|
|
1.IntroductionPhotodynamic therapy (PDT) for the treatment of solid tumors combines the use of a photosensitizing drug and light in the presence of oxygen.1 PDT involves reaction of a photoexcited (usually triplet) state of the sensitizer drug with oxygen either by transfer of excitation energy forming singlet oxygen (type II) or by electron/hydrogen atom transfer forming reactive free radical species such as superoxide (type I). The reactive oxygen species are then capable of damaging critical cellular targets. We have recently suggested an alternative combination of light and prodrug that relies on oxygen-independent photoisomerization of a combretastatin.2–4 Combretastatin drugs are based on natural products from an African bush willow that are stilbene derivatives,5 and which may therefore exist either in - (trans) or - (cis) configurations (Fig. 1). -Combretastatins such as combretastatin A4 (-CA4, Fig. 1) target microtubule assembly and ultimately tumor vasculature,6 and the prodrug -combretastatin A4 phosphate (CA4P) has recently been evaluated in clinical trials.7,8 The -isomer of CA4 is highly cytotoxic having a nanomolar in the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay while the corresponding -isomers of combretastatins are usually less toxic by 2 or 3 orders of magnitude.9 As with most stilbenes,10 photoisomerization of combretastatins occurs on illumination within their UV absorption band (ca. 320 to 340 nm)11 and offers a route to photoactivation via -isomerization that is independent of oxygen and therefore potentially useful in a wide range of tissues including hypoxic tumors. Although the optical transmission of tissues prevents the use of UV activation via usual one-photon absorption, an optical “window” in the spectra of tissues lies in the range of 600 to 900 nm,12 equivalent to red or near-infrared (NIR) wavelengths, permitting two- or three-photon absorption and activation of the -combretastatin at approximately 640 or 960 nm, respectively. Although such multiphoton absorption requires high light intensities, these are now readily achieved with femtosecond or picosecond pulsed lasers that are widely used in multiphoton microscopy.13 Because multiphoton excitation of phototherapeutic drugs widens the choice of chromophore, considerable effort is now underway to develop new sensitizers with suitably high two-photon cross sections in the red/NIR tissue window.14,15 Fig. 1Structures of combretastatins referred to in the text showing photoreversible isomerization between - (trans, upper) and - (cis, lower) isomers. 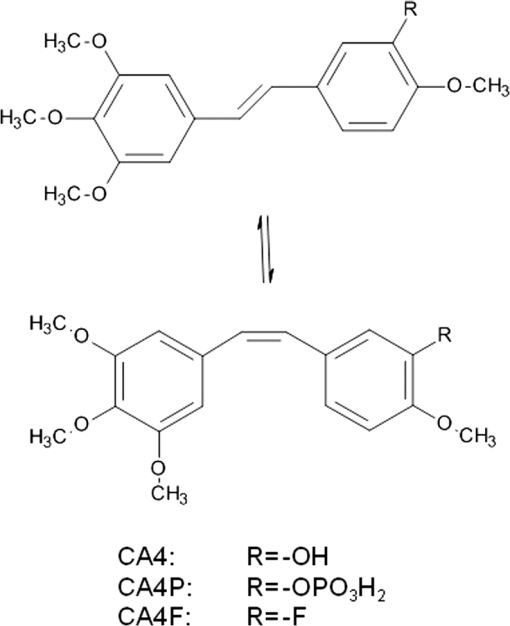 We have previously used fluorescence lifetime imaging microscopy (FLIM) with two-photon excitation (2PE) at 625 nm to study uptake and intracellular accumulation of -combretastatins including CA4 and a fluorinated derivative (CA4F) in live mammalian cells using the native UV fluorescence of the -isomers.3,4 Because of their hydrophobic nature, -combretastatins accumulate to intracellular concentrations 2 to 3 orders of magnitude higher than in solution and are mainly located in lipidic droplets and membranes. It is now shown that following uptake of -combretastatins into mammalian cells, 2PE is capable of converting the low activity -isomer to the highly cytotoxic -isomer to induce cell death. 2.Materials and Methods2.1.Synthesis of Combretastatin Derivatives- and -isomers of CA4 (CA4, 1-(3′,4′,5′-trimethoxyphenyl)-2-(4″-methoxy-3″-hydroxy-phenyl)ethene) and CA4F (1-(3′,4′,5′-trimethoxyphenyl)-2-(3″-fluoro-4″-methoxyphenyl)ethene) were synthesized as previously described.9,16 Structures and purity were determined by TLC and and NMR. 2.2.Confocal and Multiphoton FLIMConfocal images, two-photon excited FLIM images and two-photon emission spectra used a combined laser scanning confocal multiphoton FLIM and spectral detection apparatus based on a Nikon TE2000-U inverted fluorescence microscope3,17 with a water immersion objective (numerical aperture 1.2). For lifetime and FLIM measurements with -CA4 and -CA4F, BG3 (Comar) and a narrowband interference (400IU25) filters were used to isolate the light transmitted to the photomultiplier. Excitation for 2PE at 625 nm was provided by a titanium-sapphire laser (Mira, Coherent Ltd., Santa Clara, California; 180-fs laser pulses at 76 MHz) pumping an optical parametric oscillator (Coherent Ltd.). Samples were irradiated on the motorized and temperature controlled (Peltier heater/cooler) microscope stage. Confocal imaging using 488- and 543-nm excitation was carried out using Nikon eC1-Si or eC2 confocal laser scanning accessories mounted on the same Nikon microscope. 2.3.One-Photon Photoisomerization Quantum Yield MeasurementsSpectroscopic grade solvents (Sigma-Aldrich, Gillingham, United Kingdom and Alfa Aesar, Heysham, United Kingdom) were used as supplied. Aberchrome 540 was used for actinometry.18 UV–visible spectra were measured using a PerkinElmer Lambda 25. Steady-state fluorescence emission measurements were recorded using a Horiba FluoroMax-3 using the manufacturer supplied spectral correction curves. Irradiations were carried out within the fluorimeter sample cell with a 5-nm excitation slit width. Combretastatin solutions in a 1 cm quartz cuvette were deaerated by nitrogen bubbling inside an AtmosBag (Sigma-Aldrich) to minimize phenanthrene formation. Quantum yields () for the -isomerization of the different combretastatins were determined from the photon flux and the initial changes in absorbance.19 The concentrations of - and -isomers ( and ) at the photostationary state were calculated using extinction coefficients ( and ) and quantum yields derived from the kinetic measurements [Eq. (1)], and were found to be consistent with experimental measurements at the establishment of the photostationary state.10 2.4.Cell CultureChinese hamster ovary (CHO) cells were obtained from the European Collection of Cell Cultures and were grown and maintained in phenol-red free Dulbecco’s modified Eagle medium (Gibco, Paisley, United Kingdom) and minimal essential medium (Gibco), respectively, supplemented with foetal calf serum (FCS; 10%), penicillin (), streptomycin (), and l-glutamine (2 mM). Cells were seeded at densities of cells/dish on MatTek glass-bottom culture dishes (35 mm , No. 1.5, uncoated, -irradiated) (MatTek Corporation, Ashland, Massachusetts) and placed in an incubator under a humidified atmosphere (37°C, 5% ) for 24 h to adhere. 2.5.Combretastatin Induced Apoptosis Monitored by Confocal Imaging of Annexin V AlexaFluor488 Conjugate and Propidium Iodide Staining on Live CellsCombretastatin induced apoptosis on CHO cell monolayers was determined using annexin V AlexaFluor 488 conjugate (Invitrogen, Paisley, United Kingdom) as an indicator for the loss of phospholipid asymmetry in the plasma membrane of apoptotic cells in the presence of (2.5 mM), and propidium iodide (PI) as a DNA intercalating fluorescent dye that is permeable to membranes of apoptotic or necrotic cells. The cytotoxicity of -combretastatins in the CHO cell monolayers was induced by addition of aliquots of a stock solution in dimethyl sulfoxide (DMSO) (). Following incubation for 48 h, the tissue culture medium was aspirated and replaced with and HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] supplemented medium. The annexin V conjugate was added at 0.5% final concentration ( medium). A PI stock solution () was added, and the samples incubated for 15 min in the dark and imaged using confocal laser scanning microscopy (excitation at 543 nm for PI and 488 nm for annexin V conjugate). For irradiations in the presence of -combretastatins, a field was exposed to 625-nm light by raster-scanning the multiphoton beam for 10 min allowing a total of 20 scans with a pixel dwell time of 2 ms. Samples were incubated for 24 h and examined for apoptosis as described above. 3.Results3.1.Photoisomerization Induced by One- and Two-Photon Excitations In Vitrophotoisomerization of -combretastatin A4 (-CA4) (1 mM) in deaerated methanol on irradiation at 340 nm is illustrated in Fig. 2 through changes in absorption spectra. As the irradiation progresses, absorbance of the -isomer at (329 nm) decreases and is replaced by that of the -isomer with 287 nm with a lower extinction coefficient. The spectra show good isosbestic points showing that only the two species are involved and that phenanthrene formation was effectively suppressed by deaeration. A plot of A (340 nm; inset to Fig. 2) indicates progression to the stationary state mixture of - and -isomers. Quantum yields for photoisomerization were calculated from both the initial rate of absorbance change and the absorbance at the stationary state as detailed in Sec. 2, and are shown in Table 1 for CA4 and CA4F in methanol solution. The values show that photoisomerization is relatively efficient with quantum yields in the range of 0.27 to 0.48 and are comparable with published data for a wide range of other stilbenes in fluid solution at room temperature.10 Fig. 2Changes in the absorption spectrum of -CA4 () in -saturated methanol on irradiation at 340 nm. The inset shows the change in absorbance at 340 nm with irradiation time. 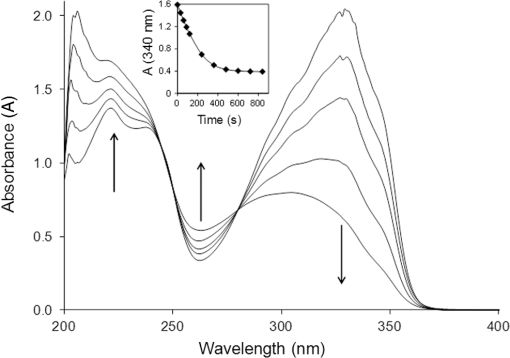 Table 1Quantum yields for photoisomerization of E- and Z-isomers of CA4 and CA4F in methanol solution. The E/Z ratios at the photostationary state were measured experimentally based on the extinction coefficients and compared with those calculated according to Eq. (1).
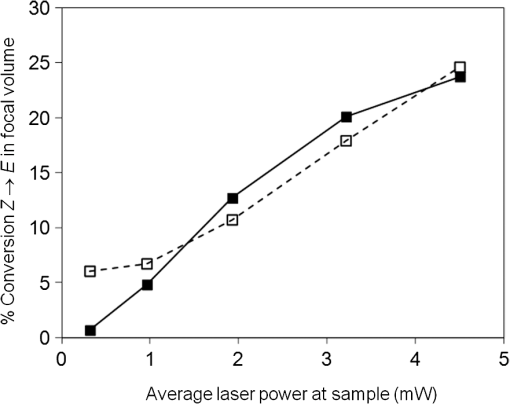
Photoisomerization of combretastatins by 2PE was inferred from measurements of fluorescence intensity versus laser power on irradiation at 624 nm in the microscope system using femtosecond laser pulses. Under these conditions, 2PE excitation takes place within the femtoliter focal volume, from which the product diffuses into bulk solution, away from the detectable zone, resulting in an equilibrium concentration balancing formation and diffusion. The fluorescence intensity () from a solution of -CA4F versus laser power (; inset to Fig. 3) shows the expected quadratic relationship ( with ) at low laser powers up to about 1.5 mW. However, a plot of (Fig. 3) shows deviation from the expected linear behavior because of saturation at higher laser powers that is indicative of depletion of the ground state -CA4F molecules as a result of photoisomerization. The saturation threshold of laser power is less than that expected () from simple excitation and fluorescence decay20 and strongly suggests that the observed saturation results from conversion of the fluorescent -isomer to the nonfluorescent -isomer by photoisomerization. For the nonfluorescent -combretastatins, fluorescence was observed as the laser power was increased and is interpreted as arising from photoisomerization to the fluorescent -isomer. Through a series of calibrations using solutions of increasing concentrations of -combretastatin, the photoisomerization could be quantified and shown to produce up to 25% conversion within the focal volume at an average laser power of 4.5 mW (Fig. 4). These measurements probe only the focal volume, from which photochemical products will rapidly diffuse, and it is concluded that in solution sufficient amounts of -combretastatins are formed by 2PE photoisomerization of -combretastatins to exert a cytotoxic effect. Fig. 3Effect of average laser power () on fluorescence intensity (, at 390 nm) from a solution of -CA4F (0.5 mM) in ethanol on illumination with the femtosecond laser beam at 624 nm. The main figure shows a plot of versus with the solid line showing the expected linear plot for two-photon excitation. The inset shows a direct plot of versus data showing the expected quadratic relation at low laser powers confirming the 2PE process under these conditions. 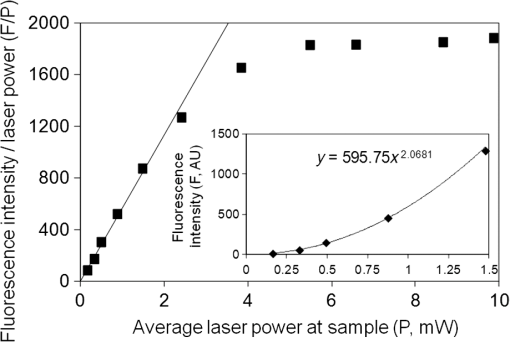 3.2.Cell Death Induced by Combretastatin Two-Photon Isomerization-CA4 induced apoptosis has been reported in human endothelial cells.21 Staining for cell death (PI, red fluorescence) and apoptosis (annexin V AlexaFluor488 conjugate, green fluorescence) in cultured CHO cells following exposure to -CA4F [Fig. 5(A)] shows significant apoptosis at drug. The combined effects of laser irradiation at 625 nm and exposure to -CA4 on live cell CHO monolayers are shown in Fig. 5(B). The central area of each field was illuminated by raster scanning a focused laser beam (625 nm). PI and annexin V staining reveal the effects of increasing laser power and -CA4 concentration. Control cells [no -CA4, Fig. 5(B), panels a1–e1] show only occasional staining for cell damage at low laser powers (), but at the higher powers () there are signs of light-induced cell damage in the central illuminated region. Cells subjected to combined incubation with -CA4 and laser illumination display positive staining for cell death within the laser exposed central regions of the fields shown, whereas the unexposed peripheral areas remain largely unaffected. These unirradiated border regions represent the control of cells in the presence of the drug without light. At -CA4 concentrations of and below, these peripheral areas show little cell damage, emphasizing the difference in toxicity between the -isomer [Fig. 5(B)] and the -isomer [Fig. 5(A)]. Optimal induction of cell damage by combined effects of light and -CA4 appears at 4.7 mW laser power and -CA4 (panel c2). It is noticeable that even in the presence of higher concentrations of -CA4, the highest laser power damages the cells to such an extent that they show less overall staining with both labels (e1–e4). Similar results were obtained with -CA4F. Fig. 5Apoptosis and cell permeability induced in CHO cell monolayers by combretastatins assessed by staining with annexin V AlexaFluor488 conjugate (green) and propidium iodide (red), respectively. (A) Effect of increasing concentration of -CA4F (0, 0.1, and ) after 48 h. (B) Effects of -CA4 (0, 10, 25, and ) added 2 h before irradiation of a field for 10 min at 625 nm with increasing laser powers at the sample (1.6, 3.1, 4.7, 6.3, and 9.4 mW). 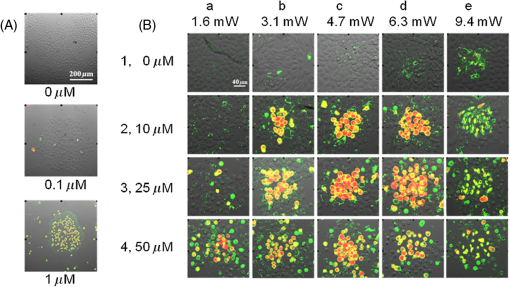 4.DiscussionThe low saturation threshold value of observed for the multiphoton-induced fluorescence of -CA4F described in Sec. 3.1 suggests considerable -isomerization conversion in fluid solution at higher laser powers. The efficiency of similar isomerization of -CA4 in the cellular experiment may be estimated from an appropriate calculation taking into account the 2PE cross section ( at ), the pulse parameters (76-MHz repetition, 180-fs pulse duration), the focal area ( diameter), and a typical overall power (5 mW). With these values, the peak photon fluence within the pulse () is . At a concentration of solute, Eq. (2) gives the rate of excitation per () as in a 1 mM solution. The concentration of -CA4 within cells is not uniform, but may readily exceed 1 mM locally within lipid droplets and other hydrophobic regions according to our FLIM data3 The number of excitations per molecule within a single 200-fs laser pulse is . In our cell experiments, each voxel was exposed to the laser for 40 ms (2 ms dwell time and 20 scans) equivalent to laser pulses. Therefore, the total number of excitations per molecule in the experiment was 64. Allowing for the quantum yield for photoisomerization (ca. 0.4), this shows sufficient energy is deposited at each voxel to convert a significant fraction of -CA4 to the active -isomer, although at present we do not know the composition of the photostationary state since for the nonfluorescent -isomer is unknown, but is expected to be slightly less than that of the -isomer based on the one-photon absorption cross section in the UV (Sec. 3.1). Because the laser beam was scanned in the -plane with a step size of , this resulted in deposition of a fairly uniform plane of converted drug with a depth of about (twice the wavelength) within the cell monolayer. The results demonstrate that using a combination of -CA4 and red light leads to effective cell killing attributable to two-photon induced isomerization of -CA4 to -CA4. The findings provide evidence for the development of a new form of phototherapy based on light-induced oxygen-independent prodrug activation. Activation of the -combretastatins occurs via a singlet excited state with subnanosecond lifetime; it is oxygen-independent as demonstrated for one-photon isomerization and therefore goes beyond current PDT limitations to treat hypoxic cancerous tissue. Recently -CA4 has been studied in clinical trials8 and while proven to have good anticancer activity, it has indiscriminate systemic toxicity that limits wide clinical application.22 Locally targeted two-photon prodrug activation is a promising route to overcoming this problem: drug activation is limited to the area of illumination (i.e., tumor targeted to the single cell level), which can be precisely controlled using well-established features of two-photon illumination. The use of 2PE overcomes the problem of competitive background absorption of UV light by other cellular/tissue constituents that would naturally prevent sufficient light penetration to activate the drug. The extent of penetration of focused light for effective two-photon activation depends on the extent of scattering and wavelength,1 but there are several reports in the literature of effective tumor and vascular control using two-photon PDT.15,23–26 Starkey et al.15 show that effective PDT might be attained at up to 4 cm depth in tissue models with unfocussed lasers. Development of more effective combretastatins for the purpose described here would benefit from being able to use longer wavelengths () to activate the isomerization process and take advantage of reduced scattering and greater transmission than at the wavelength presently used (625 nm) with three-photon activation (at ) being a further option. -combretastatins with higher two-photon absorption cross sections are also seen as desirable, and we are currently investigating new molecules with this in mind.27 AcknowledgmentsThe authors acknowledge the Central Laser Facility, Science and Technology Facilities Council (STFC) for providing access to the experimental facilities. The work was jointly funded by STFC and the University of Salford. ReferencesP. Agostiniset al.,
“Photodynamic therapy of cancer: an update,”
CA Cancer J. Clin., 61
(4), 250
–281
(2011). http://dx.doi.org/10.3322/caac.v61:4 CAMCAM 0007-9235 Google Scholar
R. H. Bisbyet al.,
“Fluorescence lifetime imaging of E-combretastatin uptake and distribution in live mammalian cells,”
Eur. J. Cancer, 48
(12), 1896
–1903
(2012). http://dx.doi.org/10.1016/j.ejca.2011.11.025 EJCAEL 0959-8049 Google Scholar
R. H. Bisbyet al.,
“Time-resolved nanosecond fluorescence lifetime imaging and picosecond infrared spectroscopy of combretastatin A-4 in solution and in cellular systems,”
Meas. Sci. Technol., 23
(8), 084001
(2012). http://dx.doi.org/10.1088/0957-0233/23/8/084001 MSTCEP 0957-0233 Google Scholar
G. G. Darket al.,
“Combretastatin A-4: an agent that displays potent and selective toxicity toward tumor vasculature,”
Cancer Res., 57
(10), 1829
–1834
(1997). CNREA8 0008-5472 Google Scholar
E. L. Schwartz,
“Anti-vascular actions of microtubule-binding drugs,”
Clin. Cancer Res., 15
(8), 2594
–2601
(2009). http://dx.doi.org/10.1158/1078-0432.CCR-08-2710 CCREF4 1078-0432 Google Scholar
J. H. Bilenkeret al.,
“Phase I trial of combretastatin A-4 phosphate with carboplatin,”
Clin. Cancer Res., 11
(4), 1527
–1533
(2005). http://dx.doi.org/10.1158/1078-0432.CCR-04-1434 CCREF4 1078-0432 Google Scholar
G. J. Rustinet al.,
“A phase Ib trial of CA4P (combretastatin A-4 phosphate), carboplatin, and paclitaxel in patients with advanced cancer,”
Br. J. Cancer, 102
(9), 1355
–1360
(2010). http://dx.doi.org/10.1038/sj.bjc.6605650 BJCAAI 0007-0920 Google Scholar
K. Gaukrogeret al.,
“Structural requirements for the interaction of combretastatins with tubulin: how important is the trimethoxy unit?,”
Org. Biomol. Chem., 1
(17), 3033
–3037
(2003). http://dx.doi.org/10.1039/b306878a OBCRAK 1477-0520 Google Scholar
H. GörnerH. J. Kuhn,
“Cis-trans photoisomerization of stilbenes and stilbene-like molecules,”
Adv. Photochem., 19 1
–117
(1995). http://dx.doi.org/10.1002/9780470133507.ch1 ADPCA2 0065-3152 Google Scholar
A. Torricelliet al.,
“In vivo optical characterization of human tissues from 610 to 1010 nm by time-resolved reflectance spectroscopy,”
Phys. Med. Biol., 46
(8), 2227
–2237
(2001). http://dx.doi.org/10.1088/0031-9155/46/8/313 PHMBA7 0031-9155 Google Scholar
A. UstioneD. W. Piston,
“A simple introduction to multiphoton microscopy,”
J. Microscopy, 243
(3), 221
–226
(2011). http://dx.doi.org/10.1111/jmi.2011.243.issue-3 JMICAR 0022-2720 Google Scholar
M. Pawlickiet al.,
“Two-photon absorption and the design of two-photon dyes,”
Angew. Chem. Int. Ed., 48
(18), 3244
–3266
(2009). http://dx.doi.org/10.1002/anie.v48:18 ACIEF5 1433-7851 Google Scholar
J. R. Starkeyet al.,
“New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse,”
Clin. Cancer Res., 14
(20), 6564
–6573
(2008). http://dx.doi.org/10.1158/1078-0432.CCR-07-4162 CCREF4 1078-0432 Google Scholar
N. J. Lawrenceet al.,
“Synthesis and anticancer activity of fluorinated analogues of combretastatin A-4,”
J. Fluorine Chem., 123
(1), 101
–108
(2003). http://dx.doi.org/10.1016/S0022-1139(03)00117-9 JFLCAR 0022-1139 Google Scholar
S. W. Botchwayet al.,
“Laser-induced radiation microbeam technology and simultaneous real-time fluorescence imaging in live cells,”
Methods Enzymol., 504 3
–28
(2012). http://dx.doi.org/10.1016/B978-0-12-391857-4.00001-X MENZAU 0076-6879 Google Scholar
P. J. Darcyet al.,
“Photochromic heterocyclic fulgides. Part 2. Electrocyclic reactions of (E)-α-2,5-dimethyl-3-furylethylidene(alkyl-substituted methylene)succinic anhydrides,”
J. Chem. Soc. Perkin Trans. 1,
(1), 202
–205
(1981). http://dx.doi.org/10.1039/p19810000202 JCPRB4 1364-5463 Google Scholar
M. Baydaet al.,
“Kinetics of reversible photoisomerization: determination of the primary quantum yields for the E-Z photoisomerization of silylenephenylenevinylene derivatives,”
Photochem. Photobiol. Sci., 8
(12), 1667
–1675
(2009). http://dx.doi.org/10.1039/b907242j PSHCB 1474-905X Google Scholar
C. Xuet al.,
“Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy,”
Proc. Natl. Acad. Sci. U. S. A., 93
(20), 10763
–10768
(1996). http://dx.doi.org/10.1073/pnas.93.20.10763 PNASA6 0027-8424 Google Scholar
S. Iyeret al.,
“Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4,”
Cancer Res., 58
(20), 4510
–4514
(1998). CNREA8 0008-5472 Google Scholar
M. Busket al.,
“Combretastatin-induced hypertension and the consequences for its combination with other therapies,”
Vasc. Pharmacol., 54
(1–2), 13
–17
(2011). http://dx.doi.org/10.1016/j.vph.2010.10.002 VPAHAJ 1537-1891 Google Scholar
M. Khuranaet al.,
“Quantitative in vitro demonstration of two-photon photodynamic therapy using photofrin and visudyne,”
Photochem. Photobiol., 83
(6), 1441
–1448
(2007). http://dx.doi.org/10.1111/php.2007.83.issue-6 PHCBAP 0031-8655 Google Scholar
H. A. Collinset al.,
“Blood-vessel closure using photosensitizers engineered for two-photon excitation,”
Nat. Photonics, 2
(7), 420
–424
(2008). http://dx.doi.org/10.1038/nphoton.2008.100 1749-4885 Google Scholar
M. Gary-Boboet al.,
“Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors,”
Angew. Chem. Int. Ed., 50
(48), 11425
–11429
(2011). http://dx.doi.org/10.1002/anie.v50.48 ACIEF5 1433-7851 Google Scholar
J. R. Starkeyet al.,
“Vascular targeting to the SST2 receptor improves the therapeutic response to near-IR two-photon activated PDT for deep-tissue cancer treatment,”
Biochim. Biophys. Acta, 1830
(10), 4594
–4603
(2013). http://dx.doi.org/10.1016/j.bbagen.2013.05.043 BBACAQ 0006-3002 Google Scholar
K. M. Schereret al.,
“Spectroscopy and fluorescence lifetime imaging in live cells of a cyano-substituted combretastatin,”
Biomed. Spectrosc. Imaging, 3
(3), 211
–218
(2014). http://dx.doi.org/10.3233/BSI-140067 BSIIAX 2212-8794 Google Scholar
BiographyKathrin M. Scherer received her BSc (2009) and PhD (2013) degrees from University of Salford. Since November 2012, she has been a postdoctoral researcher within the Central Laser Facility at the Rutherford Appleton Laboratory STFC, using advanced fluorescence imaging techniques including single-molecule total internal reflection fluorescence and superresolution stimulated emission depletion imaging to study the structure and function of the epidermal growth factor receptor family. She has authored four peer-reviewed publications and one patent. Roger H. Bisby received his BSc (1969) and PhD (1973) degrees from the University of Nottingham. He is an emeritus professor at the University of Salford and a fellow of the Royal Society of Chemistry. He has authored over 90 scientific publications that reflect his lifelong interest in fast reactions and biochemical spectroscopy. Stanley W. Botchway received his BSc degree in chemistry (1990) and his PhD degree (1996) from the University of Leicester, followed by a fellowship at Harvard Medical School. He is currently a visiting professor at Oxford Brookes University and STFC senior scientist. He has developed laser applications including instruments for ultrafast microbeam irradiation of cellular DNA and multiphoton fluorescence lifetime imaging microscopy. He has authored over 70 papers and is a member of the Royal Society of Chemistry and Radiation Research Society. John A. Hadfield is a senior lecturer in medicinal chemistry at the University of Salford. He received his BSc degree in chemistry from the University of Nottingham (1979) and his PhD degree in organic chemistry from Trent Polytechnic (1984). He is the author of more than 50 journal papers and was a chairman of Onco-NX (2011–2014). His research interests include the development of anticancer drugs, especially antivascular agents. Anthony W. Parker received his PhD degree (University of Warwick, photochemistry of DNA) and was a postdoctoral fellow at the Royal Institution of Great Britain. He joined the Rutherford Appleton Laboratory in 1986. He is now an STFC Fellow applying Raman spectroscopy to bone disease, coinventor of spatially offset Raman spectroscopy with five patents and 250 publications. He has honorary chairs at UCL and the Universities of Salford and Stellenbosh and is a fellow of the Royal Society of Chemistry. |