene core polyplexes (PrS DNA core), and shelled CD gene cores (NP DNA PrS core) for 18 h. Spheroids were followed for 2 weeks. 5-FC was not present in culture medium. Each data point represents the mean of triplicate experiments. Error bars denote standard errors. 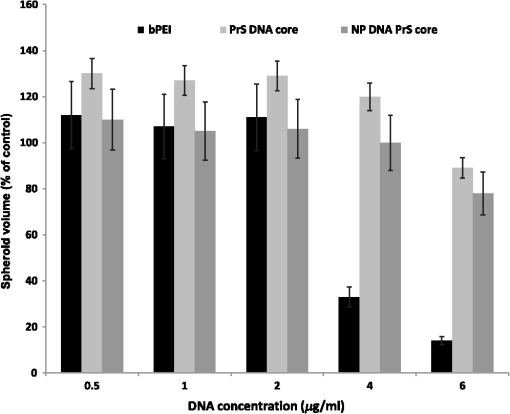 3.2.PTEN Restoration3.2.1.Inhibition of Colony Formation of U251 Glioblastoma CellsCell growth inhibition following PCI-mediated PTEN gene transfer was investigated in U251 glioma cells transfected using either PTEN polyplex cores or NP. Clonogenic survival assays using either of the two forms of gene carrier clearly demonstrated that the PCI-enhanced PTEN restoration significantly inhibited colony size and number compared to control cultures that received no light (Table 1). In the absence of, or at low, radiant exposures (0, ), inhibition of colony formation was significantly greater () when the gene carrier was in the form of NP compared with PrS-PTEN cores alone. At increased radiant exposure (), PCI could enhance the PTEN transfection effects of both gene carriers, but no significant differences were observed between the two (). Table 1Inhibition of U251 colony formation by photochemical internalization (PCI)-PTEN protomine sulfate (PrS cores) or shelled cores [nanoparticle (NP)] as gene carrier. 3.2.2.Inhibition of Spheroid GrowthAlthough the results employing glioma cell monolayer cultures indicated a positive effect (Table 1), the results obtained using this model have limitations since these cultures are unable to mimic oxygen gradients and complex intracellular interactions found in 3-D tumors. Experiments were, therefore, carried out by performing growth assays on multicell tumor spheroids of the human glioma cell line U87 since spheroids formed from U251 cells failed to grow in vitro. Experiments were performed on three groups: (1) AlPcS2a-PDT, (2) PCI PTEN transfection with “naked” cores, (3) PCI PTEN transfection with shelled cores (NP). of PTEN DNA was used in both the naked cores (group 2) and shelled cores (group 3). Light radiant exposure was varied from 0 to . Figure 4(a) shows the mean percentage of spheroids showing growth after a 3-week period. Three identical experiments were performed with 8 spheroids in each group per experiment, i.e., 24 spheroids per group. As seen in Fig. 4(a), in the absence of and at low levels of exposure (0, ), 90% and 75% of the spheroids survived, respectively following PrS/PTEN transfection. In contrast, the average survival following PTEN transfection with NP gene delivery was 70% and 40% at the two light levels. At optimal light exposure () no significant differences between cores and shelled cores were observed: in both cases, spheroid survival was below 20%. Fig. 4Inhibition of U87 spheroid growth following PCI-mediated PTEN or CD gene transfection. U87 spheroid growth (a) after PTEN transfection, and (b) after CD transfection and addition of 5-FC to the cultures after light exposure. For both, the results show the average spheroid survival as a percentage of untreated controls after a 21-day period. Each data point represents the mean of triplicate experiments of 8 spheroids. Error bars denote standard errors. * indicates significant difference with PDT control (). 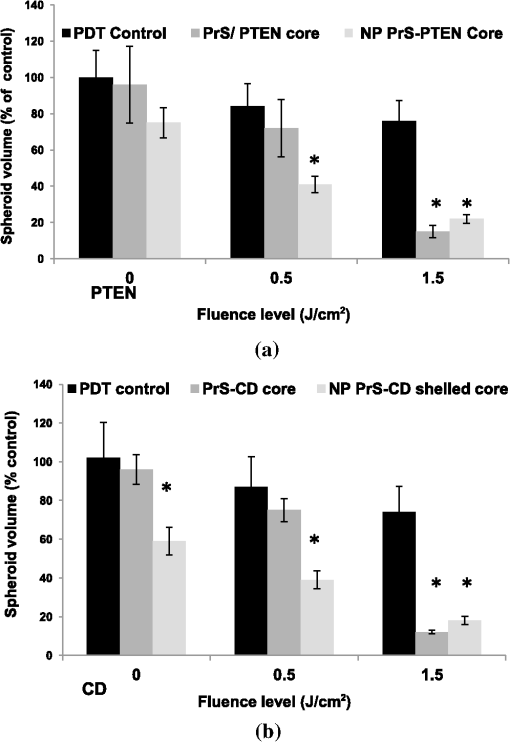 3.3.PCI CD Suicide Gene TransfectionGene-directed enzyme pro-drug therapy activating strategies employing the CD gene mediate a high concentration of toxic 5-FU mainly at the tumor site potentially reducing drug side effects. Additionally, the bystander effect, where the activated drug is exported from the transfected cancer cells into the tumor microenvironment, plays an important role. To ascertain the effects of 5-FC on spheroid survival following PCI CD gene transfection, experiments were performed consisting of three groups similar to the ones used for the PTEN transfection: (1) AlPcS2a-PDT, (2) PCI CD transfection with “naked” cores, and (3) PCI CD transfection with shelled cores (NP). U87 glioma spheroids were incubated for 18 h with the PrS-CD polyplex cores alone or shelled (DNA concentration ) together with , and following 4-h incubation in fresh medium, treated with 0, 0.5, and of laser light for groups 2 and 3. 5-FC was added to the cultures 24 h after light treatment. Figure 4(b) shows the average spheroid survival as a percentage of untreated controls measured after a 3-week period. Three identical experiments were performed with 8 spheroids in each group per experiment. At radiant exposures of 0 (dark control) and in the presence of 5-FC, the average spheroid survival was 95% and 75% respectively with PrS-CD cores as gene carrier. The survival rate for the cultures where the NP was the gene carrier was significantly lower (60% and 40% respectively). As seen with the PCI PTEN transfection, at a radiant exposure of , survival was reduced to under 20% for both the cores and NP. The growth kinetics of the U87 spheroids using optimal light () and 2 and CD gene DNA concentrations are shown in Fig. 5. Compared to controls receiving only light treatment and photosensitizer (PDT control), spheroid growth was significantly inhibited at a CD DNA concentration of with NP proving superior to the cores at this suboptimal gene DNA concentration. No significant differences in the transfection efficiency between the cores alone or as shelled core NPs could be demonstrated at a CD DNA concentration of . At this light level and gene concentration, spheroid growth was almost completely inhibited. Fig. 5The effects of 5-FC on spheroid volume growth kinetics following CD gene transfection. CD gene transfection with PrS cores or NP (shelled cores) at DNA plasmid concentrations of 2 and . Spheroids were incubated in incubation for 18 h and subsequently exposed to a radiant exposure of ; irradiance of (). 5-FC was added 24 h following light treatment. Results are shown as a percent of dark controls. Each data point represents the mean of triplicate 8 spheroids/experiment trials. Error bars denote standard errors. * indicates significant difference with PDT control (). 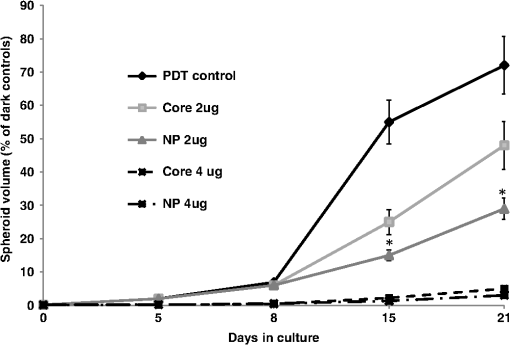 4.DiscussionAdjuvant treatments including chemotherapy for brain tumors only achieve modest clinical outcomes. The effectiveness of systemic delivery of therapeutic agents to brain tumors is hampered by several physiological barriers. The three most important are the blood brain barrier, the blood–cerebrospinal fluid barrier, and the blood–tumor barrier.24,25 The latter, caused by the leaky tumor vasculature, results in high intratumoral interstitial pressure (edema), which severely limits drug penetration from the bloodstream into the tumor.26 This is particularly true for chemotherapeutic agents that rely on free diffusion from the blood stream. In contrast, nanometer-sized particles within the size range of 10 to 100 nm can accumulate selectively at the site of brain tumors due to the enhanced permeability and retention (EPR) effect caused by the hyper-vascularized, leaky, and compromised lymphatic drainage system found in brain tumors.27 The EPR phenomenon is generally not seen with small molecular weight compounds like chemotherapeutic agents. Additionally, gene DNA polyplexes inside a nanocapsule are protected from destruction during cell internalization and transport in the circulation, allowing intravenous injection. The use of NPs, as nonviral gene delivery systems, therefore, has many advantages. The aim of the present research was to evaluate PCI-mediated nonviral gene transfection efficiency employing virus mimicking NPs as gene carriers. Polyamines, such as PrS, have high biocompatibility (low cytotoxicity), form stable PrS-DNA polyplexes, but have a very limited gene transfection capability due to their inability of inducing endosomal escape.17,19,20,28,29 Using shelled cores of PrS-DNA plasmids as gene carriers, the results of the experiments presented here demonstrated that PCI-mediated nonviral gene transfection (1) significantly enhanced the effects of the suppressor gene PTEN on PTEN-mutated glioma cells and (2) inhibited the effects of 5-FC following CD gene insertion. PCI-PTEN transfection significantly inhibited both the U251 transforming ability to produce secondary colonies (Table 1), as well as significantly inhibiting the growth of U87 multicell tumor spheroids [Fig. 4(a)]. At suboptimal light levels for both monolayers and spheroid cultures, the growth inhibiting effects of PTEN gene transfection were increased when NP were used as the gene vector compared with the PrS polyplexes [Table 1 and Fig. 4(a)]. This was also the case for CD gene transfection of U87 spheroids [Fig. 4(b)]. Additionally, at relatively low DNA concentrations, the PCI-mediated CD gene transfection, as evaluated by the growth inhibiting effects of 5-FC, was increased with NP as gene carrier compared with PrS-CD polyplexes (Fig. 5). At higher light and DNA concentrations, there were no significant differences between NP and polyplex transfection for both gene types. This would indicate that the DNA plasmid core in the NP was released into the endosome in an efficient manner as the shell dissolved and then was subsequently released into the cell cytoplasm by PCI. The superior ability of NP gene delivery, at suboptimal light and DNA concentration levels, compared to DNA polyplexes, is in all probability due to multiple effects. In this study, polyamine/DNA polyplexes were shelled with an acid degradable PK layer, which protects the gene-carrying core during cellular internalization. More importantly, at the lower pH found in the endosome, the outer PK shell hydrolyzes. Acid-hydrolysis of the shell causes release of the core along with an increase in the osmotic pressure and subsequent swelling of the endosome (the proton sponge effect hypothesis), resulting in an enhanced gene transfection compared to polyplex alone.20,30 The dark control results (0 J, no light) shown in Table 1 and Fig. 4(a), and Fig. 4(b) clearly demonstrate that, even in the absence of light treatment, (PCI) NP vectored gene delivery displays a significant transfection effect. In contrast, PrS-DNA polyplexes without PCI are more or less incapable of transfecting cells under the conditions used in these experiments. It is hypothesized that the relatively high transfection efficiency of NP vectors may be due to their ability to produce osmotic swelling similar to that seen with PEI.30,31 This, in turn, would lead to partial disruption of the endosome membrane making it more susceptible to PCI-induced rupture at lower irradiance levels. Since light at these wavelengths () attenuates very rapidly in tissue, increased gene transfection at low light levels would allow deeper penetration into tumors. On the other hand, the attenuation of light penetration would make PCI-mediated gene transfection site specific, limited to the vicinity of the light source. An indwelling balloon light applicator, implanted in the resection cavity following cytoreductive tumor surgery, would, therefore, limit the effects of gene transfection to the resection borders where most recurrences occur.3,32,33 This is of special importance for CD gene transfection where unwanted gene transfection of normal cells coupled to the pronounced bystander effect caused by the produced 5-FU could potentially damage normal brain. The gene carrying NPs used in this study have several advantages when considering translating the in vitro results presented here to in vivo models and potential patient protocols. Since the photosensitizer is present in both tumor and normal endothelial cells, light treatment for PCI would also have a direct effect on the vasculature in the vicinity of the illuminated volume; i.e., the PDT effect. Both PDT and PCI have been shown to open the blood brain barrier in a site specific manner, both to drugs34 and to cells carrying magnetic NPs.35 This could greatly enhance delivery of the gene carrying NPs in a targeted manner compared to nontargeted EPR NP delivery. 5.ConclusionThe results presented herein show that the PCI can enhance the delivery of both tumor suppressor (PTEN) and suicide (CD) genes in in vitro human glioma monolayers and spheroids. The transfection efficiency, as measured from cell survival and spheroid growth inhibition, was found to be significantly greater at suboptimal light and DNA levels for shelled NPs compared to PrS polyplexes. This has significant clinical implications since it suggests that shelled NPs are ideally suited for nonviral gene transfection of glioma cells in low light level environments such as those encountered at 5 to 10 mm in a postoperative resection cavity. AcknowledgmentsThis work was supported by grants from the Norwegian Radium Hospital Research Foundation and the Chao Family Comprehensive Cancer Center, University of California, Irvine. (National Cancer Institute of the National Institutes of Health under award number P30CA062203). Portions of this work were made possible through access to the Laser Microbeam and Medical Program (LAMMP) at the Beckman Laser Institute and the Chao Cancer Center Optical Biology Shared Resource at UCI. ReferencesS. A. Grossmanet al.,
“Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States,”
Clin. Cancer Res., 16
(8), 2443
–2449
(2010). http://dx.doi.org/10.1158/1078-0432.CCR-09-3106 CCREF4 1078-0432 Google Scholar
K. Petreccaet al.,
“Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma,”
J. Neurooncol., 111
(1), 19
–23
(2013). http://dx.doi.org/10.1007/s11060-012-0983-4 JNODD2 0167-594X Google Scholar
M. G. CastroP. R. Lowenstein,
“Neuro-oncology: the long and winding road--gene therapy for glioma,”
Nat. Rev. Neurol., 9
(11), 609
–610
(2013). http://dx.doi.org/10.1038/nrneurol.2013.198 NRNACP 1759-4758 Google Scholar
R. EndersbyS. J. Baker,
“PTEN signaling in brain: neuropathology and tumorigenesis,”
Oncogene, 27
(41), 5416
–5430
(2008). http://dx.doi.org/10.1038/onc.2008.239 ONCNES 0950-9232 Google Scholar
C. B. KnobbeA. MerloB. Reifenberger,
“Pten signaling in gliomas,”
Neuro Oncol., 4
(3), 196
–211
(2002). http://dx.doi.org/10.1215/15228517-4-3-196 JNODD2 0167-594X Google Scholar
A. Di CristofanoP. P. Pandolfi,
“The multiple roles of PTEN in tumor suppression,”
Cell, 100
(4), 387
–390
(2000). http://dx.doi.org/10.1016/S0092-8674(00)80674-1 CELLB5 0092-8674 Google Scholar
K. Geet al.,
“Transduction of cytosine deaminase gene makes rat gliomas cells highly sensitive to 5-fluorocytosine,”
Int. J. Cancer, 71
(4), 675
–679
(1997). http://dx.doi.org/10.1002/(ISSN)1097-0215 IJCNAW 1097-0215 Google Scholar
C. R. Milleret al.,
“Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas,”
Cancer Res., 62
(3), 773
–780
(2002). CNREA8 0008-5472 Google Scholar
D. Ostertaget al.,
“Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector,”
Neuro Oncol., 14
(2), 145
–159
(2012). http://dx.doi.org/10.1093/neuonc/nor199 JNODD2 0167-594X Google Scholar
Y. Tsuchiyaet al.,
“Characterization of protamine as a transfection accelerator for gene delivery,”
J. Bioact. Compat. Polym., 21
(6), 519
–537
(2006). http://dx.doi.org/10.1177/0883911506070816 JBCPEV 0883-9115 Google Scholar
X. SunN. Zhang,
“Cationic polymer optimization for efficient gene delivery,”
Mini Rev. Med. Chem., 10
(2), 108
–125
(2010). http://dx.doi.org/10.2174/138955710791185109 1389-5575 Google Scholar
A. Høgsetet al.,
“Light induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy,”
Cancer Gene Ther., 9
(4), 365
–371
(2002). http://dx.doi.org/10.1038/sj.cgt.7700447 CGTHEG 0929-1903 Google Scholar
K. Berget al.,
“Photochemical internalization: a new tool for gene and oligonucleotide delivery,”
Top. Curr. Chem., 296 251
–281
(2010). http://dx.doi.org/10.1007/978-3-642-16430-9 TPCCAQ 0340-1022 Google Scholar
P. K. Selboet al.,
“Photochemical internalization provides time and space-controlled endolysosomal escape of therapeutic molecules,”
J. Control Release., 148
(1), 2
–12
(2010). http://dx.doi.org/10.1016/j.jconrel.2010.06.008 JCREEC 0168-3659 Google Scholar
A. Maurice-Duelliet al.,
“Enhanced cell growth inhibition following PTEN nonviral gene transfer using polyethylenimine and photochemical internalization in endometrial cancer cells,”
Technol. Cancer Res. Treat., 3
(5), 459
–465
(2004). TCRTBS 1533-0346 Google Scholar
A. Ndoyeet al.,
“Eradication of p53-mutated head and neck squamous cell carcinoma xenografts using nonviral p53 gene therapy and photochemical internalization,”
Mol. Ther., 13
(6), 1156
–1162
(2006). http://dx.doi.org/10.1016/j.ymthe.2006.02.003 1525-0016 Google Scholar
M. S. Mathewset al.,
“Glioma cell growth inhibition following photochemical internalization enhanced non-viral PTEN gene transfection,”
Lasers Surg. Med., 44
(9), 746
–754
(2012). http://dx.doi.org/10.1002/lsm.v44.9 LSMEDI 0196-8092 Google Scholar
L. Prasmickaiteet al.,
“Photochemically enhanced gene transfection increases the cytotoxicity of the herpes simplex virus thymidine kinase gene combined with ganciclovir,”
Cancer Gene Ther., 11
(7), 514
–523
(2004). http://dx.doi.org/10.1038/sj.cgt.7700720 CGTHEG 0929-1903 Google Scholar
F. Wanget al.,
“Increased sensitivity of glioma cells to 5-fluorocytosine following photo-chemical internalization enhanced nonviral transfection of the cytosine deaminase suicide gene,”
J. Neurooncol., 118
(1), 29
–37
(2014). http://dx.doi.org/10.1007/s11060-014-1410-9 JNODD2 0167-594X Google Scholar
S. K. ChoY. J. Kwon,
“Polyamine/DNA polyplexes with acid-degradable polymeric shell as structurally and functionally virus-mimicking nonviral vectors,”
J. Control Release, 150
(3), 287
–297
(2011). http://dx.doi.org/10.1016/j.jconrel.2010.12.004 JCREEC 0168-3659 Google Scholar
I. K. Koet al.,
“Acid-degradable cationic methacrylamide polymerized in the presence of plasmid DNA as tunable non-viral gene carrier,”
Biomaterials, 29
(28), 3872
–3881
(2008). http://dx.doi.org/10.1016/j.biomaterials.2008.06.003 BIMADU 0142-9612 Google Scholar
A. IvascuM. Kubbies,
“Rapid generation of gingle-tumor spheroids for high-throughput cell function and toxicity analysis,”
J. Biomol. Screen., 11
(8), 922
–932
(2006). http://dx.doi.org/10.1177/1087057106292763 JBISF3 1087-0571 Google Scholar
M. S. Mathewset al.,
“Photochemical internalization of bleomycin for glioma treatment,”
J. Biomed. Opt., 17
(5), 058001
(2012). http://dx.doi.org/10.1117/1.JBO.17.5.058001 JBOPFO 1083-3668 Google Scholar
D. R. Groothuis,
“The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery,”
Neuro Oncol., 2
(1), 45
–59
(2000). JNODD2 0167-594X Google Scholar
R. NauF. SorgelH. Eiffert,
“Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections,”
Clin. Microbiol. Rev., 23
(4), 858
–883
(2010). http://dx.doi.org/10.1128/CMR.00007-10 CMIREX 0893-8512 Google Scholar
T. Rooseet al.,
“Solid stress generated by spheroid growth estimated using a linear poroelasticity model,”
Microvasc. Res., 66
(3), 204
–212
(2003). http://dx.doi.org/10.1016/S0026-2862(03)00057-8 MIVRA6 0026-2862 Google Scholar
H. Maedaet al.,
“Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review,”
J. Control. Release, 65
(1–2), 271
–284
(2000). http://dx.doi.org/10.1016/S0168-3659(99)00248-5 JCREEC 0168-3659 Google Scholar
Y. W. ChoJ. D. KimK. Park,
“Polycation gene delivery systems: escape from endosomes to cytosol,”
J. Pharm. Pharmacol., 55
(6), 721
–734
(2003). http://dx.doi.org/10.1211/002235703765951311 JPPMAB 0022-3573 Google Scholar
C. Welzet al.,
“Nuclear transport of oligonucleotides in HepG2-cells mediated by protamine sulfate and negatively charged liposomes,”
Pharm. Res., 17
(10), 1206
–1211
(2000). http://dx.doi.org/10.1023/A:1026410612600 PHREEB 0724-8741 Google Scholar
A. Akincet al.,
“Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis,”
J. Gene Med., 7
(5), 657
–663
(2005). http://dx.doi.org/10.1002/(ISSN)1521-2254 JGMEFG 1099-498X Google Scholar
S. YangS. May,
“Release of cationic polymer-DNA complexes from the endosome: a theoretical investigation of the proton sponge hypothesis,”
J. Chem. Phys., 129
(18), 185105
(2008). http://dx.doi.org/10.1063/1.3009263 JCPSA6 0021-9606 Google Scholar
S. J. Madsenet al.,
“Development of a novel balloon applicator for optimizing light delivery in photodynamic therapy,”
Lasers Surg. Med., 29
(5), 406
–412
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
M. S. EljamelC. GoodmanH. Moseley,
“ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial,”
Lasers Med. Sci., 23
(4), 361
–367
(2008). http://dx.doi.org/10.1007/s10103-007-0494-2 LMSCEZ 1435-604X Google Scholar
H. Hirschberget al.,
“Targeted delivery of bleomycin to the brain using photo-chemical internalization of Clostridium perfringens epsilon prototoxin,”
J. Neurooncol., 95
(3), 317
–329
(2009). http://dx.doi.org/10.1007/s11060-009-9930-4 JNODD2 0167-594X Google Scholar
S. J. Madsenet al.,
“Increased nanoparticle-loaded macrophage migration into the brain following PDT-induced blood-brain barrier disruption,”
Lasers Surg. Med., 45
(8), 524
–532
(2013). LSMEDI 0196-8092 Google Scholar
|

