|
|
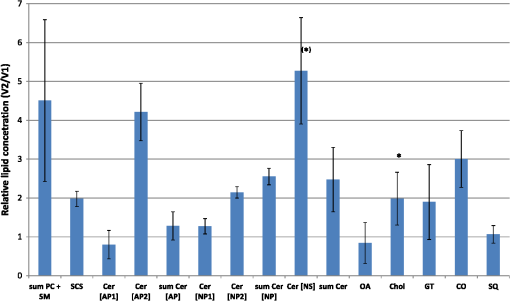
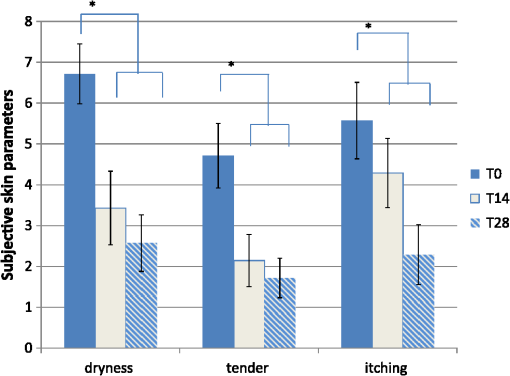
1.IntroductionAtopic dermatitis (AD) is a chronic inflammatory skin disease with an increasing prevalence in industrialized countries.1 AD is characterized by pruritus and eczematous lesions, which are accompanied by excessive infiltration of inflammatory cells.2,3 Although the pathogenesis of AD has not yet been fully understood, genetic, environmental, pharmacological, psychological, immunological, and skin barrier dysfunction factors are believed to contribute to the underlying pathogenic mechanisms.4–6 Topical glucocorticoids or calcineurin inhibitors are commonly used to treat moderate to severe AD, but the long-term use of steroids at high concentrations is associated with a number of side effects.7 In addition, various natural sources, i.e., plant extracts, are widely used in the treatment of AD, as certain plants have anti-inflammatory properties that may reduce the symptoms of AD or can extend the interval between acute inflammatory phases.8 A lipophilic extract from St. John’s wort (Hypericum perforatum) is used as an active ingredient for the treatment of atopic skin. A major constituent of the lipophilic extract is hyperforin, a phloroglucinol derivative, which is known to provide anti-inflammatory, antitumor, and antibacterial properties.9,10 Laser scanning microscopy (LSM), commonly implemented in the diagnosis of skin tumors,11,12 can be used to investigate inflammatory skin diseases.13–16 The cellular structures of the epidermis and the capillaries yield information regarding the skin status, even if the skin surface does not show any distinct pathological features. In this study, volunteers with dry to atopic skin who did not use any therapeutic cream or drugs were investigated before and after treatment with the hyperforin-containing ointment, and skin parameters were analyzed with several techniques. Typical skin parameters, including the barrier function, moisture content of the stratum corneum (SC), and the composition of skin lipids were measured using bioengineering techniques. Furthermore, subjective parameters, such as itching, skin dryness, and tension, were evaluated. The structure of the skin surface and the epidermis were analyzed using LSM. 2.Method and Materials2.1.Investigational OintmentThe investigational cosmetic ointment provided by Klosterfrau Healthcare Group, Cologne, Germany, contains a hypericum perforatum extract (1.5% w/w) that is rich in hyperforin. The extract also includes the following ingredients (International Nomenclature of Cosmetic Ingredients): aqua, petrolatum, propylene gycol, caprylic/capric triglyceride, PEG-20 glyceryl stearate, cetyl alcohol, glyceryl stearate, panthenol, phenoxyethanol, tocopherol, tocopheryl acetate, and allantoin. On average, of the ointment were applied by the volunteers on their forearms. 2.2.SubjectsFourteen volunteers with dry or atopic skin were investigated before and after the application of the cream. Seven subjects were recruited at the Department of Dermatology of the Charité-Universitätsmedizin Berlin and seven subjects at the Department of Dermatology of University Medical Center Freiburg. The treatment period was 14 days in Freiburg and 28 days, with an intermediate visit on day 14, at the Charité. The study design was evaluated and approved by the Ethics Committees of the Charité-Universitätsmedizin Berlin and the University Medical Center Freiburg. All volunteers had given their written informed consent for participation in the study. 2.3.Study ProcedureFor each volunteer, a mask of the inner forearm was prepared on an overhead foil using natural markers (i.e., naevi) to later find the same areas at all measurement time points. First, the evaluation of the subjective parameters was performed and the transepidermal water loss (TEWL) and SC moisture were measured. Subsequently, the LSM investigations were performed. The SC lipids were extracted in proximity to the investigated area to avoid contaminations with marker and immersion oil in the extraction fluid. 2.4.Parameters of the LSM InvestigationsFor laser scanning microscopy, seven volunteers were investigated at day zero and after 28 days using a commercially available LSM system (VivaScope® 1500 Multilaser, MAVIG, Munich, Germany) with the reflectance and fluorescent modes. The system is equipped with three low-power diode lasers emitting at 488, 658, and 785 nm (max. 15 mW, laser class 1M) and a lens with a numerical aperture of 0.9. In this study, the wavelength of 488 nm was used for the investigation in the fluorescence mode and 658 nm for the reflectance confocal microscopy (RCM). The device provides a lateral resolution of 0.5 to and an axial resolution of 3 to with a maximum imaging depth of 200 to . A drop of fluorescein was applied, and after a 5-min penetration time, it was removed to enable the fluorescent measurements. A drop of immersion oil was applied to the surface of the skin in order to minimize spherical aberration. Then, an objective steel ring with an adhesive plastic ring was applied to the skin. First, a dermatoscopic image was obtained by using an integrated dermatoscopic camera. Subsequently, ultrasound gel was applied between the objective ring and the objective, and the LSM imaging process was started. Horizontal composite images of (confocal mosaics) were obtained on the surface in the fluorescence and reflectance modes to present the SC structure. Four representative images of the SC were taken in the fluorescent mode. Furthermore, sequential horizontal images of were obtained in reflectance mode on the areas of special interest, starting at the SC and continuing through the stratum granulosum (SG), stratum spinosum (SS), and the dermal-epidermal junction (DEJ) to the superficial dermis. The frame rate was nine frames per second. From the mosaic of the surface, the uniform arrangement of the furrows was determined. Zero was related to a normal uniform arrangement, 1 to slight derangement, and 2 to strong derangement. The skin surface dryness was determined using the fluorescent images of the SC. A score from 1 to 3 was developed; 1 = well-tended skin, 2 = slightly dry, 3 = strongly dry and disturbed corneocyte structure. The quality of the honeycomb structure of the SS was determined by a score, taking the first images of the SS; 0 = regular structure, 1 = slightly irregular, 2 = strongly irregular. The spongiosis is mainly an intercellular edema that is characterized by dark areas; 1 = no spongiosis, 2 = small spongiosis, 3 = strong spongiosis. Other criteria for inflamed skin are the visibility of the epidermal papillae or the elongated papilla. As most of the papillae were hardly visible, the size of the papillae was not estimated. The score used for the recognition of the papilla was 1 = clear visibility, 2 = difficult to recognize, 3 = no demarcation. 2.5.Subjective ParametersThe subjective parameters, like skin dryness, itching, and tension, were determined at the time points T0 (), T14 (), and T28 (); the volunteers were asked to estimate the parameters on a scale from 0 to 10, whereby 0 was related to no dryness, no itching, and no tension and 10 to the strongest effects of the parameters. 2.6.Biophysical Skin ParametersTo characterize the skin barrier function, the TEWL (Tewameter TM 300, Courage + Khazaka electronic GmbH, Cologne, Germany) was measured at the time points T0 (), T14 (), and T28 (). The corneocyte moisture was measured using the corneometer CM 825 (Courage + Khazaka electronic GmbH, Cologne, Germany) at the same time points. 2.7.Skin Lipid DeterminationIt is well known that skin lipids are strongly modified in the skin of atopic dermatitis patients.17 To determine the skin lipid profile, lipids were directly extracted from the upper skin using ethanol as described previously.18,19 Skin lipid analysis was performed at T0 and T28 in seven volunteers. From each skin area (diameter: ), the skin lipids were extracted. The following procedure was repeated in triplicate in order to obtain 9 mL of skin lipid extract. A centrifugation tube filled with 3 mL solvent was placed on the skin and the extraction was performed by shaking the arm with the tube for 1 min. This was repeated three times on the same measuring area. Extracts were dried under a continuous stream of nitrogen and reconstituted in n-hexane/methanol, (v:v). Samples were stored at until the analysis with high-performance thin-layer chromatography (HPTLC) was conducted. The chromatographic analysis of the lipid profile was performed according to the procedures of Farwanah et al. as well as Schellenberg and Kabrodt20,21 with slight modifications. For the analysis of the skin lipid profile, of the skin extracts and the standard solutions were applied as 6 mm bands under a flow of nitrogen on silica gel plates (HPTLC, Silica Gel 60, Merck, Darmstadt, Germany, , prewashed with methanol/chloroform (v:v, ) using an HPTLC autosampler (ATS4, CAMAG, Muttenz, Switzerland). For the analysis of the cream lipid profile, the cream was dissolved in n-hexane/methanol, (v:v). Chromatographic separation was completed with a nine-step gradient using an automated development chamber (AMD 2, CAMAG). Elution solvents were methanol (Roth, Karlsruhe, Germany), chloroform (Grüssing, Filsum, Germany), toluene (Th. Geyer, Renningen, Germany), and n-hexane (Merck). 4 M acetic acid (Roth) was used as a preconditioning agent to improve the band sharpness of oleic acid. Lipids were quantified after staining with 10% (Roth), 8% (Merck), aqueous solution, and charring at 180°C for exactly 8 min using a TLC Wavelength- Scanner 3 (CAMAG) equipped with WinCATS software (version 1.4.4.6337) at a wavelength of 600 nm. For this purpose, L--phosphatidylcholine (PC), cholesterol (Chol), oleic acid, glyceryl trioleate, cholesteryl oleate, sodium cholesteryl sulfate, squalene (SQ), sphingomyeline (SM) (all Sigma Aldrich), ceramide [NS] (Sederma, Nettetal, Germany), ceramide [NP] (Cer [NP]), and ceramide [AP] (Cer [AP]) (Evonik, Essen, Germany) were used. All standards were dissolved in methanol/chloroform (v:v, ). For the determination of single compounds in SC extracts, the standard ceramides Cer [NP] and Cer [AP] were used for the quantification of two substances, each described as Cer [NP1] and Cer [NP2], and Cer [AP1] and Cer [AP2]. The amounts of L--phosphatidylcholine and sphingomyeline were determined as a sum (sum PC+SM). Calibration was conducted three times using 10 calibration solutions of the standard mixture.22 2.8.Data Analysis and Statistical EvaluationThe statistical results were calculated using Microsoft® Office Excel for determining the mean values, balances, and standard errors as well as SPSS® 19 for Windows. The descriptive exploratory data analysis of the related variables was subjected to the Friedman test and the Wilcoxon test. A significant difference was found in the case of . 3.Results3.1.Laser Scanning MicroscopyThe SC structure representing the dryness was determined using the fluorescent images of the SC, which were measured at 488 nm (Fig. 1). A honeycomb pattern of the corneocytes, as shown in Fig. 1(b), represents well-tended skin. Most of the volunteers started with a dry and disturbed SC structure, visible by highly reflecting corneocytes, which are desquamated cells [Fig. 1(a)]. Partial depressions of the surfaces were also visible at the beginning of the study. Fig. 1Selected laser scanning microscopy (LSM) images of the stratum corneum (SC) of two volunteers in the fluorescence mode. An example of (a) a dry and disturbed corneocyte structure and (b) an almost well-tended skin. 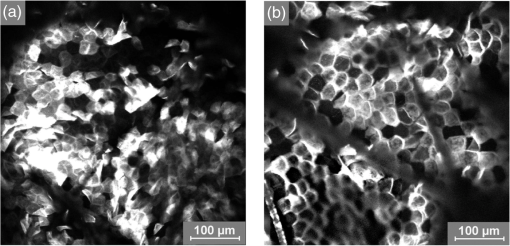 Using the reflectance mode at 658 nm, mosaics of the skin at the depth of the SS could be obtained (Fig. 2). The healthy skin showed a uniform arrangement of a polygon mesh of the furrows [Fig. 2(a)], which disappeared in atopic skin with moderate lesions [Fig. 2(b)]. In parts of the images of the lesions, disrupted skin was visible. Fig. 2Mosaics of the skin surface making the furrows on the surface visible. (a) An almost uniform arrangement of a polygon mesh of the furrows is visible. (b) Strongly atopic skin lost the uniform arrangement and mainly parallel furrows are visible. 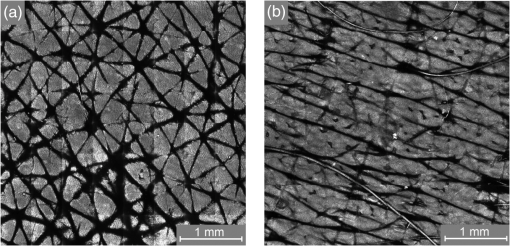 To obtain semiquantitative data, a score was developed for the skin surface dryness and the arrangement of furrows. The results of the evaluation are shown in Fig. 3. Fig. 3(a) score of SC dryness after analysis of the LSM images in the fluorescence mode: 1 corresponds to a well-tended skin, 2 to slightly dry, and 3 to strongly dry and disturbed corneocyte structure. (b) score of uniform arrangement of the furrows after analysis of the LSM images in the reflectance mode: 0 corresponds to a normal uniform arrangement, 1 to a slight derangement, and 2 to a strong derangement (). 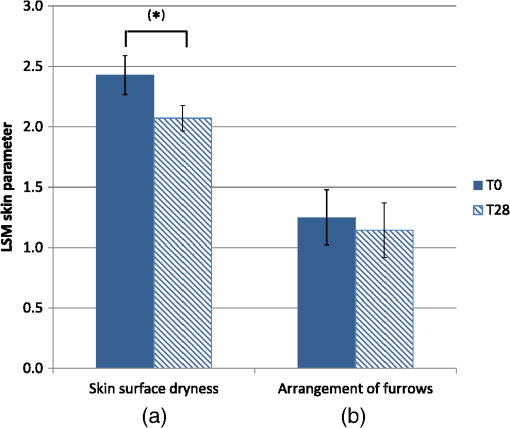 For most of the volunteers, a trend of reduction in the dryness was obtained from 2.4 to 2.0 after 28 days of application (Fig. 3, left). The uniform arrangement of the furrows did not change over the investigated time period (Fig. 3, right). After taking the mosaic images, z-scans in the reflectance mode were performed to investigate the epidermis until the DEJ zone. In Fig. 4, images of a healthy but dry skin and atopic skin with a moderate erythema are shown. Fig. 4Reflectance confocal microscopy (RCM) measurement of dry (upper panel) and moderately inflamed atopic skin (lower panel). 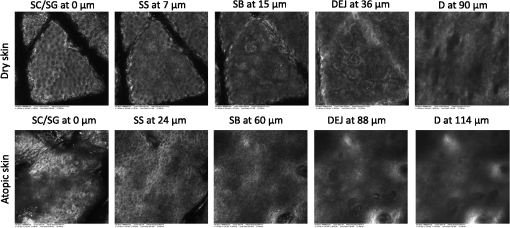 Healthy skin starts with an intact SC and SG with regularly formed cells in the SG. The atopic skin at this depth showed a disrupted skin barrier where parts of the cells of the SG lost their regular form. In healthy skin, the honeycomb structure in the SG and SS was visible, whereas in the atopic skin, spongiosis in terms of dark areas relative to the surrounding epithelium appeared. The intercellular spaces between the keratinocytes were larger than normal. In atopic skin, the SS was extended and, thus, the epidermis thickness was increased. In healthy skin, the top of the basal cell layer at was characterized by pigmented skin cells, which appeared brighter and surrounded the blood capillaries (arrow). The pigmented cells provided a higher reflective index and, therefore, appeared brighter. They were absent in inflamed atopic skin. Furthermore, the papillae consisting of basal cells were visible in intact skin at the DEJ zone. These may not be visible in inflamed atopic skin. The blood vessels appeared directly and were partially elongated, and a high throughput of erythrocytes could be observed during the z-scan measurement. The images seemed to be blurred. This continues in the dermis (D) down to a depth of , where elastic fibers and collagen should be visible as presented in intact skin at . Because of the missing structure of the papillae and the blurry images of the DEJ zone, the diameter of the papillae and epidermis thickness could not be estimated for inflamed atopic skin. To present this loss in structure at the DEJ in more detail, images before and after treatment of one volunteer starting with a superficial moderate eczema are presented in Fig. 5. The eczema was reduced after two weeks of treatment, and this was reflected clearly in the RCM images. Fig. 5RCM images of stratum papillae at T0 (a) and T28 (b) of one patient. The supposed blurring of the image is clearly visible in the left image. The bright papillae rims are almost lost and the cellular borders have vanished. The left image is taken at and the right one at , indicating the epidermal thickening. 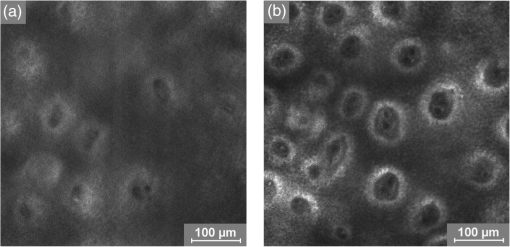 To get some statistical data for the honeycomb pattern and spongiosis of the SS, scores were developed. The mean values of these scores are shown in Fig. 6. Fig. 6Spongiosis and honeycomb structure of the stratum spinosum imaged by RCM of the inner forearm before and after the application of the ointment. Score spongiosis: 1 = no spongiosis, 2 = weak spongiosis, 3 = strong spongiosis. Score honeycomb structure: 0 = regular structure, 1 = slightly irregular, 2 = strongly irregular (). 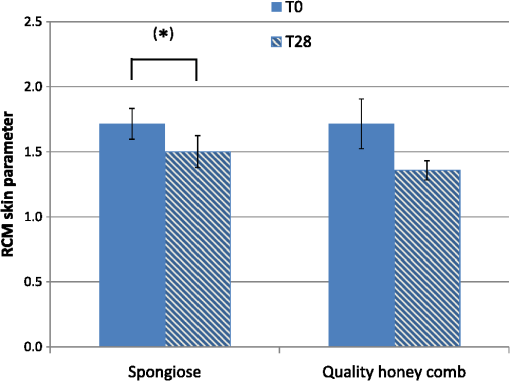 Most of the volunteers started with a relatively high irregular structure and a moderate spongiosis. After application of the investigational ointment, the spongiosis was significantly reduced and the quality of the honeycomb pattern was also improved (not significant). Going deeper into the skin, the bright papillae rimed at the DEJ in intact skin. In atopic skin, the bright papillary could be absent. The score values at the beginning at T0 were , which indicates that they are difficult to recognize and partly that there was no demarcation. At T28, the score improved to , which means that the papillae were still difficult to recognize but partly clearly visible. The changes were not significant. 3.2.Biophysical Skin ParametersThe skin parameters, TEWL and corneocytes moisture, relative to the initial values are shown in Fig. 7. The corneocyte moisture significantly increased between T0 and T14. From T14 to T28, the values were enhanced slightly (not significant). Fig. 7SC moisture and transepidermal water loss relative to the initial values () at three visits (*) [, ]. 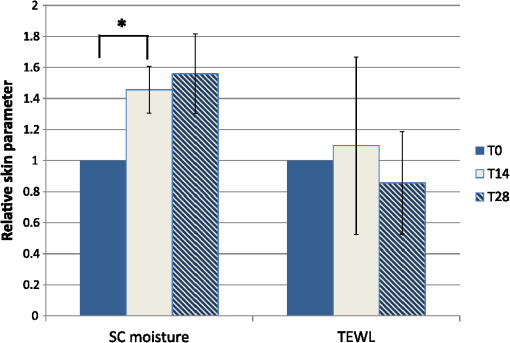 The TEWL values showed no significant changes over the study time. The values showed high variations, which was, inter alia, due to the genesis of new erythema at T28. The 15 SC lipids were analyzed before (T0) and after the application of the hyperforin-containing ointment at 28 days (T28). Several lipids increased after the application of the ointment, but only for Cer [NS], a trend () and for Chol, could a significant increase be observed. In Fig. 8, the lipid concentrations relative to the initial values (V2/V1) are shown. 3.3.Subjective Skin ParametersIn Fig. 9, the subjective parameters are shown. All parameters decreased significantly from T0 to T14 and improved further to T28, but not significantly. 4.DiscussionThe microscopic investigations with the LSM could demonstrate strong differences between healthy and inflamed atopic skin. The LSM images in the fluorescence mode showed distanced changes in the corneocytes pattern, which are partly in agreement with observations of Suihko and Serup who induced erythema by irritants.16 The increased roughness in atopic skin lesions was already observed using profilometry by Linde et al.23 The RCM measurements, in particular, can be used to characterize the status of the inflamed atopic lesion. The drastic changes in the polygon mesh of the furrows were clearly visible and have not been described in the literature thus far. The appearance of spongiosis, the loss of the honeycomb structure, acanthosis, and the absence of the bright papillary rims are described for other inflammatory skin disorders, such as acute allergic and irritant contact dermatitis, discoid lupus erythematosus, vitiligo, and psoriasis.13–15,24–27 Other well-known patterns, such as hyperkeratosis and dermal inflammation, were not detected in atopic skin lesions. The RCM results are in agreement with histological findings for AD.4 Besides the investigation of changes in the microstructure of the epidermis and dermis, the present study was aimed at correlating these parameters with classical skin parameters and the subjective parameters. Interestingly, the subjective parameters improved considerably after two weeks, and also a slight improvement at T28 was obtained. Such strong effects could not be found in any of the objective parameters. Only the SC moisture improved significantly after two weeks and the skin surface dryness and the spongiosis showed small but significant improvements after four weeks. The SC moisture and the skin surface dryness were expected to improve rapidly as the volunteers were not to use any cream three days prior to the initial visit. The measurements at visit 1 at T0, therefore, showed dry skin with a low SC moisture, and the subjective skin dryness, itching, and tenderness exhibited comparably high values. Spongiosis, the most important histological feature of acute eczema, clearly improved during treatment. This could be due to the improved barrier function of the SC subsequent to the application of the cream. In contrast, the results of the TEWL values did not show significant changes. This may be due to the small sample size and high error bars of the TEWL measurements. Also, although the volunteers did not apply the cream on the days of measurements, a barrier film could have been destroyed by putting the sensor on the skin. The LSM pattern in the deeper epidermis showed no or moderate improvement over the time of cream application. This illustrates that the inflammation was only slightly reduced in the upper layer of the epidermis but not in the deeper layer in the region of the DEJ. The nonrimmed and irregular or absent papillae combined with elongated capillaries gives strong evidence that the inflammation was still in progress. The complete reversion of inflammation may need more time. Comparable results were observed in the treatment of psoriasis where the surface of the skin looked macroscopically intact, while the inflammation was still visible in the DEJ using the RCM.13 This is of importance when therapeutic agents are used instead of daily skin care. In psoriatic patients, in whom the RCM measurements showed that no inflammation was visible anymore, no recurrence had been observed within the next six months until the end of the study. In other patients, the normal recurrence time was three months. The skin lipids improved for the subjects with dry to atopic skin with significant changes for Chol and Cer [NS]. However, this is in agreement with a study on healthy volunteers who applied the ointment for four weeks.22 Similarly, after four weeks, the Cer [NS] and Chol and cholesterol-oleate increased. In contrast to the study on healthy volunteers, in the present study, the phosphatidylcholine and sphinogmyelines (sum PC + SM), and Cer [AP2] increased after treatment of atopic skin, too, even if that increase was not significant. An increase in Chol and cholesterol-oleate was detected, whereas SQ, a precursor of cholesterol biosynthesis, also did not change. Hence, a four-week application of cream influenced the Chol and cholesterol fatty acid ester biosynthesis. This indicates a contribution toward an optimized skin barrier function. Studies with model SC lipid membranes showed that the sphingomyelin/ceramide ratio is an especially important aspect during skin barrier development. Sphingomyeline are enzymatically converted to Cer [NS] and Cer [AS] and phosphocholine by sphingomyelinases.28 In patients suffering from atopic dermatitis, a reduced activity of these enzymes has been reported, and a decreased amount of Cer in the SC lipids has been detected.29 While healthy membranes consisting of ceramides, cholesterol, free fatty acids, and cholesterol sulfate form a good natural barrier toward water, membranes with higher proportions of SM control the water loss less efficiently. This means that the importance of the natural release of Cer from SM is preventing desiccation.28 Therefore, the results of the improved skin lipids are also in agreement with the decreased SC dryness, the improved SC arrangement resulting from the fluorescent LSM investigations, and the increase in SC moisture. Because the study was not placebo controlled, it cannot be ascertained if the extract was the active compound or the vehicle. 5.ConclusionIn summary, our data obtained by biophysical methods, in particular LSM, have shown that atopic skin has distinct features compared to normal skin. Treatment with a hyperforin-rich ointment resulted in a reduction of the skin surface dryness and in an improvement of the SC moisture. The skin lipids and the subjective skin parameters indicate that the barrier is stabilized and improved by the ointment. But in contrast to the skin surface, the inflammation in the deeper epidermis/dermis still exists in most of the cases, which could be clearly shown by the RCM measurements. Therefore, RCM measurements could be useful for investigating the progress in the treatment of atopic dermatitis. AcknowledgmentsThis work was supported by the Federal Land of Berlin within the Program to Promote Research, Innovation and Technologies (ProFIT, Grant No. 10148423) for granting subsidies from the innovation promotion fund, with cofinancing by the European Fund for Regional Development (ERDF). ReferencesM. Scharioet al.,
“Children with dry skin and atopic predisposition: daily use of emollients in a participant-blinded, randomized, prospective trial,”
Skin Pharmacol. Physiol., 27
(4), 208
(2014). http://dx.doi.org/10.1159/000360546 SPPKE6 1660-5527 Google Scholar
A. Mariniet al.,
“Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: a randomised, comparator-controlled, intra-individual double-blind, multi-center trial,”
Skin Pharmacol. Physiol., 27
(2), 57
–65
(2014). http://dx.doi.org/10.1159/000351381 SPPKE6 1660-5527 Google Scholar
D. Schweigeret al.,
“Efficacy of a new tonic containing urea, lactate, polidocanol, and glycyrrhiza inflata root extract in the treatment of a dry, itchy, and subclinically inflamed scalp,”
Skin Pharmacol. Physiol., 26
(2), 108
–118
(2013). http://dx.doi.org/10.1159/000348473 SPPKE6 1660-5527 Google Scholar
N. Novak,
“New insights into the mechanism and management of allergic diseases: atopic dermatitis,”
Allergy, 64
(2), 265
–275
(2009). http://dx.doi.org/10.1111/all.2009.64.issue-2 LLRGDY 0105-4538 Google Scholar
W. Abramovits,
“Atopic dermatitis,”
J. Am. Acad. Dermatol., 53
(1), S86
–93
(2005). http://dx.doi.org/10.1016/j.jaad.2005.04.034 JAADDB 0190-9622 Google Scholar
J. Verhagenet al.,
“Absence of T-regulatory cell expression and function in atopic dermatitis skin,”
J. Allergy Clin. Immunol., 117
(1), 176
–183
(2006). http://dx.doi.org/10.1016/j.jaci.2005.10.040 JACIBY 0091-6749 Google Scholar
J. J. Russell,
“Topical tacrolimus: a new therapy for atopic dermatitis,”
Am. Fam. Physician, 66
(10), 1899
–1902
(2002). AFPYAE 0002-838X Google Scholar
D. LunterR. Daniels,
“In vitro skin permeation and penetration of nonivamide from novel film-forming emulsions,”
Skin Pharmacol. Physiol., 26
(3), 139
–146
(2013). http://dx.doi.org/10.1159/000348464 SPPKE6 1660-5527 Google Scholar
C. M. Schemppet al.,
“Antibacterial activity of hyperforin from St. John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria,”
Lancet, 353
(9170), 2129
(1999). http://dx.doi.org/10.1016/S0140-6736(99)00214-7 LANCAO 0140-6736 Google Scholar
C. M. Schemppet al.,
“Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis,”
Oncogene, 21
(8), 1242
–1250
(2002). http://dx.doi.org/10.1038/sj.onc.1205190 ONCNES 0950-9232 Google Scholar
M. Ulrichet al.,
“In vivo detection of basal cell carcinoma: comparison of a reflectance confocal microscope and a multiphoton tomograph,”
J. Biomed. Opt., 18
(6), 061229
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061229 JBOPFO 1083-3668 Google Scholar
P. Guiteraet al.,
“In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases,”
J. Invest. Dermatol., 132
(10), 2386
–2394
(2012). http://dx.doi.org/10.1038/jid.2012.172 JIDEAE 0022-202X Google Scholar
R. Archidet al.,
“Confocal laser-scanning microscopy of capillaries in normal and psoriatic skin,”
J. Biomed. Opt., 17
(10), 101511
(2012). http://dx.doi.org/10.1117/1.JBO.17.10.101511 JBOPFO 1083-3668 Google Scholar
S. Astneret al.,
“Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis,”
J. Invest. Dermatol., 124
(2), 351
–359
(2005). http://dx.doi.org/10.1111/j.0022-202X.2004.23605.x JIDEAE 0022-202X Google Scholar
S. Kolleret al.,
“In vivo reflectance confocal microscopy of erythematosquamous skin diseases,”
Exp. Dermatol., 18
(6), 536
–540
(2009). http://dx.doi.org/10.1111/exd.2009.18.issue-6 EXDEEY 0906-6705 Google Scholar
C. SuihkoJ. Serup,
“Fluorescence confocal laser scanning microscopy for in vivo imaging of epidermal reactions to two experimental irritants,”
Skin Res. Technol., 14
(4), 498
–503
(2008). http://dx.doi.org/10.1111/srt.2008.14.issue-4 0909-752X Google Scholar
J. van Smedenet al.,
“The important role of stratum corneum lipids for the cutaneous barrier function,”
Biochim. Biophys. Acta, 1841
(3), 295
–313
(2014). http://dx.doi.org/10.1016/j.bbalip.2013.11.006 BBACAQ 0006-3002 Google Scholar
M. C. Meinkeet al.,
“In vivo photoprotective and anti-inflammatory effect of hyperforin is associated with high antioxidant activity in vitro and ex vivo,”
Eur. J. Pharm. Biopharm., 81
(2), 346
–350
(2012). http://dx.doi.org/10.1016/j.ejpb.2012.03.002 EJPBEL 0939-6411 Google Scholar
M. C. Meinkeet al.,
“Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids,”
Eur. J. Pharm. Biopharm., 84
(2), 365
–373
(2013). http://dx.doi.org/10.1016/j.ejpb.2012.11.012 EJPBEL 0939-6411 Google Scholar
H. Farwanahet al.,
“Improved procedure for the separation of major stratum corneum lipids by means of automated multiple development thin-layer chromatography,”
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 780
(2), 443
–450
(2002). http://dx.doi.org/10.1016/S1570-0232(02)00654-2 1570-0232 Google Scholar
I. SchellenbergK. Kabrodt,
“Optimierung einer AMD 2-Methode zur Bestimmung von Stratum corneum-Lipiden,”
CAMAG Bibliography Service, 105 10
–12
(2010). Google Scholar
S. F. Haaget al.,
“Enhancement of skin radical scavenging activity and stratum corneum lipids after the application of a hyperforin-rich cream,”
Eur. J. Pharm. Biopharm., 86
(2), 227
–233
(2014). http://dx.doi.org/10.1016/j.ejpb.2013.06.016 EJPBEL 0939-6411 Google Scholar
Y. W. LindeA. BengtssonM. Loden,
“‘Dry’ skin in atopic dermatitis. II. A surface profilometry study,”
Acta Derm. Venereol., 69
(4), 315
–319
(1989). ADVEA4 0001-5555 Google Scholar
M. Ardigoet al.,
“Interest of reflectance confocal microscopy for inflammatory oral mucosal diseases,”
J. Eur. Acad. Dermatol. Venereol.,
(2014). http://dx.doi.org/10.1111/jdv.12540 JEAVEQ 0926-9959 Google Scholar
M. Ardigoet al.,
“Preliminary evaluation of vitiligo using in vivo reflectance confocal microscopy,”
J. Eur. Acad. Dermatol. Venereol., 21
(10), 1344
–1350
(2007). http://dx.doi.org/10.1111/jdv.2007.21.issue-10 JEAVEQ 0926-9959 Google Scholar
S. Gonzalezet al.,
“Allergic contact dermatitis: correlation of in vivo confocal imaging to routine histology,”
J. Am. Acad. Dermatol., 40
(5), 708
–713
(1999). http://dx.doi.org/10.1016/S0190-9622(99)70151-9 JAADDB 0190-9622 Google Scholar
K. Swindellset al.,
“Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo,”
J. Am. Acad. Dermatol., 50
(2), 220
–228
(2004). http://dx.doi.org/10.1016/j.jaad.2003.08.005 JAADDB 0190-9622 Google Scholar
P. Pullmannovaet al.,
“Effects of sphingomyelin/ceramide ratio on the permeability and microstructure of model stratum corneum lipid membranes,”
Biochim. Biophys. Acta, 1838
(8), 2115
–2126
(2014). http://dx.doi.org/10.1016/j.bbamem.2014.05.001 BBACAQ 0006-3002 Google Scholar
J. M. Jensenet al.,
“Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis,”
J. Invest. Dermatol., 122
(6), 1423
–1431
(2004). http://dx.doi.org/10.1111/jid.2004.122.issue-6 JIDEAE 0022-202X Google Scholar
BiographyMartina C. Meinke studied chemistry at the Free University of Berlin and did her PhD degree in 1994. After the management of a laboratory for environmental analysis, she switched to the medical diagnostics field. In 2006, she finished her postgraduate studies of medical physics and since 2007 she has been an assistant professor at the Department of Dermatology of the Charité-Universitätsmedizin Berlin. Her research fields are optical and spectroscopic methods to determine skin physiological parameters. Heike Richter was trained as a communications engineer from 1976 to 1979. From 1990 to 1991, she received her training as a technical assistant (physics). From 1986 to 1991, she was affiliated with the Academy of Sciences, Central Institute of Optics and Spectroscopy. Since 1992, she has been working with the Center of Experimental and Applied Physiology of the Department of Dermatology at the Charité-Universitätsmedizin Berlin. Her main focus is on optical and spectroscopic investigations. Anke Kleemann is a biologist who has been working in the pharmaceutical industry with focus on phytotherapeutical projects—especially St. John’s wort preparations—for more than 20 years. Recently, she was appointed as a manager of the Innovations Division at Klosterfrau Healthcare Group. Juergen Lademann studied at the Moscow State University, Faculty of Physics, Quantum Electronics Department, where he completed his master's degree. In 2000, he was appointed as a professor of dermatology at the Charité-Universitätsmedizin Berlin. He is the director of the Center of Experimental and Applied Cutaneous Physiology at the Charité and editor of the peer-reviewed journal Skin Pharmacology and Applied Skin Physiology. Kathrin Tscherch is a postdoctoral research associate in the research group of Dr. Michael Best in the Department of Chemistry at the University of Tennessee in Knoxville, Tennessee, USA. She is currently performing metabolic labeling of target lipids in cancer cells to study their function in disease onset. She is also researching cell-surface recognition events with particular interest in understanding lipid-binding proteins’ involvement in cellular events and providing approaches for therapeutic intervention. Sascha Rohn is a full professor of food chemistry at the University of Hamburg, Hamburg School of Food Science, Germany. His group is dealing with the analysis of secondary plant metabolites and their antioxidant activity, but especially with the reactivity and stability of these bioactive compounds. The group’s aim is to identify degradation products that serve as process markers during food/feed processing or as biomarkers in nutritional physiology. Christoph M. Schempp is a head of the Research Centre Skintegral® and professor of dermatology at the Department of Dermatology, University Medical Center Freiburg, Germany. His recent position is head of the inpatient department and phototherapy unit, and head of the inflammatory skin diseases study center. His scientific main focus is natural compound research, dermopharmacy, photodermatology, and inflammatory skin diseases. |