|
|
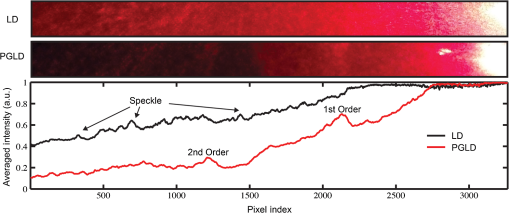
1.IntroductionOriginating from work in the 1970s,1 laser diffraction (LD)–based methods are widely used to measure sarcomere length, a key parameter that determines striated muscle function. Briefly, sarcomere length determines the active force production of individual muscle fibers2,3 and is the normalization factor used to calibrate a skeletal muscle’s architecture,4,5 which successfully predicts muscle function.6 The term sarcomere describes a unit of interdigitating contractile proteins that repeat in series along the length of a muscle fiber.7,8 This results in characteristic striations observed in skeletal muscle. Trans-illumination of muscle fibers by laser light yields a diffraction pattern, and the grating equation9 relates diffraction pattern periodicity to the periodicity of the muscle striation pattern and, therefore, sarcomere length. LD-based methods have been used to measure and understand the role of sarcomere length in a variety of physiological studies, including abnormally-long resting sarcomere lengths in muscular deformities in children with spastic cerebral palsy,10 intraoperative sarcomere lengths before and after surgical tendon lengthening,11 and in muscle architecture studies of the lumbar spine.12 The major utility of LD is the fact that it rapidly samples hundreds of thousands of sarcomeres that are illuminated by the beam. Traditional microscopy–based methods are limited to the system’s field-of-view and depth of field, which typically includes only hundreds of sarcomeres,13 nonplanar optical artifacts, and the need for significant tissue preprocessing. The use of confocal microscopy allows greater sarcomere sampling and minimizes nonplanar artifacts but is significantly more time consuming and is often cost prohibitive. Muscle and connective tissues are birefringent and heavily scattering media, therefore, diffraction signals are degraded by scattering events as light propagates through tissue.14 At distances exceeding a few mean free paths (average distance between light–tissue interactions), light enters the “diffuse”15 regime of propagation and diffracted signals cannot be detected. The reduced scattering coefficient is larger with increased connective tissue associated with many muscle pathologies,16,17 and increased scattering typically makes it impossible to measure diffraction patterns from fibrotic tissues. Some clinical and experimental examples that show connective tissue increases in muscle include: muscular dystrophies,18 developmental disorders such as cerebral palsy,10 immobilization,19 and aging.20 Detailed models of diffraction21 and diffuse propagation of polarized light14,22 suggest that they differently alter polarization state. Therefore, the signal exiting a highly scattering muscle sample is a polarization sensitive superposition of diffracted light and diffuse transmittance. As a result, we tested whether polarization-gated laser diffraction (PGLD) could extract diffracted signals from diffuse noise and thus enable sarcomere length measurement. We built a PGLD system and used it to recover diffracted signals in fibrotic soleus muscles from chronically immobilized murine hind limbs. Importantly, LD was unable to measure sarcomere lengths in these highly scattering muscles. We also compared sarcomere lengths measured by PGLD to bright field (BF) microscopy and found a significant bias. By using confocal microscopy as an additional positive control, we saw that BF measurements of these fibers, rather than PGLD measurements, suffer from sampling bias. 2.Methods2.1.Sarcomere Length Measurement by Polarization-Gated Laser DiffractionTo perform sarcomere length measurement by LD, a wavelength He–Ne laser transilluminated tissue samples (Fig. 1). The resultant diffraction pattern and diffuse-transmitted light illuminated a measurement screen and spacing between diffracted peaks (if present) was measured by digital calipers and used to solve for sarcomere length using the grating equation9 where SL is the sarcomere length, is the diffraction order, is the laser wavelength, and is the angle to diffraction peak for order . Calibration of the distance between sample and diffraction screen was performed using standardized 2 and diffraction gratings.Fig. 1Sarcomere length measured by polarization-gated laser diffraction (PGLD). Polarizing filters extract diffraction signal from superimposed noise. 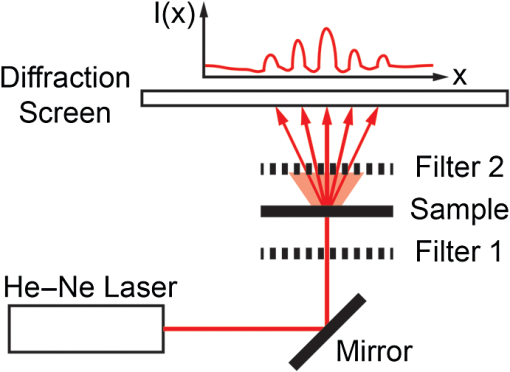 Polarization gating was performed by inserting linear polarizers in rotary optic mounts (#52-574, Edmund Optics Inc., Barrington, New Jersey) above and below the sample stage (Fig. 1). The linear polarization angle for each polarizer could be adjusted manually with 0.5-deg accuracy. Polarization angles for both polarizers were manually adjusted to visually maximize diffraction patterns. Though sarcomere length measurements were made manually, a stationary camera was used to image the diffraction screen as an example of PGLD signal extraction. Images were processed using MATLAB (The MathWorks, Inc., Natick, Massachusetts) to obtain an average signal intensity plotted against the long axis of the muscle fibers. 2.2.Hindlimb Immobilization to Generate Highly Scattering MuscleOne hindlimb each of four 8-week old wild-type (C57BL/6) mice were immobilized in paris casts for 14 days such that the ankle was in dorsiflexion () stretching the soleus muscle, following the protocol of Williams and Goldspink.23 The contralateral uncasted hindlimbs served as controls. The casts were repaired as needed to ensure that the integrity of the casts was maintained throughout two weeks. 2.3.Tissue PreparationHindlimbs from the euthanized immobilized mice were disarticulated at the hip, skinned, pinned to a cork board at a fixed angle of 90 deg at the knee and ankle and chemically fixed in 10% formalin for two days. Whole tibialis anterior (TA) and soleus muscles were surgically removed then rinsed and stored in phosphate-buffered saline. To facilitate fiber bundle dissection, muscles were partially digested in 15% for 30 min, similar to the protocol of Shah et al.24 Fiber bundles containing 1-10 myofibers were microdissected under a microscope and mounted on a glass slide for sarcomere length measurements. Ten to fifteen fiber bundles were dissected from each muscle. 2.4.Light MicroscopyA microscope (Model DM6000, Leica Microsystems Inc., Buffalo Grove, Illinois) was used to take BF images of soleus and TA muscle fiber bundles using a objective. ImageJ software25 was used to average 10 to 30 sarcomere lengths in series within an image. Measured regions were chosen based on the ability to clearly see the sarcomeres-in-series, unimpaired by the increased connective tissue. Confocal BF microscopy (Model SP5, Leica Microsystems Inc., Buffalo Grove, Illinois) was used to sample sarcomere length through a muscle fiber bundle with a objective. Focal depth was adjusted to image at least 50 focal planes through each fiber bundle (0.5 to Sol, 2.5 to TA). In each focal plane, the sarcomere length was averaged across 10 in-focus sarcomeres in series. Measured regions of muscle fiber bundles were marked on the slide, and the same volume of muscle was measured by PGLD. 2.5.Data AnalysisStatistical tests were made using Prism (GraphPad Software, Inc., La Jolla, California) using a significance level () of 0.05. The average sarcomere length measured by PGLD and BF were compared in four immobilized soleus muscles in a two-way ANOVA with the main effects of measurement technique and muscle sample. We did not use a paired -test because the exact sampled location was not the same between tests. However, we did measure the same volume of muscle with PGLD and confocal microscopy and used a paired -test for this comparison. 3.Results and Discussion3.1.Polarization Gating Rescue Diffracted SignalsSarcomere lengths from murine soleus muscles harvested from hindlimbs treated with prolonged immobilization could not be measured by traditional LD, due to increased connective tissue content that erodes the diffracted signal with scattering events and buries the signal in diffuse-transmitted light and laser speckle (Fig. 2). However, the diffraction pattern was readily visualized using PGLD, which enabled comparison between sarcomere lengths in fibrotic muscles without sampling bias, as described in Secs. 3.2 and 3.3. For our proof-of-principle study, we used simple linear polarizing filters for two reasons: they are simple to use and Li et al.26 showed that the diffuse propagation of circular polarization and linear polarization are similar in muscle. However, it is possible that the signal-to-noise ratio and, more importantly, sensitivity can be further improved by fully characterizing the scattering process in diseased muscle tissue. Different muscular diseases may have different polarization combinations that best extinguish scattered noise. 3.2.Sarcomere Lengths Measured by PGLD and Bright Field MicroscopyWe compared sarcomere lengths from muscle fiber bundles of four immobilized soleus muscles measured by PGLD and BF (Fig. 3). The sarcomere length measured in a normal TA muscle with traditional LD was included for reference. Note that the polarization gating does not shift the location of the diffracted peaks. Statistical analysis by two-way ANOVA shows a significant effect of measurement technique (), a significant effect of muscle sample (), and no significant interaction term (). Surprisingly, these data suggest a systematic measurement bias between sarcomere lengths measured by PGLD and BF ( average difference). BF under estimated the sarcomere length in every sample measured, including both healthy and diseased tissue, despite the varied sarcomere lengths in each muscle sample. This disagreement was surprising because diffraction and microscopy techniques are considered standard methods to measure sarcomere length. However, BF samples a volume of sarcomeres from the muscle fiber bundle that is in focus, whereas PGLD samples throughout the bundle thickness. Therefore, BF may under sample or have a regional sampling bias that results in the sarcomere length disagreement. As an additional positive control, we thus compared PGLD to confocal microscopy images taken throughout the muscle fiber bundle thickness, which we believed would provide a more accurate comparison. Fig. 3Sarcomere length (, to 15 measurements per muscle) measured by laser diffraction (LD), PGLD, and bright field microscopy (BF). In immobilized muscles, sarcomere length measurement by traditional LD method was not possible. Two-way ANOVA revealed a significant effect of measurement technique (), a significant effect of muscle sample (), and no significant interaction term (). 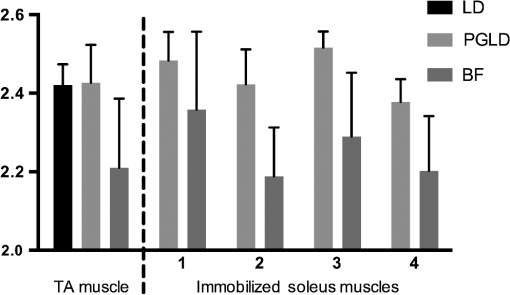 3.3.Sarcomere Lengths Measured by PGLD and Confocal MicroscopyData presented above suggested a systematic bias leading to different sarcomere length measurements between PGLD and BF. We hypothesize that relatively small numbers of sarcomeres measured by BF at specific depths of focus in the fiber bundles produced this measurement bias. We thus performed a rigorous comparison of sarcomere lengths measured by PGLD and confocal microscopy, because confocal microscopy is able to image throughout the muscle fiber bundle volume (Fig. 4). Fig. 4PGLD enables sarcomere length measurements in highly scattering muscle tissue. No significant differences were found between the two methods (). Data shown are ( muscles). 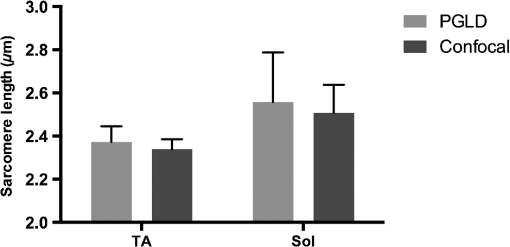 Paired -test comparing sarcomere length measured by PGLD and confocal microscopy in 10 fiber bundles (5 from normal TA, 5 from immobilized soleus) showed no significant difference () with 80% power to detect a difference. This serves as a positive control for the new PGLD sarcomere length method, and highlights the need to obtain appropriate samples to accurately perform such comparisons. 3.4.Trade-offs of Several Sarcomere Length Measurement MethodsThere are several important differences among these measurement methods (Table 1). LD was only successful in 7 of 115 fiber bundles obtained from immobilized muscles. Using PGLD drastically improved the sensitivity to 92 of 115, a 1333% improvement in measurement ability. We did not perform BF and confocal microscopy on all 115 fiber bundles, but our experience suggests that 100% sensitivity can be achieved with these techniques if enough time is spent. High sensitivity, however, is balanced by measurement time and sampling bias. BF has high sensitivity, but we yielded a significant bias of for shorter sarcomere lengths. Confocal microscopy also has high sensitivity, but it takes significantly longer to perform than PGLD. The sample size was estimated using the following parameters: diameter and length sarcomeres, 1-mm laser beam width, and depth of field. Table 1Polarization-gated laser diffraction (PGLD) enables sarcomere length measurements in highly scattering muscle tissue. 4.ConclusionWe demonstrated that the PGLD successfully and accurately recovers diffracted signals in highly scattering muscle tissue, which were previously impossible to measure by LD. This method is an example of a simple technical adjustment that can be used to increase the sensitivity of LD. Diffraction-based methods efficiently sample large numbers of sarcomeres compared with microscopy-based techniques. AcknowledgmentsResearch reported in this publication was supported by the National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R24 HD050837; by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers P30 AR061303 and R01 AR057393; and by the Department of Veteran’s Affairs Grant A9028R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Peter Dykstra for his assistance with the confocal microscope experiments. ReferencesD. CleworthK. Edman,
“Changes in sarcomere length during isometric tension development in frog skeletal muscle,”
J. Physiol., 227 1
–17
(1972). JPHYA7 0022-3751 Google Scholar
A. GordonA. HuxleyF. Julian,
“The variation in isometric tension with sarcomere length in vertebrate muscle fibres,”
J. Physiol., 184 170
–192
(1966). JPHYA7 0022-3751 Google Scholar
A. GordonA. HuxleyF. Julian,
“Tension development in highly stretched vertebrate muscle fibres,”
J. Physiol., 184 143
–169
(1966). JPHYA7 0022-3751 Google Scholar
C. Gans,
“Fiber architecture and muscle function,”
Exerc. Sport Sci. Rev., 10 160
–207
(1982). http://dx.doi.org/10.1249/00003677-198201000-00006 ESSRB8 0091-6331 Google Scholar
R. L. LieberJ. Fridén,
“Functional and clinical significance of skeletal muscle architecture,”
Muscle Nerve, 23
(11), 1647
–1666
(2000). http://dx.doi.org/10.1002/(ISSN)1097-4598 ESSRB8 0148-639X Google Scholar
P. Powellet al.,
“Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs,”
J. Appl. Physiol., 57 1715
–1721
(1984). JAPYAA 0021-8987 Google Scholar
A. F. HuxleyR. Niedergerke,
“Structural changes in muscle during contraction: interference microscopy of living muscle fibres,”
Nature, 173
(4412), 971
–973
(1954). http://dx.doi.org/10.1038/173971a0 NATUAS 0028-0836 Google Scholar
H. HuxleyJ. Hanson,
“Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation,”
Nature, 173
(4412), 973
–976
(1954). http://dx.doi.org/10.1038/173973a0 NATUAS 0028-0836 Google Scholar
R. L. LieberY. YehR. J. Baskin,
“Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter,”
Biophys. J., 45
(5), 1007
–1016
(1984). http://dx.doi.org/10.1016/S0006-3495(84)84246-0 BIOJAU 0006-3495 Google Scholar
L. R. Smithet al.,
“Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length,”
J. Physiol., 589
(10), 2625
–2639
(2011). http://dx.doi.org/10.1113/tjp.2011.589.issue-10 JPHYA7 0022-3751 Google Scholar
J. FridénR. L. Lieber,
“Physiologic consequences of surgical lengthening of extensor carpi radialis brevis muscle-tendon junction for tennis elbow,”
J. Hand Surg. Am., 19
(2), 269
–274
(1994). http://dx.doi.org/10.1016/0363-5023(94)90018-3 1753-1934 Google Scholar
S. R. Wardet al.,
“Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability,”
J. Bone Joint Surg. Am., 91
(1), 176
–185
(2009). http://dx.doi.org/10.2106/JBJS.G.01311 0021-9355 Google Scholar
M. E. Llewellynet al.,
“Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans,”
Nature, 454
(7205), 784
–788
(2008). http://dx.doi.org/10.1038/nature07104 NATUAS 0028-0836 Google Scholar
X. WangL. V. Wang,
“Propagation of polarized light in birefringent turbid media: a Monte Carlo study,”
J. Biomed. Opt., 7
(3), 279
–290
(2002). http://dx.doi.org/10.1117/1.1483315 JBOPFO 1083-3668 Google Scholar
S. BartelA. H. Hielscher,
“Monte Carlo simulations of the diffuse backscattering Mueller matrix for highly scattering media,”
Appl. Opt., 39
(10), 1580
–1588
(2000). http://dx.doi.org/10.1364/AO.39.001580 APOPAI 0003-6935 Google Scholar
J. J. Xiaet al.,
“Characterizing beef muscles with optical scattering and absorption coefficients in VIS-NIR region,”
Meat. Sci., 75
(1), 78
–83
(2007). http://dx.doi.org/10.1016/j.meatsci.2006.07.002 MESCDN 0309-1740 Google Scholar
R. L. LieberS. R. Ward,
“Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis,”
Am. J. Physiol. Cell Physiol., 305
(3), C241
–C252
(2013). http://dx.doi.org/10.1152/ajpcell.00173.2013 0363-6143 Google Scholar
L. ZhouH. Lu,
“Targeting fibrosis in Duchenne muscular dystrophy,”
J. Neuropathol. Exp. Neurol., 69
(8), 771
–776
(2010). http://dx.doi.org/10.1097/NEN.0b013e3181e9a34b JNENAD 0022-3069 Google Scholar
P. E. WilliamsG. Goldspink,
“Connective tissue changes in immobilised muscle,”
J. Anat., 138
(2), 343
–350
(1984). JOANAY 0021-8782 Google Scholar
C. J. Mannet al.,
“Aberrant repair and fibrosis development in skeletal muscle,”
Skelet. Muscle, 1
(1), 21
(2011). http://dx.doi.org/10.1186/2044-5040-1-21 SMKUAK 2044-5040 Google Scholar
E. Sidicket al.,
“Rigorous analysis of light diffraction ellipsometry by striated muscle fibers,”
Biophys. J., 66
(6), 2051
–2061
(1994). http://dx.doi.org/10.1016/S0006-3495(94)80999-3 BIOJAU 0006-3495 Google Scholar
A. ShuaibX. LiG. Yao,
“Transmission of polarized light in skeletal muscle,”
J. Biomed. Opt., 16
(2), 025001
(2011). http://dx.doi.org/10.1117/1.3536512 JBOPFO 1083-3668 Google Scholar
P. E. WilliamsG. Goldspink,
“Longitudinal growth of striated muscle fibres,”
J. Cell Sci., 9
(3), 751
–767
(1971). JNCSAI 0021-9533 Google Scholar
S. B. Shahet al.,
“Sarcomere number regulation maintained after immobilization in desmin-null mouse skeletal muscle,”
J. Exp. Biol., 204
(10), 1703
–1710
(2001). JEBIAM 0022-0949 Google Scholar
W. S. Rasband,
“ImageJ,”
U.S. National Institutes of Health, Bethesda, Maryland, USA,
(1997–2014) http://imagej.nih.gov/ij/ ( 07 2014). Google Scholar
X. LiJ. C. RanasinghesagaraG. Yao,
“Polarization-sensitive reflectance imaging in skeletal muscle,”
Opt. Express, 16
(13), 9927
(2008). http://dx.doi.org/10.1364/OE.16.009927 OPEXFF 1094-4087 Google Scholar
BiographyKevin W. Young is currently a PhD candidate in the muscle physiology lab at University of California, San Diego. He received his BS degree in physics from the University of California, Santa Barbara, and worked as a research assistant at the California Institute of Technology. His current research focuses on biomedical optics in the field of muscle physiology. Sudarshan Dayanidhi is currently a postdoctoral fellow in the muscle physiology lab at the University of California, San Diego. He received his BSc and MS degrees in physical therapy from the University of Mumbai and Drexel University respectively. He practiced as a pediatric physical therapist and obtained his MS degree in mechanical engineering in 2010 and his PhD degree in biokinesiology in 2012 from the University of Southern California. His current research focuses on muscle dysfunction in children with developmental disabilities. Richard L. Lieber is the senior vice president of research and chief scientific officer at the Rehabilitation Institute of Chicago. He is a professor at Northwestern University Feinberg School of Medicine and is senior research career scientist in the Department of Veterans Affairs. Previously, he was a professor and a vice chairman of the Department of Orthopaedic Surgery at the University of California, San Diego, and principal investigator of the San Diego Skeletal Muscle Research Center. |