|
|
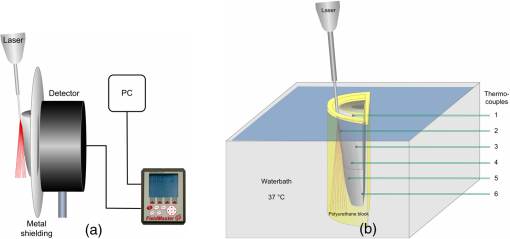
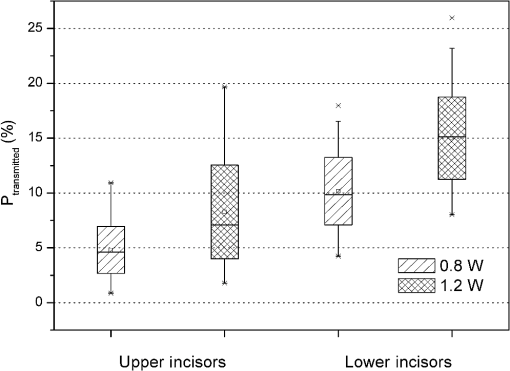
1.Introduction1.1.Diode Lasers in PeriodonticsThe treatment of periodontal diseases requires the removal of the bacterial biofilm for preventing the progression of attachment losses.1,2 Mechanical debridement of root surfaces with hand instruments and ultrasonic systems is a generally accepted method.2,3 Nevertheless, these techniques cannot guarantee complete removal of calculus and bacterial deposits.4,5 To overcome this drawback, the adjunct use of lasers in periodontal therapy has been the focus of numerous studies. Although some authors have attained promising clinical outcomes using Nd:YAG- and semiconductor lasers,6–9 others did not find beneficial results.10 In clinical applications, a laser system has to achieve appropriate bactericidal effects without causing potentially harmful heat. In 1965, Zach and Cohen11 introduced the still valid 5.6°C level for critical temperature increase. Pulpal damage can occur by transmission of irradiation through dentine and subsequent absorption in the pulp tissue. Near-infrared-wavelengths are especially subject to low absorption in dental hard tissues.12,13 On the other hand, absorbed light converted into thermal energy may cause deleterious effects. Laser tissue interactions strongly depend on the wavelength of the applied laser system. For this reason, therapeutic settings have to be redefined for new dental lasers. Diodes with 810 and 980 nm have been investigated in the past 15 years.10 However, there is still no clinical evidence for the more recently introduced 940-nm wavelength that shows a different absorption in water containing tissue. Even though the use of 810 and 980 nm has become widespread in clinical practice, only a very few studies on the potential harmful effects can be found.14–18 In particular, continuous wave (CW) mode has solely been the focus of Kreisler et al.14,15 However, studies dealing with pulsed mode16–18 cannot be compared as a pulsed beam generates significantly lower temperature rises.19 Additionally, the methodologies of the existing safety studies are often far from in vivo conditions. The common application of a therapeutic method without scientific evidence regarding its safety is negligent and ethically unacceptable. Potential side effects should be studied prior to its introduction. 1.2.Model DevelopmentSince temperatures cannot be measured in vivo, appropriate in vitro models are required. However, heat transfer inside the pulp and its surroundings is very complex and several processes have to be accounted for in these experiments. Particularly, the ambient medium type and temperature, the simulation of blood circulation, the positioning of thermocouples (TCs) and the use of split or intact teeth need to be carefully considered. Many authors performed measurements without any surrounding ambient medium directly at room temperature17,19 or inside a 37°C warm water bath to simulate body temperature.14 The low thermal conductivity of air creates an unrealistic heat up in those setups. A thermal bath, however, generates an immense cooling effect due to the large heat capacity of water. An important part of the present study was to develop a periodontal pocket model that avoids these shortcomings. Several reproducible TC positions allow the investigation the spatial temperature distribution as well as the influence of different remaining dentine thicknesses (RDTs). El Yazami et al.20 introduced an interesting model contour. However, their silicon block surrounding the root can only be utilized for the application of the photosensitizer in photodynamic therapy. A simulation of heat transfer to the surrounding tissues was not achieved. The authors intended to find a more suitable material for the tooth surrounding medium. Thermodynamic properties of a tissue depend on the thermal conductivity, density, and heat capacity.21 Bone has a thermal conductivity of 0.58 to , a density of , and a heat capacity of 1.6 to .22 In this respect, polyurethane casting resin was the most appropriate material.23 Although it cannot simulate the structure of human bone, the polyurethane block provides a limited isolating effect against the water bath and allows conduction of the generated heat. Even if the pulpal blood flow can add a certain cooling effect, its simulation can be neglected in vitro. In the case of short exposure times and low power applied, convection plays a minor role in heat transport in tissues.24,25 One or two TCs are commonly placed inside the pulp chamber or the root canal of intact teeth to detect pulp temperature,25,26 even though several limitations like an unexact positioning of the TCs, the influence of different RDTs on temperature rise, nonreal-time measurements at different positions, and a possible gap between TC and dentine, probably lead to measurement errors.27 To exclude thickness variations, the roots have to be sectioned prior or after the experiments.28,29 Although these modifications in geometry and mass have an influence on heat conduction27 and heat capacity, no significant differences in the temperature rise between split teeth and intact teeth have been reported in the literature.30,31 Biological variation was minimized by removing the crowns and apices. These adjustments ensured reduced scattering of the measured values and created a desired worst case scenario. During model development, numerous influencing factors were examined in comparative experiments. To the author’s knowledge, a similar setup that considers several factors in one model was not found yet. 1.3.ObjectiveThe aim of the present study was to analyze thermal effects during diode laser assisted periodontal treatment. For this purpose, a periodontal pocket model with a modified methodology for measuring temperatures and simulating the physiological heat transfer was developed. The model was used to examine laser settings regarding the applied power and the irradiation time with respect to pulp safety. Parameters were investigated for the thickest dentine walls in upper incisors and the thinnest walls in lower incisors in terms of light transmission and heat generation on the root canal surface. In addition, the influence of transmission on temperature elevation was assessed. 2.Materials and Methods2.1.Sample PreparationExtracted upper and lower human incisors stored in 0.9% saline solution were used in this study. Roots were cleaned mechanically by scaling and root planing (SRP) before the crowns were removed at the cemento enamel junction (CEJ) with a low-speed diamond saw (Exakt Apparatebau, Norderstedt, Germany). All samples were shortened to a length of 10 mm from CEJ. A rotary file system (VDW Gold and files, VDW GmbH, München, Germany) was used to remove pulp tissue and to give the root canals a uniform shape (upper incisors: ISO 30; 6 deg; 18 mm; lower incisors: ISO 20; 6 deg; 16 mm). Canals were rinsed with NaOCl (2.5%) and ethylenediamine tetraacetic acid (EDTA) (20%), and finally with a 2-ml flush of NaCl (0.9%). Afterward, roots were longitudinally sectioned with a band saw. Ten upper and ten lower samples were selected by mass. Upper incisors: , lower incisors: . Based on a 6-mm periodontal pocket, the irradiated surface was for upper and for lower incisors. 2.2.Laser SystemAn EZlase 940-nm diode laser (Biolase Inc., Irvine, California) with a Perio Tip E 3–7 mm was used in this study. Irradiations were performed with the following settings (CW): The fiber was guided parallel to the root surface in a constant horizontally swinging movement (). The entire surface was passed in a meandering pattern, thrice mesio-distally and simultaneously from apical to coronal. To measure maximum transmission, the fiber was moved just along the center axis of the root in transmission experiments. According to the mean surface, in lower incisors were irradiated and in upper incisors . For the fidelity of the experiments, the whole study was done by the same investigator. 2.3.Experiment I: Examination of the Transmitted PowerRoot sections were fixed with adhesive wax on a metal plate containing a slot in the middle, then positioned in front of a power detector (LM 3, Coherent, Inc., Auburn, California) covering its surface [Fig. 1(a)]. For better transmission, the samples were taken from the storage solution immediately prior to the experiment to ensure complete hydration.32 Additionally, surfaces were moistened to simulate the sulcus fluid. The detector head was connected to a power/energy meter (Fieldmaster GS, Coherent, Inc., Auburn, California). Measurements were recorded by data acquisition software (LabVIEW 2012, National Instruments Corp., Austin, Texas). Six repetitions for each power setting were accomplished. The actual emitted power remained stable at of the nominal value of 1 W. Thus, an emitted power of 0.8 and 1.2 W (80.4% of 1.0 and 1.5 W) was used for further analysis. The total transmitted energy was calculated and the result divided by irradiation time to get the mean transmitted power. 2.4.Experiment II: Temperature MeasurementsFor positioning the TCs, holes were drilled on the inner root canal surface with different RDTs (Fig. 2) using a 0.5-mm spiral drill (Proxxon GmbH, Föhren, Germany) mounted on a precision milling device (Girrbach Royal EM Parallelometer, Girrbach AG, Koblach, Austria). A 6-mm-deep and 1-mm-thick pocket was blocked out with wax as well as the root canal. The samples were fixed in a silicon mold that was filled with polyurethane mixed in a ratio of 6:1 (ISO-PUR K 760, ISO ELEKTRA, Elze, Germany). This casting resin has a thermal conductivity of and a density of . The back side of the block was closed with a cover of the same material containing holes and six K-type TCs (5TC-TT-KI-36-1M, Omega Engineering Inc., Stamford, Connecticut) were fixed. Four nails embedded in this cover fitted in holes in the model and ensured a precise and reproducible position. Edges between root canal and resin were sealed with adhesive wax. The root canal walls were covered with a thin layer of thermal conductance paste (, Fischer WLPF 50, Fischer Elektronik, Lüdenscheid, Germany). TCs were fixed and gaps sealed with adhesive wax to avoid water leakage. Figure 3(a) shows the final model. TC positions were confirmed radiographically [Fig. 3(b)]. Pockets were filled with saline solution and the models immersed in a water bath [Fig. 1(b)]. Temperature was set to at position 2, according to subgingival temperature in 3-mm-deep pockets.33 Values were recorded by a USB data acquisition module (OM-USB-TC, Omega Engineering Inc., Stamford, Connecticut). The actual power remained stable at of the nominal value for 1 W, so the emitted power was set on 0.87 and 1.3 W. Fifteen repetitions for each power setting were performed. From the recorded values, was calculated and Pearson’s correlation coefficient was used to verify any existing relation. 3.Results3.1.Transmission MeasurementsIn general, the transmission was quite low. A large scatter of the measured data was observed (Fig. 4). Table 1 illustrates the average transmitted power. At an actual emitted power of 0.8 W, 4.8% () of the irradiation was transmitted in upper incisors, at 1.2 W, the mean value increased by a factor of 1.7 (). Thinner lower incisors revealed values approximately twice as high compared with upper ones for 1 W () and 1.5 W (). This was consistent with the values of wall thickness that were 1.5 to 2 times greater in upper than in lower incisors (Fig. 2). The results indicate an exponential relation between wall thickness and transmission (, ; ) as well as a positive relation between applied power and transmission (Fig. 4). Table 1Transmitted power in upper and lower incisors.
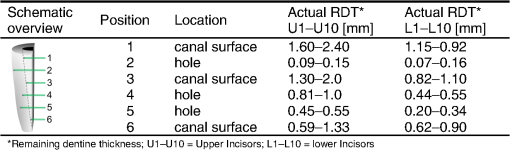
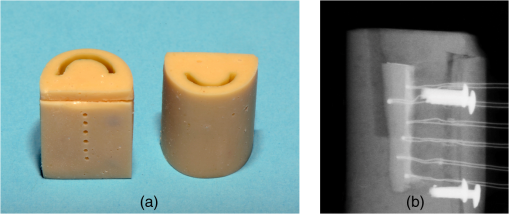
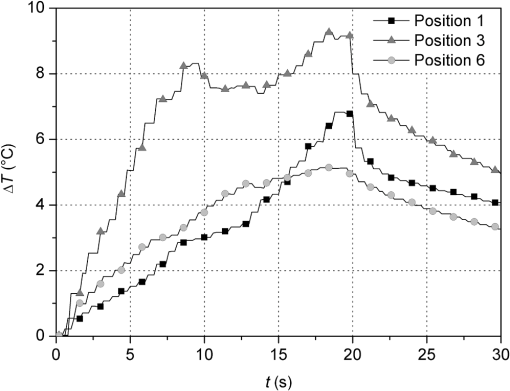
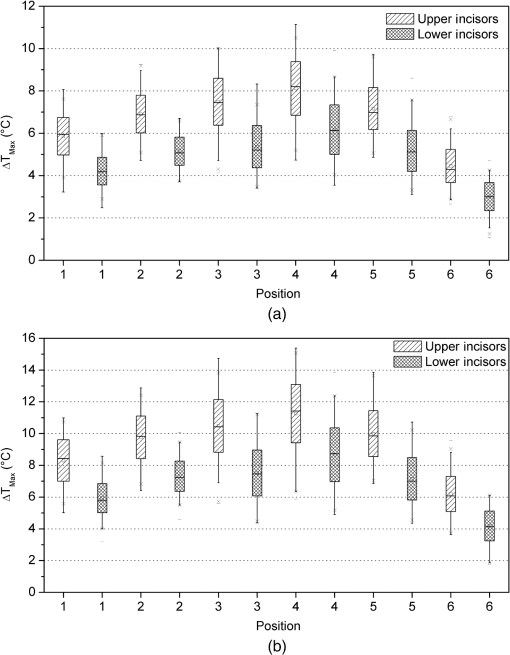
3.2.Temperature MeasurementsImmediately with the start of laser irradiation, temperatures rose at all measuring points within less than 1 s (Fig. 5). The maximum increases were detected at TC 3, 4, 5 (Fig. 2). Positions 1, 2, and 6 were located more peripherally and showed smaller gradients. Local maxima of the graphs follow the motion of the laser fiber tip. The highest temperature rises were recorded during the first 10 s of irradiation. Within the following 10 s, a slight further increase of 1°C to 2°C in the middle root area of the upper incisors was noticed. Regarding the spatial temperature distribution, the highest values for were observed in the middle of the root (Fig. 6). Despite the outstanding variation in the RDT at positions 2 to 5 (Fig. 2), differed by just 1.2°C to 1.5°C. In two-thirds of all measurements, TC 3 detected higher temperatures than TC 2. Apparently, the position of the TC has more influence on than the RDT. A correlation between and RDT could not be found as was between and (; see also Fig. 2, Fig. 6). Table 2 shows the values for at all positions. Regarding pulp safety, positions 1, 3, and 6 are most important because they were located on the inner root canal wall. In upper incisors, irradiation with 0.87 W generated temperature rises of 7.49°C and 10.49°C for 1.3 W, respectively, at position 3. Values for lower incisors were consistently lower by 1.5°C to 3°C for both power settings (Fig. 6, Table 2). Under the chosen conditions, scatter within the data was acceptable (Table 2). Table 2Maximum temperature rise (ΔTmax) in upper and lower incisors at different positions.
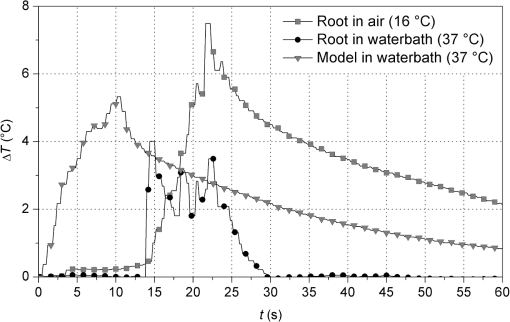
There was no carbonization of the root surfaces in either experiment. 4.Discussion4.1.Experiment I—TransmissionLight that is neither reflected at the root surface nor absorbed, spreads out in the hard tissue. As in all biological tissues, significant scattering occurs,34 mainly induced by dentine tubules whereas crystals and collagen fibers have minor influence.35 Two existing theories describe the anisotropic properties of dentine. Many authors consider the dentinal tubules as an optical fiber,30,32,36,37 based on total internal reflection due to differing refractive indices of peri- and intertubular dentine. Scattering leads to coupling of a certain portion of light into the tubules.36 However, results of Kienle et al.38 indicate multiple scattering by the cylindrical tubules instead of light guidance causing anisotropic propagation. The present study revealed large scatter of the data in repeated measurements and also between the samples. Especially, deviation in repeated measures is likely due to the small signal value that frequently lay close to the detection limit of the measuring head, mainly concerning the upper incisors. Additional bias might be due to manual movement of the laser. The greater dispersion of the values in the upper incisors can be explained by larger deviations in wall thicknesses than in lower incisors (Fig. 2). The measured values are below those of other authors for 1064 nm. Lenz and Gilde39 observed 10% to 35% transmission in halved teeth, Behrens et al.40 reported 35% in 1-mm-thick dentine slices. Deviations of the present study could be due to different methodologies such as a 90-deg working angle, the use of plane slices instead of a curved tooth surface or a measuring head-related gap between the detector surface and the sample in the present setup, which potentially resulted in a loss of a portion of diffusely scattered light. A wavelength effect cannot be the reason, since the absorption spectra for water and hydroxyapatite show just small differences between 940 and 1064 nm.12 Although anisotropic light propagation itself was found to be independent of the incident angle,36,37 differences between a 5-deg working angle and maximum transmission at 90 deg, compared in the preliminary experiments, were detected. Transmission increases at 90 deg because surface reflection and scattering decreases and a larger portion of irradiation is directed to the pulp. Particularly, in upper incisors these factors favor transmission (2.5 to 3 fold), whereas lower incisors show just a 1.6-fold increase at 90 deg (Table 1). A strong negative correlation between RDT and transmitted power was observed, which illustrates the dependence of absorption and scattering on the material thickness. Altogether, variations of the wall thickness between upper and lower incisors seem to have a larger influence on transmission than changes in the angle of the applied laser beam. According to these results, transmission, especially in thicker teeth, can be expected to have just a slight effect on heat generation in the pulp. 4.2.Experiment II—Temperature MeasurementsFrom the plot of temperature increase as a function of time (Fig. 5), strong light scattering can be concluded to occur due to the following reasons. In many test runs, temperatures started to rise immediately after the beginning of laser exposure, indicating absorption of transmitted light by the TCs as reported by Yu et al.13 But even those TCs that were definitely not directly exposed during the first half of irradiation period detected simultaneous, albeit smaller temperature increases. Furthermore, all temperatures dropped immediately when the laser was off. If heat generation had been due to conduction alone, a delay of a few seconds would have been detected, as was observed in the preliminary experiments. Scattered light also leads to absorption and diffuse transmission in the coronal parts of the root. Although the measured values are quite low (Fig. 4, Table 1), transmission has a major contribution to heat generation in the pulp. This is consistent with absorption spectra for water and hydroxyapatite.12 The abovementioned gap between the detector surface and the sample probably led to some losses in the recorded transmitted power. The results of Zach and Cohen,11 that were later verified by some additional studies,41–43 indicate that a temperature rise of 5.6°C leads to pulp necrosis in 15% of teeth. Other authors who conducted different methodologies, such as slower heating up of the teeth or the use of juvenile well-vascularized teeth, provide higher limits.44–46 In dental laser application, large energies are applied during relatively short exposure times, comparable to Zach and Cohen’s study that used exposure times between 5 and 20 s. Therefore, the limit established by them should be maintained, at least for safety reasons. The polyurethane model developed in this study provided temperature measurements that are neither high like experiments conducted at ambient temperature nor low as those carried out directly inside a water bath (Fig. 7), and thus allows a more realistic comparison to an in vivo situation. Although the critical 5.6°C threshold was exceeded at position 3 by 2°C to 5°C, one has to consider that this is a worst case scenario and these temperatures will not be achieved in vivo. Several modifications like a strongly reduced mass compared to an intact tooth, the absence of the heat capacity of the pulp tissue and, albeit small, convection due to blood flow decrease the total heat capacity of the sample. Additional substance losses caused by the endodontic treatment and widening of dentinal tubules by EDTA had a further effect. Heat transfer to the adjacent tissues is lower because of the smaller surface of the sample and the surrounding material. Although having similar thermal properties as bone, polyurethane does not have its porosity and water content. Furthermore, the use of TCs implies direct absorption and an artificial heat capacity that results in higher temperatures recorded.47,48 All these influencing factors, as well as minor ones like dentine liquor, saliva, and breathing air, favor the heat transport in vivo. Against this background, exceeding the limit by 2°C appears small. Hence, the chosen settings 1 W, 10 s and 1.5 W, 10 s for lower incisors and 1 W, 20 s for upper incisors can be considered safe. On the other hand, temperature rises of 10.5°C induced in the upper incisors by 1.5 W appear potentially harmful. However, higher settings (, 30 s), have already been applied clinically with success by Kamma et al.6 using a 980-nm diode laser. Their in vivo study demonstrates that either our model reaches its limits in simulating heat transport or the physiological heat tolerance of the pulp is greater than expected. One has to consider that the wavelength of 940 nm is strongly absorbed in hemoglobin.12 Following irradiation with an 810-nm diode laser, Kreisler et al.15 observed carbonization of root surfaces that had been wetted with blood. Therefore, the authors recommend performing SRP and lasing in different sessions. On the other hand, a slight bleeding that may occur during laser treatment limits transmission toward the pulp. In this case, the energy is already absorbed in the periodontal tissues that show greater heat tolerance than the pulp.49 As mentioned above, there are few similar studies investigating pulp temperature during adjunctive laser treatment in periodontics. Nonetheless, a direct comparison with our results cannot be done due to different methodologies. Using an 810-nm laser, Kreisler et al.14 suggested significantly lower settings for punctual irradiation of the root surface at a 90-deg angle, which cannot be realized in practical applications. Others evaluated higher power densities and exposure times to be acceptable.17 However, repeat wave mode, such as used in that study, leads to significantly less heating compared to CW.19 Highest temperatures were detected in the middle part of the root where the largest energy input occurred due to the movement of the fiber tip. In this regard, no clear relation was found between temperature distribution and the S-shaped course of the dentinal tubules in the cervical root area50 that possibly direct the incident light further apically. It is not the RDT, but the position within the irradiated surface, which determines the most susceptible area for pulp damage within a tooth. An inversely proportional relationship between RDT and temperature rise has been presented,51 implying that the wall thickness has a decisive influence.13 However, these authors applied static irradiation without any movement of the beam. Of course, different tooth types require irradiation with different energies, but differences of wall thickness within a tooth are irrelevant for clinical practice. In upper incisors, twice the amount of energy (10 J versus 20 J) generated temperatures that were higher by just 1.5°C to 3°C compared with lower incisors, since the heat capacity of upper incisors is greater (1.5-fold mass). The fact that temperatures just rise slightly after half of the total irradiation time is based on the proportional relation between thermal conductivity and temperature gradient. The latter decreases during irradiation, i.e., the greatest rises can be observed at the beginning. A linear correlation between applied power and temperature ( for upper and 0.65571 for lower incisors; ) was observed as expected.29 5.ConclusionWithin the limits of this in vitro study, the middle-third of the root could be identified to be the most susceptible area for pulp damage. In upper incisors, 1 W, 20 s generates acceptable temperature rises whereas 1.5 W, 20 s is potentially harmful to the pulp. For lower incisors, 1 W, 10 s as well as 1.5 W, 10 s can be considered safe. A correlation between RDT and temperature rise could not be found. With respect to transmission experiments, a strong conclusion cannot be drawn due to large scatter in the data. The introduced periodontal pocket model allows for well-reproducible, highly reliable, and spatially resolved temperature measurements. It provides a more realistic simulation of heat transfer to the adjacent tissues compared to the existing setups and can be suggested for further investigation on pulp temperature, irrespective of whether complete teeth or tooth sections are embedded. However, a certain heat storage in the model cannot be avoided. Future studies that implement the porous properties of bone and verify the bactericidal effect of the experimental settings should be investigated before bringing these parameters into clinical trials. AcknowledgmentsThe authors would like to thank Dr. Nicole Heussen from the Institute of Medical Statistics at RWTH Aachen University for valuable discussions. ReferencesT. J. O’Leary,
“The impact of research on scaling and root planing,”
J. Periodontol., 57
(2), 69
–75
(1986). http://dx.doi.org/10.1902/jop.1986.57.2.69 JOPRAJ 0022-3492 Google Scholar
C. M. Cobb,
“Non-surgical pocket therapy: Mechanical,”
Ann. Periodontol., 1
(1), 443
–490
(1996). http://dx.doi.org/10.1902/annals.1996.1.1.443 1553-0841 Google Scholar
G. Greenstein,
“Periodontal response to mechanical nonsurgical therapy: a review,”
J. Periodontol., 63
(2), 118
–130
(1992). http://dx.doi.org/10.1902/jop.1992.63.2.118 JOPRAJ 0022-3492 Google Scholar
P. A. Adriaenset al.,
“Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth,”
J. Periodontol., 59
(8), 493
–503
(1988). http://dx.doi.org/10.1902/jop.1988.59.8.493 JOPRAJ 0022-3492 Google Scholar
E. M. Rateitschak-Plüsset al.,
“Non-surgical periodontal treatment: where are the limits?,”
J. Clin. Periodontol., 19
(4), 240
–244
(1992). http://dx.doi.org/10.1111/j.1600-051X.1992.tb00460.x JCPEDZ 0303-6979 Google Scholar
J. J. KammaV. G. S. VasdekisG. E. Romanos,
“The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters,”
Photomed. Laser. Surg., 27
(1), 11
–19
(2009). http://dx.doi.org/10.1089/pho.2007.2233 PLDHA8 1549-5418 Google Scholar
M. KreislerH. Al HajB. d’Hoedt,
“Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing,”
Lasers Surg. Med., 37
(5), 350
–355
(2005). http://dx.doi.org/10.1002/lsm.20252 LSMEDI 0196-8092 Google Scholar
T. Qadriet al.,
“Long-term effects of a single application of a water-cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation,”
Lasers Med. Sci., 26
(6), 763
–766
(2011). http://dx.doi.org/10.1007/s10103-010-0807-8 LMSCEZ 1435-604X Google Scholar
N. Gutknechtet al.,
“Reduction of specific microorganisms in periodontal pockets with the aid of an Nd:YAG laser—an in vivo study,”
J. Oral Laser Appl., 2
(3), 175
–180
(2002). Google Scholar
F. Sgolastraet al.,
“Effectiveness of diode laser as adjunctive therapy to scaling root planing in the treatment of chronic periodontitis: a meta-analysis,”
Lasers Med. Sci., 28
(5), 1393
–1402
(2013). http://dx.doi.org/10.1007/s10103-012-1181-5 LMSCEZ 1435-604X Google Scholar
L. O. ZachG. Cohen,
“Pulp response to externally applied heat,”
Oral Surg. Oral Med. Oral Pathol., 19
(4), 515
–530
(1965). http://dx.doi.org/10.1016/0030-4220(65)90015-0 OSOMAE 0030-4220 Google Scholar
J. MeisterR. FranzenC. Apel,
“Grundlagen der Laserzahnheilkunde. Teil III: Die Licht-Gewebe-Wechselwirkung,”
Laser Zahnheilkunde, 4
(3), 199
–204
(2004). Google Scholar
D. Yuet al.,
“Comparison of three lasers on dental pulp chamber temperature change,”
J. Clin. Laser Med. Surg., 11
(3), 119
–122
(1993). http://dx.doi.org/10.1089/clm.1993.11.119 JCLSEO Google Scholar
M. KreislerH. Al-HajB. D’hoedt,
“Intrapulpal temperature changes during root surface irradiation with an 809-nm GaAlAs laser,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 93
(6), 730
–735
(2002). http://dx.doi.org/10.1067/moe.2002.124766 1079-2104 Google Scholar
M. Kreisleret al.,
“Effect of diode laser irradiation on root surfaces in vitro,”
J. Clin. Laser Med. Surg., 20
(2), 63
–69
(2002). http://dx.doi.org/10.1089/104454702753768034 JCLSEO Google Scholar
G.L. Castroet al.,
“Histological evaluation of the use of diode laser as an adjunct to traditional periodontal treatment,”
Photomed. Laser Surg., 24
(1), 64
–68
(2006). http://dx.doi.org/10.1089/pho.2006.24.64 PLDHA8 1549-5418 Google Scholar
L. H. Theodoroet al.,
“Effect of Er:YAG and diode laser irradiation on the root surface: morphological and thermal analysis,”
J. Periodontol., 74
(6), 838
–843
(2003). http://dx.doi.org/10.1902/jop.2003.74.6.838 JOPRAJ 0022-3492 Google Scholar
F. Schwarzet al.,
“In vivo and in vitro effects of an Er:YAG laser, a GaAlAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study,”
Lasers Surg. Med., 32
(5), 359
–366
(2003). http://dx.doi.org/10.1002/lsm.10179 LSMEDI 0196-8092 Google Scholar
A. M. Arrastiaet al.,
“Comparative study of the thermal effects of four semiconductor lasers on the enamel and pulp chamber of a human tooth,”
Lasers Surg. Med., 15
(4), 382
–389
(1994). http://dx.doi.org/10.1002/lsm.1900150408 LSMEDI 0196-8092 Google Scholar
H. El Yazamiet al.,
“Pulp temperature increase during photo-activated disinfection (PAD) of periodontal pockets: an in vitro study,”
Lasers Med. Sci., 25
(5), 655
–659
(2010). http://dx.doi.org/10.1007/s10103-009-0686-z LMSCEZ 1435-604X Google Scholar
A. J. WelchM. J. C. van Gemert, Optical-Thermal Response of Laser-Irradiated Tissue, 2nd ed.Springer, Netherlands
(2011). http://dx.doi.org/10.1007/978-90-481-8831-4 Google Scholar
M. B. JakubinekC. Samarasekera,
“Elephant ivory: a low thermal conductivity, high strength nanocomposite,”
J. Mater. Res., 21
(1), 287
–292
(2006). http://dx.doi.org/10.1557/jmr.2006.0029 JMREEE 0884-2914 Google Scholar
P. Elsneret al., Domininghaus—Kunststoffe. Eigenschaften und Anwendungen, VDI Buch, 8th ed.Springer, Heidelberg
(2012). Google Scholar
M. H. Niemz, Laser-Tissue Interactions: Fundamentals and Applications, 3rd ed.Springer, Berlin, Heidelberg, New York
(2007). Google Scholar
J. C. ChangP. Wilder-Smith,
“Laser-induced thermal events in empty and pulp-filled dental pulp chambers,”
Lasers Surg. Med., 22
(1), 46
–50
(1998). http://dx.doi.org/10.1002/(SICI)1096-9101(1998)22:1<46::AID-LSM11>3.0.CO;2-6 LSMEDI 0196-8092 Google Scholar
S. MankM. SteineckL. Brauchli,
“Influence of various polishing methods on pulp temperature: an in vitro study,”
J. Orofac. Orthop., 72
(5), 348
–357
(2011). http://dx.doi.org/10.1007/s00056-011-0039-y 1434-5293 Google Scholar
M. Linet al.,
“A review of heat transfer in human tooth—Experimental characterization and mathematical modeling,”
Dent. Mater., 26
(6), 501
–513
(2010). http://dx.doi.org/10.1016/j.dental.2010.02.009 DEMAEP 0109-5641 Google Scholar
H. S. Malmströmet al.,
“Effect of CO2 laser on pulpal temperature and surface morphology: an in vitro study,”
J. Dent., 29
(8), 521
–529
(2001). http://dx.doi.org/10.1016/S0300-5712(01)00028-8 JDENAB 0300-5712 Google Scholar
D. Simeoneet al.,
“The radicular dentine temperature during laser irradiation: an experimental study,”
J. Clin. Laser Med. Surg., 14
(1), 17
–21
(1996). http://dx.doi.org/10.1089/clm.1996.14.17 JCLSEO Google Scholar
A. Mehlet al.,
“3D volume-ablation rate and thermal side effects with the Er:YAG and Nd:YAG laser,”
Dent. Mater., 13
(4), 246
–251
(1997). http://dx.doi.org/10.1016/S0109-5641(97)80036-X DEMAEP 0109-5641 Google Scholar
C. Millenet al.,
“A study of temperature rise in the pulp chamber during composite polymerization with different light-curing units,”
J. Cotemp. Dent. Pract., 8
(7), 29
–37
(2007). Google Scholar
R. E. WaltonW. C. OuthwaiteD. F. Pashley,
“Magnification—an interesting optical property of dentin,”
J. Dent. Res., 55
(4), 639
–642
(1976). http://dx.doi.org/10.1177/00220345760550041601 JDREAF 0022-0345 Google Scholar
N. TrikilisA. RawlinsonT. F. Walsh,
“Periodontal probing depth and subgingival temperatures in smoker and non-smokers,”
J. Clin. Periodontol., 26
(1), 38
–43
(1999). http://dx.doi.org/10.1034/j.1600-051X.1999.260107.x JCPEDZ 0303-6979 Google Scholar
D. Friedet al.,
“The nature of light scattering in dental enamel and dentin at visible and near-IR wavelengths,”
Appl. Opt., 34
(7), 1278
–1285
(1995). http://dx.doi.org/10.1364/AO.34.001278 APOPAI 0003-6935 Google Scholar
J. R. ZijpJ. J. Ten Bosch,
“Theoretical model for the scattering of light by dentin and comparison with measurements,”
Appl. Opt., 32
(4), 411
–415
(1993). http://dx.doi.org/10.1364/AO.32.000411 APOPAI 0003-6935 Google Scholar
G. B. Altshuleret al.,
“Human tooth as an optical device,”
Proc. SPIE, 1429 95
–104
(1991). http://dx.doi.org/10.1117/12.44658 PSISDG 0277-786X Google Scholar
T. M. Odoret al.,
“Pattern of transmission of laser light in teeth,”
Int. Endod. J., 29
(4), 228
–234
(1996). http://dx.doi.org/10.1111/j.1365-2591.1996.tb01374.x IENJEA 1365-2591 Google Scholar
A. KienleR. MichelsR. Hibst,
“Magnification—a new look at a long-known optical property of dentin,”
J. Dent. Res., 85
(10), 955
–959
(2006). http://dx.doi.org/10.1177/154405910608501017 JDREAF 0022-0345 Google Scholar
P. LenzH. Gilde,
“Temperaturverlauf im pulpakavum bei schmelzversiegelung mit laserstrahlen,”
Dtsch. Zahnarztl. Z., 33
(9), 623
–628
(1978). Google Scholar
V. G. Behrenset al.,
“Die transmission und absorption der temperature und energie des Nd:YAG lasers im dentin,”
ZWR, 102
(9), 629
–634
(1993). Google Scholar
M. PohtoA. Scheinin,
“Microscopic observations on living dental pulp II. The effect of thermal irritants on the circulation of the pulp in the lower rat incisor,”
Acta Odontol. Scand., 16
(3), 315
–327
(1958). http://dx.doi.org/10.3109/00016355809064116 AOSCAQ 0001-6357 Google Scholar
W. H. RaabH. Müller,
“Temperature-dependent changes in the microcirculation of the dental pulp,”
Dtsch. Zahnarztl. Z., 44
(7), 496
–497
(1989). Google Scholar
G. L. PowellT. H. MortonB. K. Whisenant,
“Argon laser oral safety parameters for teeth,”
Lasers Surg. Med., 13
(5), 548
–552
(1993). http://dx.doi.org/10.1002/lsm.1900130509 LSMEDI 0196-8092 Google Scholar
P. BaldissaraS. CatapanoR. Scotti,
“Clinical and histological evaluation of thermal injury thresholds in human teeth: a preliminary study,”
J. Oral Rehabil., 24
(11), 791
–801
(1997). http://dx.doi.org/10.1111/j.1365-2842.1997.tb00278.x JORHBY 0305-182X Google Scholar
H. NyborgM. Brännström,
“Pulp reaction to heat,”
J. Prosthet. Dent., 19
(6), 605
–612
(1968). http://dx.doi.org/10.1016/0022-3913(68)90262-X JPDEAT 0022-3913 Google Scholar
V. F. LisantiH. A. Zander,
“Thermal injury to normal dog teeth: in vivo measurements of pulp temperature increases and their effect on the pulp tissue,”
J. Dent. Res., 31
(4), 548
–558
(1952). http://dx.doi.org/10.1177/00220345520310040501 JDREAF 0022-0345 Google Scholar
W. B. Nowaket al.,
“On the use of thermocouples for temperature measurements during laser irradiation,”
Life Sci., 3
(12), 1475
–1481
(1964). http://dx.doi.org/10.1016/0024-3205(64)90091-8 LIFSAK 0024-3205 Google Scholar
H. J. FothM. Lüke,
“Model calculations for three-dimensional heat conduction in a real tooth,”
Proc. SPIE, 4950 83
–90
(2003). http://dx.doi.org/10.1117/12.476664 PSISDG 0277-786X Google Scholar
A.R. ErikssonT. Albrektsson,
“Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit,”
J. Prosth. Dent., 50
(1), 101
–107
(1983). http://dx.doi.org/10.1016/0022-3913(83)90174-9 JPDEAT 0022-3913 Google Scholar
L. Moss-SalentijnM. Hendricks-Klyvert, Dental and Oral Tissues: An Introduction, 2nd ed.Lea & Febiger, Philadelphia
(1985). Google Scholar
D. S. CobbD. N. DederichT. V. Gardner,
“In vitro temperature change at the dentin / pulpal interface by using conventional visible light versus argon laser,”
Lasers Surg. Med., 26
(4), 386
–397
(2000). http://dx.doi.org/10.1002/(SICI)1096-9101(2000)26:4<386::AID-LSM7>3.0.CO;2-C LSMEDI 0196-8092 Google Scholar
BiographyFabian Falkenstein studied dentistry at RWTH Aachen University. Since January 2012, he has been working under the supervision of Prof. Dr. Norbert Gutknecht. Norbert Gutknecht studied medicine and dentistry. After receiving his PhD in 1993 he became associate professor at the Department of Conservative Dentistry, Periodontology and Preventive Dentistry, at RWTH Aachen University in 1998 and subsequently full professor in 2003. He is director of Aachen Dental Laser Centre, president of the German Society for Laser Dentistry, and CEO of the World Federation for Laser Dentistry (WFLD). He has authored more than 155 publications in international journals. René Franzen studied physics at the University of Düsseldorf and received his PhD in theoretical medicine from the Medical Faculty at RWTH Aachen University in 2004. Besides a research position at the University Hospital of RWTH he works at the Aachen Dental Laser Centre and has authored or co-authored more than 70 publications in national and international journals. |