|
|
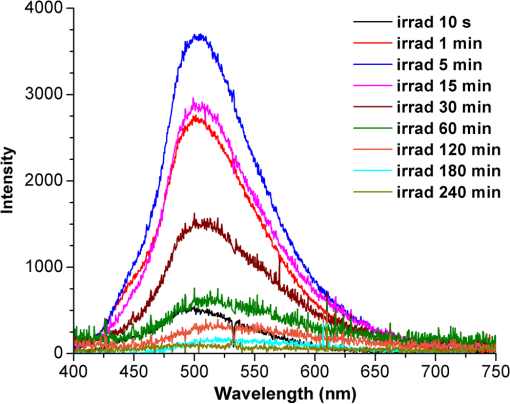
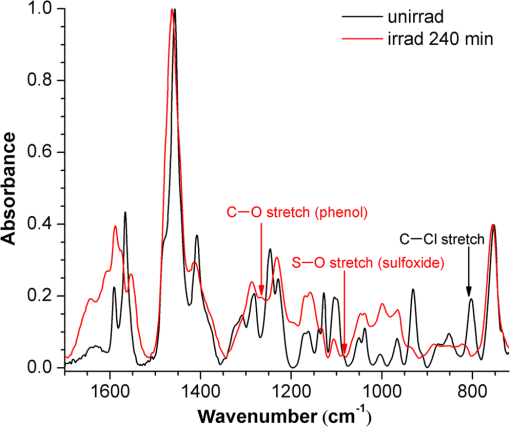
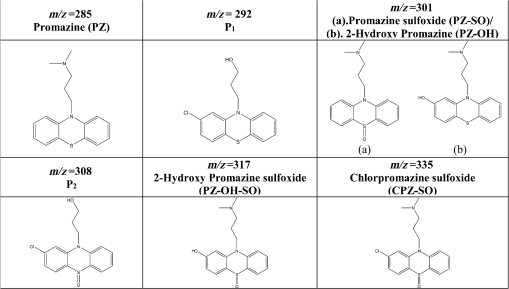
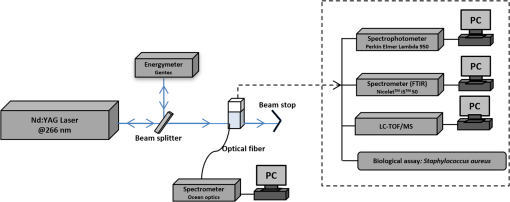
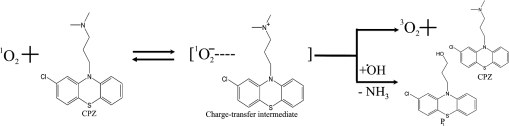
1.IntroductionPhenothiazines are compounds that contain two benzene rings ortho-fused with a thiazine ring, known for their pharmaceutical properties. They are used as sedative, tranquilizer, antituberculotic, or bactericide agents1–4 and can form radical-cations due to their low oxidation potential.5 A more recent application of phenothiazines uses them as markers for DNA.6 Chlorpromazine (CPZ) is a phenothiazine derivative, well known for its intensive use in psychotherapy,7 which was lately discovered to have a potential use in cancer treatment. It inhibits the proliferation or induces the apoptosis in cultured cells like leukemia cells without affecting the viability of normal lymphocyte8 or melanoma cells9 where CPZ induces a dose-dependent decrease in the cell viability. It can enhance the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells,10 as well. Besides these properties, it was demonstrated that CPZ acts as an efflux pump inhibitor, playing an important role in the fight against the resistance at antibiotics acquired by bacteria.11 A recently reported property of some phenothiazines is that under controlled exposure to ultraviolet (UV) laser beam, CPZ for instance undergoes molecular modifications, new photoproducts are generated, and the irradiated solutions gain activity against a Staphylococcus aureus reference strain,12,13 which is otherwise insensitive to the CPZ parent compound. An even more recent study showed that a mixture of the photoproducts obtained after irradiation with a 266-nm laser beam of a CPZ water solution enhances the antimicrobial activity against S. aureus and Escherichia coli compared with the unirradiated compound and becomes a good efflux pump inhibitor for Salmonella Enteritidis.14 Though, for future applications on bacteria, it is important to identify each newly generated molecules and separate the photoproducts in order to know which of them has bactericide properties and if they have synergistic effects on bacteria cultures or not. To do this, we diversified the methods and techniques used to analyze the composition of the CPZ liquid samples exposed to laser radiation. In this paper, the study of photocleavage of CPZ in order to obtain photoproducts with enhanced antibacterial properties is reported. The products generated by CPZ as a consequence of exposure to UV laser radiation were analyzed by steady-state absorption, laser-induced fluorescence (LIF), Fourier transform infrared spectroscopy (FTIR), and liquid chromatography—tandem time-of-flight mass spectroscopy (LC-TOF/MS). Singlet oxygen generation quantum yield was determined relative to the standard ZnPc in dimethyl sulfoxide (DMSO), and the CPZ fluorescence quantum yield was determined relative to the standard Coumarin 1 in ethanol. Compared with Ref. 13, the same concentration of of CPZ was investigated but more methods and techniques (mainly LC-TOF/MS correlated with LIF, FTIR, and standard optical absorption) were applied in order to elucidate the formation pathways of the photoproducts resulted from the irradiation. Using LC-TOF/MS, the concentrations of each photoproduct in percentage were extracted for the respective time intervals of irradiation. In Ref. 13, the main focus was set on CPZ irradiated 24 h and the high-performance liquid chromatography with diode-array detection (HPLC-DAD) and high-performance liquid chromatography (HPLC) with tandem mass spectrometric (MS/MS) detection (HPLC-MS/MS) analyses have shown that 200 compound were produced/recorded as the result of the laser beam interaction with the sample. Compared with Ref. 13, in this study was chosen a lower concentration, , and a shorter exposure time of 240 min in order to analyze the formation pathways and to lower the number of unknown factors used in data analysis. This was made because literature reports show that CPZ in water is most probably the critical micellar concentration (cmc) of CPZ in water, although a large range of cmc values is mentioned.15,16 The exposure to UV laser beams of CPZ in water reported in Ref. 13, although dominated by the interaction of laser radiation with monomers, does not exclude the contribution of micelles to this process since at production of micelles in the first steps may be present. So we have chosen to work at in order to avoid the possible contribution of micelles at the interaction of the laser radiation with CPZ molecules in water solution. We also used exposure time durations of, at most, 240 min, since this was long enough from the point of view of the generation of photoproducts of interest for our experiments on bacteria. In the following, the article will be organized in three sections: Results, Materials and Methods, and Discussions and Conclusions, showing the data from the biological results to the identification of the photoproducts generated in CPZ solutions in water by exposure to UV laser radiation. 2.Results2.1.Biological Assay. Antimicrobial Activity of CPZ Solutions in Water Exposed to UV Laser Beam Against Staphylococcus aureus ATCC 25923The biological assay consists of using the agar disk diffusion method,17 where the evaluation of the activity of the test drug was analyzed by determining the growth response of the microorganism. The bacteria were swabbed on the agar, after which the paper disks were placed and increasing quantities of irradiated samples were applied. After the incubation of the plates, the inhibition zones around the disks were measured for different irradiation time intervals of CPZ. In Fig. 1, the biological assay shows that of CPZ in ultrapure water irradiated 60 and 240 min presents the antimicrobial activity against S. aureus ATCC 25923 wild-type strain. The inhibitory effect for both irradiation time intervals appears starting with the fifth filter paper () and the area around it is increasing slightly as the quantity of CPZ is increased. The activity of the irradiated compound for different time intervals against this strain shows that for the same amount of mixture applied, 25 and , the 240-min irradiated CPZ presents a larger zone of inhibition. Above applied on the disks, the 60-min irradiation sample presents higher diameter of inhibition than the 240-min irradiated CPZ. In Ref. 12, it is shown that unirradiated CPZ tested against S. aureus has no antimicrobial activity and consequently for a smaller concentration of CPZ, the antimicrobial effect/activity is not present. Fig. 1The antimicrobial activity of CPZ in water irradiated 60 and 240 min against Staphylococcus aureus ATCC 25923 strain. Diameter on -axis is the diameter of the inhibition zones around the disks for different quantities of samples represented on -axis. 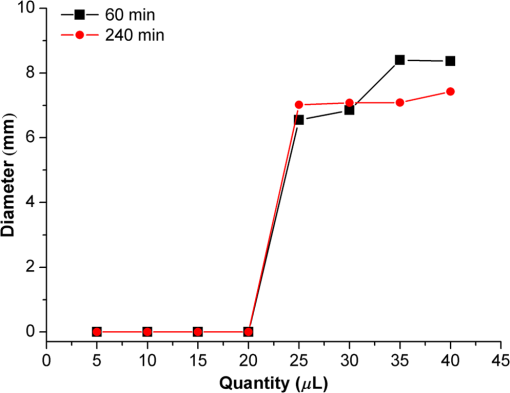 For 60-min irradiated CPZ, the major compound is Promazine (PZ) followed by Promazine sulfoxide/2-hydroxy Promazine (PZ-SO/PZ-OH) and for 240-min irradiated CPZ, the major compound is PZ-SO/PZ-OH (see Sec. 2.6). The rest of the photoproducts have higher concentrations for 60-min irradiated CPZ than the one at 240 min. The 60-min irradiated CPZ presents higher diameters of inhibition than the 240-min irradiated sample above of mixture applied on the disks. This is probably due to two factors: one is the toxic concentrations of the photoproducts in the samples which are different between 60 and 240 min and the second may be the different synergistic action of the photoproducts in the same two cases, due to their difference in concentrations. 2.2.UV-Vis Absorption SpectraThe UV-Vis absorption spectrum of unirradiated CPZ is characterized by two absorption bands with peaks at, respectively, 254 and 307 nm [Fig. 2(a)]. The 254-nm band intensity shows a hypochromic effect until 120 min and then it starts to increase until 240 min. The band at 307 nm presents a bathochromic shift to 344 nm by the end of the irradiation and its shape starts to change after 30 min, becoming broader. The changes in the UV-Vis spectra indicate that the substituents of the phenothiazine ring are changing and at the same time exhibit the effect of the structural modification, new compounds being formed.18 Fig. 2UV-Vis absorption spectra of the samples. (a) UV-Vis absorption spectra of CPZ solution in water measured between 200 and 800 nm for irradiation time intervals between 1 and 240 min. In the inset, a part of the spectrum is plotted in close-up; (b) First-order derivative of the absorption spectrum in the range 450 to 570 nm for 240-min irradiated CPZ. 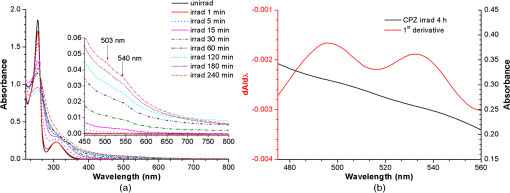 Due to the interaction of the laser beams with the sample, after 30 min of irradiation, two other peaks appear at 503 and 540 nm [Fig. 2(a)]. According to Ref. 19, these peaks could be responsible for the oxidized forms of CPZ (525 to 530 nm) and PZ (510 to 515 nm). Due to the overlapping of the bands in the absorption spectra, the wavelengths are shifted to 540 and 503 nm. One method to solve the overlapping bands from a spectrum can be the use of derivative spectra to enhance the differences within the spectrum.20 Figure 2(b) presents the first-order derivative of the absorption spectrum in the range 450 to 570 nm for 240-min irradiated CPZ, obtained using Savitzky–Golay algorithm. This reveals two individual bands that overlap to obtain the peaks at 503 and 540 nm, respectively. 2.3.LIF SpectraThe LIF spectrum of solutions of CPZ in ultrapure water irradiated for 240 min shows a band with a peak at 504 nm. The fluorescence intensity increases for exposure times up to 5 min, after which it starts to decrease (Fig. 3), and the peak shows a bathochromic shift of 38 nm at the end of the irradiation time suggesting the formation of new photoproducts. The intensity of the fluorescence is not influenced by the substituents at position 10, as shown in Ref. 19, so that no changes can be observed in the LIF spectra. A different substituent at position 2 of the phenothiazine ring may induce changes in the florescence spectra.19 The change in the wavelength for the maximum of the emission spectra indicates the modifications in the CPZ molecular structure at position 2. Factors that can influence fluorescence intensity are the pH and the irradiation time.19 The change in pH affects the reconfiguration of the fluorophore’s -electron system that occurs upon protonation.21 The pH analysis shows a decrease for the CPZ solution from 5.75 to 2.23 during irradiation. This behavior can be justified by the formation of the hydronium cation due to the cleavage of the chlorine from the aromatic structure of CPZ.22 The fluorescence spectra show a decrease in intensity until total bleaching, which takes place after 120-min exposure, at the same time with the total photodestruction of CPZ from the sample. 2.4.FTIR SpectraThe solutions of unirradiated/irradiated CPZ were also analyzed by FTIR and the stretching vibrations of different bonds belonging to CPZ have been identified (Fig. 4). The spectrum of unirradiated CPZ was compared with that of the 240-min irradiated sample and differences were envisaged. The formation of new compounds can be proven by the appearance of new bands in FTIR spectra. For CPZ solutions at in ultrapure water, while irradiating 240 min, the new band formed at is attributed to the stretching vibration of the C–O bond of a phenol, indicating the formation of a hydroxyl—photoproduct. The band at is due to the stretching vibration of S–O bond from sulfoxide, indicating the formation of oxidized compounds. The band at assigned to C–Cl disappeared in the spectrum of the irradiated samples, this fact indicating the formation of PZ. The shift of toward longer wavenumbers observed for all bands is due to the total disappearance of CPZ and the formation of new products that present different vibrational characteristics. 2.5.Singlet Oxygen Generation: CPZ Fluorescence Quantum YieldSinglet oxygen generation quantum yield was determined relative to the standard ZnPc in DMSO (0.67% reported in Ref. 23). The quantum yield for the singlet oxygen generation by CPZ in solution of 3.3% was measured. To estimate the interaction of the singlet oxygen with CPZ, time-resolved phosphorescence transients were registered for different CPZ concentrations in the range to . From the Stern–Volmer plot, a dynamic quenching rate constant of the singlet oxygen for CPZ results in . The CPZ fluorescence quantum yield was determined relative to the standard Coumarin 1 in ethanol (0.5 reported in Ref. 24); 15% fluorescence quantum yield was obtained out of the total rates of de-excitation processes that take place during irradiation. 2.6.LC-TOF/MS AnalysisExtremely accurate mass measurements () obtained with a TOF detection were used to provide quick and accurate qualitative information and to deduce the identity of the compounds with a high degree of certainty. Based on exact mass measurements of the generated ions and subsequent fragment ions of TOF/MS after in-source collision-induced dissociation, there were evaluated the extracted ions chromatograms and were identified the corresponding compounds after the exposure of CPZ to the laser beam. The solution of CPZ was analyzed after laser irradiation for 1, 5, 15, 30, 60, 120, and 240 min. The full-scan chromatogram revealed the presence of seven species and a gradual decrease of CPZ, after different irradiation times. In extracted ion chromatogram, it was possible to detect the , 292.1371, 300.1360, 301.1440, 308.1321, 317.1370, 319.1114, and 335.1044 amu. The evaluation of the results showed that after 120 to 240 min, the signal of CPZ disappeared, and the concentration of the known products increased or decreased. This is function of the time of irradiation and of the competitive photoreactions. Figure 5 presents the LC-TOF/MS spectra of irradiated and irradiated chlorpromazine and the inset graphs show the spectra in which the most important modifications could be observed. The spectrum shows the single-molecular ion at 319 amu and by comparing mass spectra of unirradiated CPZ with those of irradiated sample, photoproducts resulting from the interaction of CPZ with the UV laser radiation were identified. Fig. 5LC-TOF/MS spectra of CPZ solution for unirradiated and irradiated samples between 1 and 240 min. 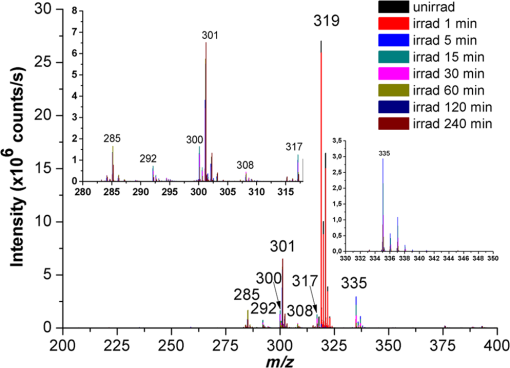 Figure 6 presents the proposed structures for photoproducts resulted from the interaction of UV radiation with CPZ water solutions. In general, the primary photochemical transfer reactions in an aromatic structure are fragmentation of -bond to the aromatic ring, fragmentation of -bond to the aromatic ring, and addition and substitution reactions directly in the aromatic ring and cicloaddition.25 Upon photolysis, the aryl halides undergo homolysis to produce radicals and halogen atoms, so that the formed photoproducts contain hydrogen from the solvent instead of the halogen.26 The compounds are: PZ, PZ-SO, PZ-OH, 2-hydroxy promazine sulfoxide (PZ-OH-SO), chlorpromazine sulfoxide (CPZ-SO), and other three compounds with values of 292, 300, and 308 amu labeled , , and , respectively. For and , the structures are shown in Fig. 6, but the chemical structure is not yet identified. From the total ion chromatogram, the concentrations in percentage units of each photoproduct at the irradiation time intervals mentioned above were extracted and they are shown in Fig. 7. In the case of CPZ, at the end of 120 min of irradiation, there is no compound left in the sample. The time dependence of CPZ-SO shows a maximum of concentration at 5 min (6.84%), after which it starts to decrease, being 1.1% at the end of the irradiation. PZ shows a maximum of its concentration at 60 min (46.58%) and is 28.31% at the end of the experiment. The same behavior is present in the case of but at different concentrations: at 60 min is 2.36% and at 240 min is 0.56%. As for and , a maximum is at 15 min (2.5%) and 30 min (6.02%) and at 240 min is 1.39% and 0.93%, respectively. PZ-OH-SO has a maximum concentration at 120 min (5.41%) and at the end of irradiation, it starts to decrease, reaching 4.26%. The most abundant compound in the sample until 60 min was PZ, but after all the amount of CPZ disappeared from the sample, at 120 min, PZ-OH/PZ-SO became the major compound. The most significant change in concentration is that of PZ-OH/PZ-SO, which from the beginning until the end of the irradiation presents an increase in concentration, reaching, at 240 min, 63.41%. Fig. 7The percent concentration of CPZ and of each obtained photoproduct during irradiation, extracted from total ion chromatogram data. 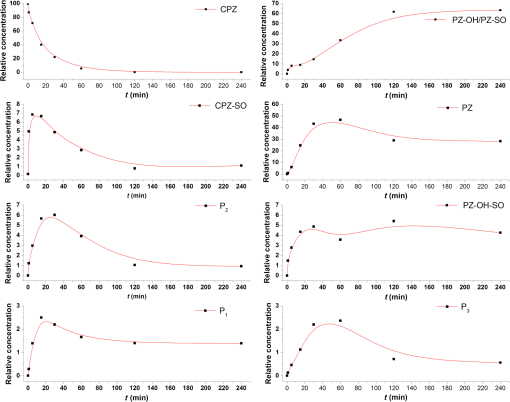 The second compound at 240 min, looking at its abundance, is the PZ. These results suggest that there is a competition among the photoproducts newly generated. 3.Materials and MethodsThe compound studied is a phenothiazine derivative, CPZ in the hydrochloride form , purity 98.9% (Sigma Aldrich, St Louis, Missouri). For the measurement of singlet oxygen quantum yield and fluorescence quantum yield, Zn-Phthalocyanine (ZnPc) (Sigma Aldrich) and Coumarin 1 (Coumarin 460, Exciton, Dayton, Ohio) were used, respectively. In Fig. 8, the result about the CPZ geometry optimization is depicted after running calculations using Gaussian 09.27 In order to obtain an accurate geometry optimization of the CPZ molecule, the density functional theory and the B3LYP functional were used, along with the 6-31G* basis set, a combination of which is known as yielding accurate results when used for organic compounds.28 In order to reach an optimization as close as possible to the experimental conditions, the IEFPCM module for solvation was used, with water as the solvent. Fig. 8The optimized geometry of CPZ obtained using Gaussian 9.0. The out of plane distribution of the S, Cl atoms, and the benzene rings, as well as the side chain are evidenced. The bending and stretching vibrations coupled with the rotation of the atoms and groups of atoms are shown (Video 1, MP4, 8.5 MB) [URL: http://dx.doi.org/10.1117/1.JBO.20.5.051002.1]. 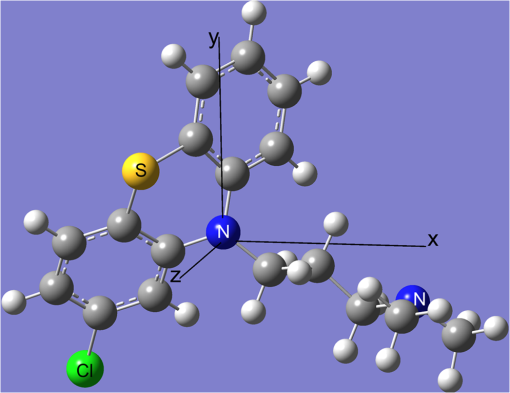 For the photochemical study of CPZ solutions in ultrapure water, was irradiated with a pulsed laser beam having 6.5-mJ average pulse energy at 266 nm [the fourth harmonic of a Nd:YAG laser, Continuum (San Jose, California), Excel Technology laser, model Surelite II, 6-ns full time width at half maximum (FTWHM), 10-Hz pulse repetition rate]. The experimental setup is given in Fig. 9. The optical path length was 1 cm, the area of the beam spot on the cuvette was , and the beam fluence was . The laser pulse energy was measured during the experiment by monitoring a part splitted from the laser beam with a Gentec Laser (Quebec, Canada) energymeter equipped with a QE25LP-H-MB-D0 head. The irradiation was made different time intervals: 1, 5, 15, 30, 60, 120, 180, and 240 min. Each sample was investigated by steady-state UV-Vis absorption spectroscopy, FTIR spectroscopy, LIF, and LC-TOF/MS. The absorption spectra were registered between 200 and 800 nm using a Perkin Elmer Spectrophotometer, model Lambda 950, having a standard error of . For the LIF studies, the same irradiation beam was used to excite the fluorescence emission.29 The LIF spectra were acquired in real time by an Ocean Optics (Winter Park, Florida) spectrometer, model HR4000. The IR spectra of unirradiated and irradiated solutions were recorded using a FTIR Nicolet™ (Madison, Wisconsin) iS™ 50 spectrometer in the range 750 to at resolution. The samples of CPZ solutions in water were dried on a KRS-5 (thallium bromo-iodide) supports, and the background due to KRS-5 was subtracted from all the spectra. The singlet oxygen time-resolved phosphorescence was detected by a cooled NIR photomultiplier Hamamatsu (Iwata, Japan) H-10330, the output of which was fed to a digital oscilloscope Tektronix (Beaverton) DPO 7254. This experimental setup is described in detail in Ref. 30. For the singlet oxygen generation study, the CPZ was dissolved in deuterium oxide at 0.2 () and the excitation was made at 355 nm (the third harmonic of the Nd:YAG laser fundamental beam). The solvent used was due to the longer lifetime of the singlet oxygen in it, compared with the same parameter in water ( and, respectively, 3 to ).31 The quantum yield was obtained by a relative method, using as standard the ZnPc in DMSO. The time-resolved phosphorescence signals were measured under the same conditions, and the singlet oxygen quantum yield was given in Ref. 32: where stands for the quantum yield of the singlet oxygen and for the phosphorescence intensity of it. is the absorbance of the solutions at 355 nm, is the singlet oxygen lifetime, and is the solvent refractive index, while subscript ref corresponds to the standard ZnPc in DMSO. The phosphorescence intensity is obtained by extrapolating to , the mono-exponential fitting curve of the phosphorescence kinetics.For the determination of the fluorescence quantum yield, the CPZ was dissolved in ultrapure water at (), which corresponds to 0.1 absorbance at 355 nm. In this way, the fluorescence intensity is free from reabsorption and self-quenching effects. The excitation wavelength was set at 355 nm in order to obtain a excitation so that nonradiative transitions between higher electronic levels are avoided. The quantum yield was obtained using the Coumarin 1 in ethanol as standard and the same conditions were assured as in the CPZ case. The fluorescence spectra were recorded using an UV-Vis optical fiber ( core diameter), fixed at 90 deg with respect to the incident beam that passes the cuvette, connected to a spectrograph coupled with an ICCD camera for detection and analysis of the emitted light. The spectrograph used is an Acton Research (Trenton, New Jersey) model, SpectraPro SP-2750 (Czerny–Turner configuration), focal length 750 mm, and resolution 2.2 nm when a grating is utilized. The attached camera detector was an ICCD from Princeton Instruments (Trenton, New Jersey), model PIMAX 1024RB (25-nm intensifier, resolution , 2-ns gate speed) provided with a programmable controller unit (2-ns time resolution). The gate of the detection system was opened for 20 ns after the laser pulse. The average energy of the laser pulse was 7.4 mJ at 355 nm wavelength, and 100 pulses were averaged to obtain each spectrum. The time-resolved fluorescence spectra of the sample and the standard were measured under the same conditions, and the CPZ fluorescence quantum yield was calculated using the equation from Ref. 33: where stands for the fluorescence quantum yield, for the fluorescence-integrated intensities (areas), for the absorption factor of the solutions at 355 nm, and for the solvent refractive index, while subscript ref corresponds to the standard Coumarin 1 in ethanol.The solution of CPZ irradiated at the time intervals mentioned above was analyzed by LC-TOF/MS, using an Agilent Technologies (Santa Clara, California) 1200 Infinity Series LC system, consisting of 1260 quaternary pump, a 1260 ALS auto-sampler coupled to an electrospray ionization (ESI) source, and a 6224 TOF/MS, controlled by the Mass Hunter Acquisition software. The chromatographic separation was accomplished using a Zorbax Extend-C18-1.8 () (Agilent Technologies, California) column, and as mobile phase a mixture of 30% acetonitrile and 70% water containing 0.15% heptafluorobutyric acidat a flow rate of is used. For MS, the ESI was used in the positive mode with the following settings: dual spray needles for continuous infusion of reference mass solution, drying gas flowing at , nebulizer pressure of 40 psig, capillary voltage of 3000 V, and fragmentor voltage of 100 V. The TOF was tuned and calibrated using Agilent ESI-TOF calibration and tuning mix. The system was flushed with 100% HPLC flushing solvent for 30 min after every sample injection to wash off accumulated nontarget analytes. The data acquisition mass range was 200 to , at and . For the biological assay, CPZ irradiated 60 and 240 min were tested against S. aureus ATCC 25923 in order to determine the antimicrobial activity of the mixture of the generated photoproducts. The protocol for this study is presented in detail in Ref. 13. The eight filter papers were impregnated with multiple aliquots solution for 60- and 240-min irradiated samples after being placed on top of the agar containing the swabbed bacteria. In this respect, disk number 1 contained of CPZ irradiated for 4 h, disk number , disk number , disk number , disk number , disk number , disk number , and disk number 8 contained . The impregnation with was made at 5- to 10-min intervals to ensure the evaporation of the water and care was taken not to overflow the compound from the filter paper. The results were analyzed by measuring the zone of inhibition diameter for each quantity of CPZ irradiated for 60 and 240 min, having as reference the diameter of the filter paper of 6.19 mm. The combination of these techniques was used to achieve a better understanding of how the photoproducts are formed and the possible pathways that lead to their formation and to determine if, at the used concentration, the obtained mixture in solution is a good antimicrobial agent against S. aureus ATCC 25923 strain. 4.Discussions and ConclusionsThis work is part of a larger effort to develop new antimicrobial platforms to be used in the treatment of the multiple drug resistance (MDR) cases acquired by bacteria. Along this line, it is necessary to first understand the interaction of the CPZ with laser radiation whereas the understanding of the formation pathways of the photoproducts becomes mandatory; so, one may establish a relation between the antimicrobial activity and the newly formed photoproducts. This method could be a fast and inexpensive approach of developing new antimicrobial agents, having as final products a new model to obtain in a short time, without any use of chemical agents and time-consuming synthetic chemistry, new compounds that are able to fight MDR of bacteria and/or cancer. CPZ was chosen for this study because previous experiments showed that exposing it to UV radiation, its antimicrobial activities are enhanced compared with the unirradiated compound.13 In those studies, CPZ at in ultrapure water was exposed to 266-nm radiation for 24 h and tested on a S. aureus strain. The result showed an enhanced antimicrobial activity as compared with the unirradiated CPZ. In a different study,14 the CPZ at the same concentration and 4-, 8-, 16-, and 24-h exposure time was tested against Gram-negative and Gram-positive bacteria strains, both having a wild-type and MDR representatives. The microbiology analysis includes the minimum inhibitory concentration (MIC) and the real-time ethidium bromide accumulation assays. It was demonstrated that the mixture of photoproducts which resulted at the end of irradiation was active against S. aureus and E. coli and with respect to the Salmonella Enteritidis strains it had a significant activity against the organism’s efflux pump.14 An important step in obtaining a better antimicrobial compound is to identify if it has a lower toxicity. This study is shown in Ref. 34, where the same concentration and irradiation time as above were used. The best in vitro cytotoxicity against human cells assay was obtained for CPZ exposed 4 h at , suggesting that this method produces compounds with greater bioactivity and lower toxicity than the parental species.34 At the same time, studies performed on species of Mycobacteria of human interest showed that irradiated CPZ could be a good candidate in treating tuberculosis.34 Further studies to elucidate the generation of the photoproducts resulted from the interaction of CPZ with laser radiation considered a semiquantitative theoretical approach. This was made to calculate the maximum amount of molecules needed to absorb the radiation in order to obtain molecular modifications of CPZ.35 These results lead to another kind of analysis, which considers the modifications at molecular level following the interaction of CPZ in water with a laser beam emitted at 266 nm. Compared with our previous studies, Ref. 13, the photochemical reactions were studied using additional techniques: FTIR, TOF-LC/MS, and LIF. It was also evidenced that the absorption spectra present two other bands, which are highlighted by the first-order derivative of them. The concentration and the time interval of irradiation were selected in order to have a low number of possible photocombinations to create a starting point in the rates of photoproducts formations and to establish a scheme of reaction. After the absorption of the photons by CPZ, the possible de-excitation pathways of excited molecules are fluorescence and phosphorescence emissions, photochemical transformation, conformational changes, energy transfer, internal conversion, and singlet oxygen generation. All these processes are in competition to each other. The fluorescence quenching is a result of combined processes such as singlet oxygen generation, phosphorescence emission, photodegradation of the parental compound, and pH modification. In Fig. 10, we present the possible pathways of the CPZ evolution as a result of its interaction with the laser beam at 266 nm. Fig. 10Possible pathways of CPZ that result in the formation of the photoproducts. CPZ: chlorpromazine; : excited singlet state chlorpromazine; isc: intersystem crossing; : excited triplet state chlorpromazine; : promazyl radical; : phenazathioniumcation; CPZ-SO:chlorpromazine sulfoxide; : excited triplet-state chlorpromazine sulfoxide; : hydroxyl radical; : singlet oxygen; :ground state oxygen; PZ-OH:hydroxypromazine; :promazylcation; PZ-SO: promazinesulfoxide; PZ-OH-SO:hydroxypromazinesulfoxide; : chloride anion; PZ: promazine. 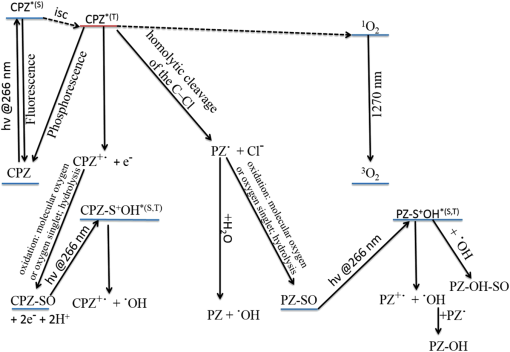 The figure with possible pathways of the CPZ photodecomposition was drawn using authors’ experimental results as well as literature reports.18,36–46 Ultrapure water was chosen as solvent due to its compatibility with the biological systems. The exposure of CPZ was performed in aqueous solution without extraction of the molecular oxygen so that, in this respect, its presence can lead to the formation of free radicals and singlet oxygen.36 There are three proposed ways for the formation of sulfoxide compounds following the CPZ interaction with the optical radiation. In aqueous solution, in Ref. 37, it is suggested that the molecular oxygen is incorporated as the oxygen atom in the sulfoxide due to aerial oxidation; in Refs. 38 and 39, it is proposed the possibility of the participation of singlet oxygen as an oxidant agent of CPZ ground state; and in Ref. 40, the hydrolysis of the cation radical in order to obtain CPZ-SO is considered. After the interaction of aqueous CPZ solution with the photons, the phenazathionium cation has a tendency to form CPZ-5-oxide due to the low-electron density at the sulfur atom in water.18 At the same time, the sulfoxide compound cannot be formed if water and/or oxygen is not involved.41 The formation of PZ is obtained by dehalogenation of CPZ from the triplet state [], the proposed process being the homolytic cleavage of the C–Cl bond followed by the hydrogen atom abstraction from the solvent by the promazinyl radical.42 The percentage change of the CPZ-SO during irradiation is an indication that this compound is also affected by the UV radiation. In Ref. 43, it is indicated that at the sulfur–oxygen bond of CPZ-SO, a homolytic cleavage takes place and hydroxyl radicals can be formed. The cleavage of the CPZ-SO at the sulfur–oxygen bond is also supported in Ref. 44, where it is postulated that the electronic excitation weakens the bond in the excited-state protonation resulting in a single bond with charge separation (). The formation of PZ-OH can be due to a recombination of the promazinyl and the hydroxyl radicals and is supported by the visible absorption peaks shifted to longer wavelengths by hydroxylation.18 Another process involved in the generation of photoproducts is the quenching of the singlet oxygen by tertiary amine. This is possible via two mechanisms that result from the formed charge-transfer intermediate: chemical quenching where photoproducts are obtained and physical quenching where the tertiary amine and the singlet oxygen in ground state result.45,46 The and compounds can be formed by the interaction of the singlet oxygen with the tertiary amine from dimethyl-amino-propyl side chain. Figure 11 presents the reaction path for CPZ–oxygen singlet interaction that leads to the formation. From the same reaction path, can be obtained as a result of CPZ-SO/singlet oxygen interaction or as a result of the oxidation process. The increase in intensity in the FTIR spectra of the band at , responsible for the O–H stretch vibration (alcohol), supports the formation of . As for their antimicrobial activity, it is known that CPZ is not a good candidate in MDR bacteria assays14,34 and also there is inhibition of the bacteria (Fig. 1) for the 240-min irradiated sample even though there is no CPZ left in it. At the same time, PZ has a greater MIC value than CPZ for all the bacteria tested in Ref. 14 (unpublished data), so that this compound is not responsible for the inhibition of the S. aureus strain used in this study. In the CPZ-SO case, in Ref. 47, it is demonstrated that the surface activity of this compound at interfaces covered with insoluble monomolecular films, simulating the orientated structure of biological membranes, is lower than that of CPZ. This suggests that CPZ-SO may have a smaller antimicrobial activity against bacteria than CPZ, due to the lower interaction with the simulated biological membrane. For the rest of the photoproducts, no interaction with biological membranes or MIC values was found in literature. The interaction of these compounds with bacterial strains should be subject for further studies. The mechanism of action of these compounds against bacterial strains could be the following: action on the permeability of the bacterial membrane, blocking the efflux capacity of the bacterial cell,48 secretion of toxins,49 reverse resistance to antibiotics,50 inhibition of bacterial enzymes, and elimination of plasmids from infected Gram-negative bacteria51 or the lysis of bacteria.52 In conclusion, the photochemical breakdown schemes of CPZ in aqueous solution during irradiation with a laser beam emitted at 266 nm and the formation of photoproducts have been suggested. The rates of photoproducts formation are influenced by the generation of singlet oxygen and fluorescence emission by CPZ. In this paper, the singlet oxygen quantum yield and the fluorescence quantum yield of CPZ are measured, obtaining 3.3% and 15%, respectively. The UV-Vis absorption, FTIR, and LIF techniques can be good spectroscopic tools in the investigation of the mixtures of compounds formed during irradiation. Combining them offered an insight of the transformations which occur in the CPZ molecular structure:
This is in agreement with the data obtained by LC-TOF/MS analysis. These techniques also allow to follow the time stability of the solutions exposed to laser radiation. After ending the irradiation sequences, the obtained solutions/compounds are stable in time for weeks and even months53,54 which do not recommend restrictions about the time interval in which they may be used for applications, provided the samples are kept between the room (20°C) or fridge (4°C) temperatures and in dark. The discovery of compounds with direct antimicrobial effects or of compounds with synergistic effects, obtained by laser irradiation of a drug, that are good candidates in MDR bacterial treatment is a further step in overcoming the gap created in antimicrobial research. Developing new efflux pump inhibitor could have a major benefit in allowing the reuse of the existing antibiotics affected by these systems. AcknowledgmentsThe authors from NILPRP acknowledge the financial support of the Romanian National Authority for Scientific Research, CNCS-UEFISCDI by project number PN-II-ID-PCE-2011-3-0922 and PN-II-PT-PCCA-2013-3.1-1350. T. Alexandru was supported by the strategic grant POSDRU/159/1.5/S/137750, “Project Doctoral and Postdoctoral programs support for increased competitiveness in Exact Sciences research,” co-financed by the European Social Found within the Sectorial Operational Program Human Resources Development 2007–2013. ReferencesE. KarlssonL. E. LarssonK. Nilsson,
“The effects of prophylactic dixyrazine on postoperative vomiting after two different anaesthetic methods for squint surgery in children,”
Acta. Anaesthesiol. Scand., 37
(1), 45
–48
(1993). http://dx.doi.org/10.1111/aas.1993.37.issue-1 AANEAB 0001-5172 Google Scholar
R. L. SmithR. P. MaickelB. B. Brodie,
“Acth-hypersecretion induced by phenothiazine tranquilizers,”
J. Pharmacol. Exp. Ther., 139
(2), 185
–190
(1963). JPETAB 0022-3565 Google Scholar
L. Amaralet al.,
“Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy,”
J. Antimicrob. Chemother., 47
(5), 505
–511
(2001). http://dx.doi.org/10.1093/jac/47.5.505 JACHDX 0305-7453 Google Scholar
J. MolnárY. MándiJ. Király,
“Antibacterial effect of some phenothiazine compounds and R-factor elimination by chlorpromazine,”
Acta Microbiol. Acad. Sci. Hung., 23
(1), 45
–54
(1976). AMAHA5 0001-6187 Google Scholar
L. A. TinkerA. J. Bard,
“Electrochemistry in liquid sulfur dioxide. 1. Oxidation of thianthrene, phenothiazine, and 9, 10-diphenylanthracene,”
J. Am. Chem. Soc., 101
(9), 2316
–2319
(1979). http://dx.doi.org/10.1021/ja00503a012 JACSAT 0002-7863 Google Scholar
S. Ronaldet al.,
“Phenothiazine inhibitors of TLKs affect double-strand break repair and DNA damage response recovery and potentiate tumor killing with radiomimetic therapy,”
Genes Cancer, 4
(1–2), 39
–53
(2013). http://dx.doi.org/10.1177/1947601913479020 0165-4608 Google Scholar
W. W. GoodM. SterlingW. H. Holtzman,
“Termination of Chlorpromazine with schizophrenic patients,”
Am. J. Psychiatry, 115
(5), 443
–448
(1958). AJPSAO 0002-953X Google Scholar
Z. Zhelevet al.,
“Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Phenothiazines and leukemia,”
Cancer Chemother. Pharmacol., 53 267
–275
(2004). http://dx.doi.org/10.1007/s00280-003-0738-1 CCPHDZ 0344-5704 Google Scholar
I. Gil-Adet al.,
“Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth,”
Oncol. Rep., 15
(1), 107
–112
(2006). OCRPEW 1021-335X Google Scholar
C. W. Ydeet al.,
“The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells,”
Anti-Cancer Drugs, 20
(8), 723
–735
(2009). http://dx.doi.org/10.1097/CAD.0b013e32832ec041 ANTDEV 0959-4973 Google Scholar
M. Martinset al.,
“Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections: mechanisms for their direct and indirect activities,”
Int. J. Antimicrob. Agents, 31
(3), 198
–208
(2008). http://dx.doi.org/10.1016/j.ijantimicag.2007.10.025 IAAGEA 0924-8579 Google Scholar
M. L. Pascuet al.,
“Direct modification of bioactive phenothiazines by exposure to laser radiation,”
Recent Pat. Rev. Anti-Infect. Drug Discov., 6
(2), 147
–157
(2011). http://dx.doi.org/10.2174/157489111796064515 RPADCX 1574-891X Google Scholar
M. L. Pascuet al.,
“Exposure of chlorpromazine to 266 nm laser beam generates new species with antibacterial properties: contributions to development of a new process for drug discovery,”
PLoS One, 8
(2), e55767
(2013). http://dx.doi.org/10.1371/journal.pone.0055767 1932-6203 Google Scholar
T. Alexandruet al.,
“Biological evaluation of products formed from the irradiation of chlorpromazine with a 266 nm laser beam,”
Biochem. Pharmacol., 2
(1), 1
–4
(2013). http://dx.doi.org/10.4172/2167-0501.1000109 BCPCA6 0006-2952 Google Scholar
M. P. McIntoshet al.,
“Impact of chlorpromazine self-association on its apparent binding constants with cyclodextrins: effect of SBE(7)-beta-CD on the disposition of chlorpromazine in the rat,”
J. Pharm. Sci., 99
(7), 2999
–3008
(2010). http://dx.doi.org/10.1002/jps.22064 JPMSAE 0022-3549 Google Scholar
S. SchreierS. V. P. MalheirosE. de Paula,
“Surface active drugs: self-association and interaction with membranes and surfactants,”
Biochim. Biophys. Acta, 1508
(1–2), 210
–234
(2000). http://dx.doi.org/10.1016/S0304-4157(00)00012-5 BBACAQ 0006-3002 Google Scholar
M. B. Coyleet al., Manual of Antimicrobial Susceptibility Testing, 39
–53 American Society for Microbiology Press, Washington, DC
(2005). Google Scholar
R. R. Gupta, Phenothiazines and 1, 4-Benzothiazines. Chemical and Biomedical Aspects, Elsevier, Amsterdam
(1988). http://dx.doi.org/10.1002/recl.19891080412 Google Scholar
J. KarpinskaB. StarczewskaH. Puzanowska-Tarasiewicz,
“Analytical properties of 2- and 10-disubstituted phenothiazine derivatives,”
Anal. Sci., 12 161
–170
(1996). http://dx.doi.org/10.2116/analsci.12.161 ANSCEN 0910-6340 Google Scholar
T. Owen, Fundamentals of Modern UV-Visible Spectroscopy: A Primer, Agilent Technologies, Waldbronn, Germany
(2000). Google Scholar
A. Ghouiliet al.,
“Synthesis, crystal structure and spectral characteristics of highly fluorescent chalcone-based coumarin in solution and in polymer matrix,”
J. Phys. Chem. Solids, 75
(2), 188
–193
(2014). http://dx.doi.org/10.1016/j.jpcs.2013.09.011 JPCSAW 0022-3697 Google Scholar
G. BuettnerR. Hall,
“The stepwise biphotonic photoionization of chlorpromazine as seen by laser flash photolysis,”
Photochem. Photobiol., 49
(3), 249
–256
(1989). http://dx.doi.org/10.1111/php.1989.49.issue-3 PHCBAP 0031-8655 Google Scholar
N. Kuznetsovaet al.,
“Relationship between the photochemical properties and structure of pophyrins and related compounds,”
Russ. J. Gen. Chem., 70
(1), 133
–140
(2000). RJGCEK 1070-3632 Google Scholar
G.A. ReynoldsK.H. Drexhage,
“New Coumarin dyes with rigidized structure for flashlamp-pumped dye lasers,”
Opt. Commun., 13
(3), 222
–225
(1975). http://dx.doi.org/10.1016/0030-4018(75)90085-1 OPCOB8 0030-4018 Google Scholar
J. Malkin, Photophysical and Photochemical Properties of Aromatic Compounds, CRC Press, Boca Raton, Florida
(1992). Google Scholar
J. GrimshawA. P. de Silva,
“Photochemistry and photocyclization of aryl halides,”
Chem. Soc. Rev., 10 181
–203
(1981). http://dx.doi.org/10.1039/cs9811000181 CSRVBR 0306-0012 Google Scholar
M. J. Frischet al., Gaussian09, Revision C.01, Gaussian Inc., Wallingford, Connecticut
(2010). Google Scholar
J. G. LeeC. SaguiC. Roland,
“Quantum chemistry simulations of glycopeptide antibiotics,”
Lect. Notes Comput. Sci. Eng., 49 343
–351
(2006). http://dx.doi.org/10.1007/3-540-31618-3 LNCSA6 1439-7358 Google Scholar
M. Boniet al.,
“Laser beams interaction with liquids in optofluidic experiments,”
Rom. Rep. Phys., 64
(S), 1179
–1194
(2012). RORPED 1221-1451 Google Scholar
A. Staicuet al.,
“Photophysical study of Zn phthalocyanine in binary solvent mixtures,”
J. Mol. Struct., 1044 188
–193
(2013). http://dx.doi.org/10.1016/j.molstruc.2012.12.010 JMOSB4 0022-2860 Google Scholar
I. B. C. MathesonJ. LeeA. D. King,
“The lifetime of singlet oxygen () in heavy water, a revised value,”
Chem. Phys. Lett., 55
(1), 49
–51
(1978). http://dx.doi.org/10.1016/0009-2614(78)85129-X CHPLBC 0009-2614 Google Scholar
F. WilkinsonW. P. HelmanA. B. Ross,
“Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution,”
J. Phys. Chem. Ref. Data, 22 113
–262
(1993). http://dx.doi.org/10.1063/1.555934 JPCRBU 0047-2689 Google Scholar
A. M. Brouwer,
“Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report),”
Pure Appl. Chem., 83
(12), 2213
–2228
(2011). http://dx.doi.org/10.1351/PAC-REP-10-09-31 PACHAS 0033-4545 Google Scholar
A. M. Armadaet al.,
“The in vitro activity of products formed from exposure of Chlorpromazine to a 266nm laser beam against species of Mycobacteria of human interest,”
In Vivo, 27
(5), 605
–610
(2013). IVIVE4 0258-851X Google Scholar
A. DinacheM. BoniM.L. Pascu,
“Phenothiazine derivatives interaction with laser radiation,”
Rom. Rep. Phys., 65
(3), 1078
–1091
(2013). RORPED 1221-1451 Google Scholar
D. MooreS. Tamat,
“Photosensitization by drugs: photolysis of some chlorine-containing drugs,”
J. Pharm. Pharmacol., 32
(3), 172
–177
(1980). http://dx.doi.org/10.1111/jphp.1980.32.issue-1 JPPMAB 0022-3573 Google Scholar
T. IwaokaM. Kondo,
“Mechanistic studies on the photooxidation of chlorpromazine in water and ethanol,”
Bull. Chem. Soc. Jpn, 47 980
–986
(1974). http://dx.doi.org/10.1246/bcsj.47.980 BCSJA8 0009-2673 Google Scholar
I. Rosenthalet al.,
“Photochemical reactions of chlorpromazine: chemical and biochemical implications,”
Photochem. Photobiol., 28
(4–5), 591
–594
(1978). http://dx.doi.org/10.1111/php.1978.28.issue-4-5 PHCBAP 0031-8655 Google Scholar
A. K. DavisS. NavaratnamG. O. Phillips,
“Photochemistry of chlorpromazine (2-chloro-N-(3dimethylamino propyl) phenothiazine) in propan-2-ol solution,”
J. Chem. Soc., Perkin Trans. 2, 25 25
–29
(1976). http://dx.doi.org/10.1039/p29760000025 JCPKBH 0300-9580 Google Scholar
H. Y. ChengP. H. SackettR. L. McCreery,
“Kinetics of chlorpromazine cation radical decomposition in aqueous buffers,”
J. Am. Chem. Soc., 100
(3), 962
–967
(1978). http://dx.doi.org/10.1021/ja00471a051 JACSAT 0002-7863 Google Scholar
A. FelmeisterC. A. Discher,
“Photodegradation of Chlorpromazine hydrochloride,”
J. Pharm. Sci., 53
(7), 756
–762
(1964). http://dx.doi.org/10.1002/(ISSN)1520-6017 JPMSAE 0022-3549 Google Scholar
C. Garcíaet al.,
“Photodegradation of 2-chloro substituted phenothiazines in alcohols,”
Photochem. Photobiol., 85
(1), 160
–170
(2009). http://dx.doi.org/10.1111/php.2009.85.issue-1 PHCBAP 0031-8655 Google Scholar
G. R. Buettneret al.,
“Free radical production by chlorpromazine photolysis sulfoxide, an ESR spin-trapping study,”
Photochem. Photobiol., 44
(1), 510
(1986). http://dx.doi.org/10.1111/php.1986.44.issue-1 PHCBAP 0031-8655 Google Scholar
H. J. ShineE. E. Mach,
“Ion radicals. V. Phenothiazine, phenothiazine 5-oxide, and 3Hphenothiazin- 3-one in acid solutions,”
J. Org. Chem., 30
(7), 2130
–2139
(1965). http://dx.doi.org/10.1021/jo01018a004 JOCEAH 0022-3263 Google Scholar
M. V. EncinasE. LempE. A. Lissi,
“Interaction of singlet oxygen [] with aliphatic amines and hydroxylamines,”
J. Chem. Soc. Perkin Trans. 2, 2
(8), 1125
–1127
(1987). http://dx.doi.org/10.1039/p29870001125 JCPKBH 0300-9580 Google Scholar
E. BaciocchiT. Del GiaccoA. Lapi,
“Quenching of singlet oxygen by tertiary aliphatic amines. Structural effects on rates and products,”
Helv. Chim. Acta, 89
(10), 2273
–2280
(2006). http://dx.doi.org/10.1002/(ISSN)1522-2675 HCACAV 0018-019X Google Scholar
G. ZografiD. E. Auslander,
“Surface activity of chlorpromazine and chlorpromazine sulfoxide in the presence of insoluble monomolecular films,”
J. Pharm. Sci., 54
(9), 1313
–1318
(1965). http://dx.doi.org/10.1002/(ISSN)1520-6017 JPMSAE 0022-3549 Google Scholar
A. Mahamoudet al.,
“Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy,”
J. Antimicrob. Chemother., 59
(6), 1223
–1229
(2007). http://dx.doi.org/10.1093/jac/dkl493 JACHDX 0305-7453 Google Scholar
R. M. NelsonH. E. NoyesJ. P. Sanford,
“Effect of chlorpromazine and dibenzyline on bacterial toxins,”
Proc. Soc. Exp. Biol. Med., 92
(3), 617
–621
(1956). http://dx.doi.org/10.3181/00379727-92-22562 PSEBAA 0037-9727 Google Scholar
H. V. AposhianN. S. PointerM.M. Aposhian,
“Reversal of inhibitory activity of chlorpromazine by calcium,”
Proc. Soc. Exp. Biol. Med., 100
(3), 512
–513
(1959). http://dx.doi.org/10.3181/00379727-100-24679 PSEBAA 0037-9727 Google Scholar
G. Spengleret al.,
“The mechanism of plasmid curing in bacteria,”
Curr. Drug Targets, 7
(7), 823
–841
(2006). http://dx.doi.org/10.2174/138945006777709601 CDTUAU 1389-4501 Google Scholar
R. P. AgarwalA. Guha,
“Lytic action of chlorpromazine hydrochloride on Escherichia coli g. cells,”
Br. J. Pharmacol. Chemother., 24
(2), 466
–469
(1965). http://dx.doi.org/10.1111/(ISSN)1476-5381a BJPCBM 0007-1188 Google Scholar
A. Smarandacheet al.,
“Optical studies of the spectral properties of phenothiazines,”
Lett. Drug Des. Discovery, 9
(4), 352
–360
(2012). http://dx.doi.org/10.2174/157018012799859918 LDDDAW 1570-1808 Google Scholar
A. Dinache,
“The optical and spectral study of the stability of the solutions of drugs in variable environment conditions,”
University of Bucharest,
(2013). Google Scholar
BiographyTatiana Alexandru is an assistant researcher at National Institute for Lasers, Plasma and Radiation Physics, Bucharest. She received her BS/MS degrees in physics from the Faculty of Physics, University of Bucharest, in 2010/2012, respectively; she is currently a PhD student in optics and spectroscopy. Her research interests include laser interaction with biomolecular systems and drug solutions by means of laser spectroscopy. She is currently the president of the SPIE Romanian Student Chapter for the time interval 2013–2014. Angela Staicu is a senior scientist at National Institute for Lasers, Plasma, and Radiation Physics, Bucharest. She obtained her PhD degree in physics at University of Bucharest, Faculty of Physics, specialization in “Optics Spectroscopy,” in 2002. Her research activity was concentrated on laboratory spectroscopy in the directions: spectroscopic techniques for investigation of pollutants and intermediate species formed in combustion processes, gas-phase laser spectroscopy of astrophysically relevant molecules, spectroscopic studies of molecules relevant in biomedical science. Alexandru Pascu is a scientist at National Institute for Lasers, Plasma and Radiation Physics, Bucharest, Romania. Holding a MS degree from the Faculty of Physics, University of Bucharest, in 1972, his research interests cover spectroscopy, laser–matter interaction, biophotonics, and biomedicine. Elena Radu is a scientific research 3rd degree at National Research & Development Institute for Chemistry and Petrochemistry, Department of Bioresources, Bucharest. She received her BS degree in biochemistry from Faculty of Chemical Technology, University POLITEHNICA of Bucharest since 1983, and she is currently a PhD student in analytical chemistry. Her competences include applied research in chemistry field and organic chemistry technologies, chemistry, and instrumental analysis. She is member of Romanian Society of Chemistry. Alexandru Stoicu is an assistant researcher at National Institute for Lasers, Plasma and Radiation Physics, Bucharest. He received his BS degree in chemical engineering from the Faculty of Veterinary Medicine, University of Agronomic Science and Veterinary Medicine Bucharest, in 2010, and MS degree in drug chemistry from the Faculty of Chemistry, University of Bucharest, in 2014. His research interests include laser interaction with drug solutions, laser spectroscopy, drug chemistry, and quantum chemistry. Viorel Nastasa is a research scientist at National Institute for Lasers, Plasma and Radiation Physics from Bucharest, Romania. He received his BS degree in biophysics, his MS degrees in biophysics and medical physics in 2009, and his PhD degree in optics, spectroscopy, lasers and plasma physics in 2012 from the Faculty of Physics, University of Bucharest. His research interests include optofluidics and microfluidics, laser spectroscopy, and laser interaction with medicines. He is an active member of SPIE Romanian Student Chapter. Andra Dinache is a research scientist at National Institute for Lasers, Plasma and Radiation Physics, Bucharest. She received her BS/MS degrees in biophysics in 2008/2010, respectively, and her PhD degree in optics, spectroscopy, lasers, plasma in 2013 from the Faculty of Physics, University of Bucharest. Her research interests include spectroscopy of biomolecules, laser spectroscopy, and laser interaction with biomolecular systems and medicine solutions. She is an active member of SPIE Romanian Student Chapter. Mihai Boni is a research scientist at National Institute for Lasers, Plasma and Radiation Physics, Bucharest, Romania. He received his BS degree in physics-informatics and his MS degree in optics, spectroscopy, lasers and plasma in 2009/2011 from the Faculty of Physics, University of Bucharest. His research interests include optofluidics and microfluidics, laser spectroscopy, and laser interaction with medicines. He is an active member of SPIE Romanian Student Chapter. Leonard Amaral is a professor emeritus of the Institute of Hygiene and Tropical Medicine/Universidade Nova de Lisboa, where he was a professor and director of Mycobacteriology (1999–2010) and prior to that director of Clinical Laboratories of the Bronx-Lebanon Hospital Center, Bronx, New York (1977–1999). His main interest is the development of drugs for therapy of multidrug resistant pathologies and this interest has resulted in almost 300 publications in international journals with citations that exceed 4500. Mihail Lucian Pascu is a senior scientist at National Institute for Laser, Plasma and Radiation Physics, Bucharest, where he heads Laser Spectroscopy Group, he founded in 1975. He is a professor at Physics Faculty, University of Bucharest, where he is PhD head. His scientific interests are laser physics, spectroscopy, optofluidics, and biophotonics, in which he published over 200 papers and made over 250 communications. He was SO at EC-DG-RTD and ESF serving COST Office (2001–2006). |