|
|
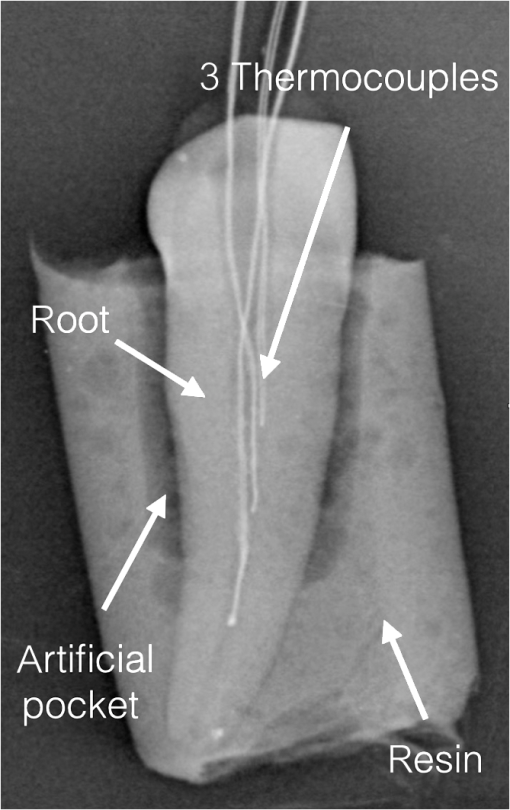
1.IntroductionMechanical modification and debridement of the root surface is considered the gold standard of nonsurgical periodontal treatment for periodontally diseased teeth, which is accomplished through manual or ultrasonic instruments so as to remove bacterial biofilms, calculus, and deposits supra- and subgingivally.1–6 However, this goal is not completely achieved since access to areas like furcations, concavities, grooves, and distal sites is limited; therefore, bacterial toxins, biofilms, and plaques may be left over the root surfaces, which consequently impair the predictable healing result and cause reinfection.6–10 Many different root modification methods have been applied to improve and stimulate the regeneration of periodontal ligament fibers on root surfaces. These include chemical, mechanical, growth factors, and different lasers.1–4,6,8 Lasers are one of the most promising new advancements for nonsurgical periodontal treatment due to tissue modification, detoxification, and bactericidal effects. Due to transmission and scattering effects, laser light may reach the areas, as mentioned above, where conventional instruments cannot.10,11 Scaling with erbium lasers has demonstrated comparable12–15 and even better outcomes than conventional scaling in that laser scaling not only removes calculus, plaques, reduces bacterial numbers, and has a positive effect in reduction of bacterial endotoxins, but also removes biofilms and provides a stable rough surface for better attachment of fibroblasts, blood clot, and periodontal ligament fibers.5,10,16–20 Dental calculi comprise -substituted apatite crystals, water, and some other crystalline forms of phosphate and calcium; due to high absorptions of erbium laser in water and hydroxyapatite, erbium lasers have positive effects on the obliteration of mineralized bacterial plaques.18 Nevertheless, to remove hard tissue deposits and calculus, more intensity is needed; therefore, some side effects like overheating, microcracks, pit, grooves, and craters over the root surfaces have been reported.10 Any thermal changes of within the pulp cavity can lead to tooth necrosis if the temperature persists for too long, which must be avoided.21 As such, in the literature, minimal thermal changes on root surface, in pulp, bone, and adjacent tissues via utilization of erbium laser have been reported.10,22–25 Temperature elevation depends on time of exposure and, in consequence, on the total energy applied; the higher the power and the longer the treatment duration, the higher are the temperatures expected. Naturally, severe damages and altered root surfaces are expected when inappropriate laser parameters are utilized in either in vitro or in vivo situations.26–29 Therefore, the purpose of the present study is to examine the thermal changes in the pulp of extracted human teeth by simultaneous application (dual wavelength) of Er,Cr:YSGG and 940-nm diode lasers using a thermal bath and thermocouples in vitro, compare the measured values with the critical threshold of 5.6°C and to critically discuss the applied parameters. Null hypothesis (): applications of combined 2.78- and laser radiation increase the temperature in pulp cavity beyond critical point of 5.6°C.21 Alternative hypothesis (): application of combined 2.78- and laser radiation is safe for pulp in root debridement within the use of the investigated parameters. 2.Materials and MethodsFor this cross-sectional laboratory study, three groups, consisting of 10 extracted human teeth per group, were examined. All samples () were single-rooted maxillary and mandibular teeth, which were free of caries, gross cracks, and restorations on root surfaces. The teeth were always kept in a water-based solution with 0.1% thymol and 0.9% NaCl to prevent drying and bacterial growth. For temperature measurements, the teeth were prepared to be fitted with thermocouples (K-Type, model OMEGA, 5TC-TT-36, 0.13 mm diameter). Therefore, the root canals were prepared and enlarged with K-files up to ISO 40. To simulate a physiological situation, an artificial periodontal pocket model was manufactured by using cold-curing two-component polyurethane cast resin (ISO-PUR K 760), which was chosen because of its thermal conductivity being similar to cortical bone () when mixed in a ratio of 5:1, resin:hardener () as described by Hmud et al.30 Before pouring the resin, the whole tooth, except for the lowest 3 mm of the apical root, was wrapped in Teflon tape () 10 times to shape a periodontal pocket of 1 mm width and at least 6 mm of depth around the teeth. Then, three thermocouples per tooth were placed at the cervical, middle, and apical third of each root canal; the access cavity was sealed with sticky wax; the locations of the three thermocouples’ ends were confirmed with a radiograph. The ends of the thermocouples were dipped into thermal compound (ARCTIC, MX-2) before insertion into the specimens to ensure optimal heat transfer from the dentin to the thermocouples. An exemplary specimen radiograph is shown in Fig. 1. The other ends of respective thermocouples were connected to a digital thermometer (OM-USB-TC, OMEGA ENGINEERING, INC, USA), which is, in turn, connected to a computer to record the temperature in real time. To provide an environment with similar temperatures as the human oral cavity, a thermally stabilized water bath (GFL, waterbath type 1086, Germany) was used to host the specimens for the experiments. All laser irradiation procedures were done by the same person. 3.Laser Parameters and ProtocolsFor the three groups, the following parameters and protocols were used (Table 1). Table 1Laser parameters for the groups.
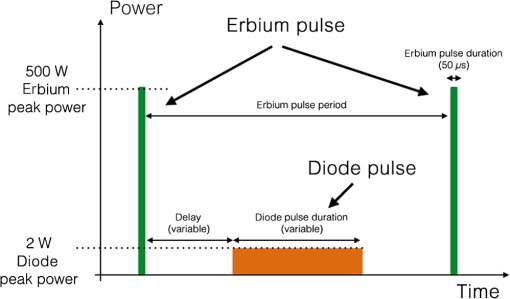
Note: W/A is the water air ratio. Delay is the delay time after the Er,Cr:YSGG pulse to start the diode pulse. Duration is the pulse duration of the laser. Pulse period is 1/repetition rate. The repetition rate is the same (50 Hz) for both lasers, as the diode laser emits exactly between the erbium laser emissions. The laser setup for the first group was Er,Cr:YSGG 2780 nm (serving as a control group) without any adjunct diode laser irradiation; the second one was Er,Cr:YSGG whose pulses were alternated with pulses of the 940-nm diode laser operating in chopped (gated) mode with a duty cycle of 0.2 (pulse duration to pulse period ratio of 20%), and the third group was Er,Cr:YSGG alternating pulses of the 940-nm diode laser with a duty cycle of 0.5. For the Er,Cr:YSGG 2780 nm, the pulse energy was 25 mJ with a pulse repetition rate of 50 Hz and pulse duration of (designated as H-mode by the manufacturer). For the 940-nm diode laser, all groups utilized a peak power of 2 W, with average powers resulting from multiplication with the respective duty cycle. In group 2, this yielded 0.4 W and in group 3, 1 W. Radial firing periodontal tips (RFPT 5, BIOLASE, 6201176, LOT NO: 12. 04. 19) with a diameter of and length of 14 mm were utilized. The alternating pulses emitted through the same laser tip are illustrated in Fig. 2 (here an exemplary duty cycle of 0.5 is shown for illustration, as is used in group 3). Individual pulse durations are listed in Table 1. The water/air spray was set as water 80% and air 10% as a pilot study had shown the least temperature increases with this setting. As seen in Table 1, samples in the first group were only irradiated by the Er,Cr:YSGG laser without any diode laser pulses, while specimens in the second and third groups were lased with both Er,Cr:YSGG and alternating diode laser pulses. The whole irradiation time for each tooth specimen, with a pocket depth of 6 mm from the cementum-enamel-junction, was 60 s, including 30 s for the buccal pocket and 30 s for the lingual pocket side. To be exact, each 2 mm depth of pocket, at the labial and/or lingual root surface, was irradiated for 10 s. The laser fiber tip was held parallel to the long axis of the tooth and was moved in a scanning motion as in the standard clinical protocol with just one wavelength. All irradiations were performed with the resin pocket-model inserted into a thermally stabilized water bath (GFL, waterbath type 1086, Germany) set to a temperature of 37°C. The actually measured temperature just outside the model was subtracted from the measured values at the intrapulpal thermocouples. These temperature rises above the baseline are called delta () hereinafter. Following each irradiation, 7 min were allowed for the specimens to return to the baseline temperature of the water bath. In order to compare the groups statistically regarding the temperature rises at the apical, middle, and cervical positions of the thermocouples, a two-tailed paired test was performed in Microsoft Excel (version 14.2.4) and the value was obtained ( was considered as significant, confidence level 99%). 4.ResultsThe mean values for the highest temperature rises were 1.68°C with a standard deviation of 0.98°C in group 3. Lower temperature increases were measured in groups 1 and 2, with and , respectively. The highest temperature increase was found in one specimen in group 3, in the apical third (specimen #10) with 3.81°C. The maximum, mean, and standard deviation of maximum temperature changes () for laser groups (groups 1, 2, and 3) in cervical, middle, and apical thirds of root canals have been described in Centigrade in Table 2. Tables 3Table 4–5 list the results of the groups in detail. Table 2Maximum, mean, and standard deviation (SD) for groups within 60 s of irradiation in the cervical, mesial, and apical position of the thermocouples.
Note: Nr, number of specimen; ΔTmax, recorded maximum temperature rise in respective area; SD, standard deviation. Table 3Recorded temperature rises for group 1 specimens including means and standard deviations.
Note: S, sample. Final mean value and final standard deviation (over all specimen in that group) are in bold. Table 4Recorded temperature rises for group 2 specimens including means and standard deviations.
Note: S, sample. Final mean value and final standard deviation (over all specimen in that group) are in bold. Table 5Recorded temperature rises for group 3 specimens including means and standard deviations.
Note: S, sample. Final mean value and final standard deviation (over all specimen in that group) are in bold. In groups 1 and 2, there was no significant difference between the means of delta temperature maximum of these two groups at all, suggesting that the proposed settings do cause almost the same temperature rises in the apical, middle, and cervical regions of the tooth. Hence, intermitted 20% diode laser radiation with a peak power of 2 W does not significantly elevate the temperature in any tested area of the anterior teeth. In the comparison of groups 1 and 2 with group 3, there was a significant difference between the means of delta temperature maximum of apical and middle thirds (), but no significant differences were observed on the cervical thirds of the above-mentioned groups. 5.DiscussionThe most important criterion that one should consider in nonsurgical periodontal therapy is to have a clean root surface, which clinically means a root surface without bacterial calculus deposits, biofilm, and smear layer. In this condition, it would be a favorable place for gingival fibroblast attachment, which is considered the critical point for periodontal regeneration, in order to assure a new healthy connective tissue attachment.1–6,29–32 A myriad of instruments have been proposed for scaling and root planing, including lasers, especially in the erbium laser family (YAG and YSGG host crystals), that have shown promising results in periodontal treatment and fibroblast adhesion. Additionally, Er:YAG and Er,Cr:YSGG lasers have different morphological changes on the root surface compared to chemical means.1,10,12,31–36 In the present study, it was demonstrated that the accompanying use of a diode laser emitting at 940 nm alternating with Er,Cr:YSGG in groups 2 and 3 does not show any adverse thermal effects on the pulp in comparison to group 1, in which only Er,Cr:YSGG laser radiation was applied. Although temperature changes in group 3 showed a significantly higher difference () from the other laser groups—only in middle and apical third of specimens—all temperature measurements were lower than the critical physiological limit of 5.6°C, i.e., these statistical significant differences were not clinically relevant. Mean in the cervical thirds does not show any significant differences () among laser groups, which could be explained with the sufficient presence of water spray in the cervical thirds rather than the mesial and apical thirds. Articles are mostly concerned with the morphological and thermal changes of root surfaces following the laser applications, while there are only few articles regarding temperature measurements within the pulp cavity when using erbium and diode laser irradiation (Table 6). To the best of our knowledge, this is the first study to report on the thermal effects of a dual-wavelength laser ( and 940 nm) within the pulp cavity. Table 6Literature overview of basic studies on temperature elevation with the combination of erbium and diode lasers including the present study.
Theodoro et al.37 compared the morphological and thermal effects of Er:YAG and a diode of 810 nm on scaled and root-planed single-rooted teeth. No significant morphological alterations like melting, fusion, charring, or carbonizations were observed on SEM pictures, although root surfaces were more irregular in the Er:YAG laser group. A diode laser with an average power of 1 W and 1.4 W cw for 30 s caused a thermal increase of 1.6 and 3.3°C, respectively, while a temperature decrease of 2.2°C was recorded for the Er:YAG laser group. In our study, a 940-nm diode laser emitting at 2 W in the chopped mode in the second and third groups with duty cycles of 20 and 50% was applied, respectively, yielding an average power of 0.4 and 1 W. Thus, temperature increase in the third group of the our study () was very similar to the findings of Theodoro et al.’s37 study (), although the time of irradiation in the presented study was 60 s, two times more than Theodoro et al.’s study, which can be explained by the presence of the water spray and the use of a stabilized thermobath, creating a more realistic model for the periodontal system. Aoki et al. reported a temperature rise of 1.4°C during Er:YAG laser scaling under a water coolant.38 They also mentioned that the laser caused superficial microroughness over the root surface under SEM analysis. Kreisler et al. investigated an 810-nm diode laser and recommended that a power output of 0.5 W for 10 s would be suited for lower incisors and upper first premolars, while a power of 1 W, also for 10 s, is the maximum output for other teeth; otherwise, by using more power, the pulp vitality may be jeopardized.27 Due to presence of water/air spray in our study, the time of irradiation could be increased to 60 s per tooth, without any adverse effects on temperature elevation in the pulp chamber. The maximum temperature rise, which was recorded for the third group, was 3.81°C, which is , and underscores the importance of a water/air spray.20,22 Hmud et al. assessed the temperature elevation on the external root surface with a 940-nm diode laser while irradiating within the canal for 5 s to generate shock waves in water to disrupt the biofilm in endodontic treatment.30 They utilized 4 W peak power and 10 Hz, with an average power of 0.4 W, similar to group 2 of our study; the maximum temperature elevation in their study was 4°C, while the maximum temperature change was only 1.18°C in our study, which also emphasizes the positive effect of water/air spray in controlling the temperature. Indeed, the importance of a correct setting for the water/air spray is to be emphasized at this point. While our parameters have shown no significant temperature increase above any critical physiological limit, preliminary experiments before our study showed that with otherwise identical irradiation parameters, insufficient water to air ratios can lead to higher temperatures of up to 5.99°C in our case with a spray with a ratio setting of water 60% to air 30%. 6.ConclusionWithin the scope of this study, it can be concluded that Er,Cr:YSGG laser with a pulse energy of 25 mJ, 50 Hz, 80% water, 10% air spray is safe as an adjunct periodontal therapy to root-plane while removing the biofilm covering the root surface; additionally, since a diode laser is used in between erbium laser pulses, disinfection and bactericidal effects can be applied without any thermal damage to the pulp vitality. This new combination therapy concept might be a promising approach in periodontology. The proper portion of water/air spray plays a crucial role in controlling the temperature changes within the pulp during a laser-based scaling and root debridement. The alternative hypothesis () formulated beforehand can be confirmed: the application of the dual wavelength is safe for pulp in root debridement; while the null hypothesis () is rejected. The application of this dual-wavelength laser under the mentioned parameters does not increase the temperature in the pulp cavity beyond the critical point of 5.6°C according to Zach and Cohen.21 ReferencesC. Galliet al.,
“Effect of laser-induced dentin modifications on periodontal fibroblasts and osteoblasts: a new in vitro model,”
J. Periodontol., 80 1648
–1654
(2009). http://dx.doi.org/10.1902/jop.2009.090152 JOPRAJ 0022-3492 Google Scholar
A. D. Walmsleyet al.,
“Advances in power driven pocket/root instrumentation,”
J. Clin. Periodontol., 35 22
–28
(2008). http://dx.doi.org/10.1111/cpe.2008.35.issue-s8 JCPEDZ 0303-6979 Google Scholar
R. C. V. Casarinet al.,
“Root surface defect produced by hand instruments and ultrasonic scaler with different power settings: an in vitro study,”
Braz. Dent. J., 20 58
–63
(2009). http://dx.doi.org/10.1590/S0103-64402009000100010 0103-6440 Google Scholar
J. LindheN. P. LangT. Karring, Clinical Periodontology and Implant Dentistry. Initial Periodontal Therapy (Infection Control), John Wiley & Sons, Hoboken, NJ
(2009). Google Scholar
R. Crespiet al.,
“Effects of Er: YAG laser compared to ultrasonic scaler in periodontal treatment: a 2-year follow-up split-mouth clinical study,”
J. Periodontol., 78 1195
–2000
(2007). http://dx.doi.org/10.1902/jop.2007.060460 JOPRAJ 0022-3492 Google Scholar
A. M. Polsonet al.,
“The production of a root surface smear layer by instrumentation and its removal by citric acid,”
J. Periodontol., 55 443
–446
(1984). http://dx.doi.org/10.1902/jop.1984.55.8.443 JOPRAJ 0022-3492 Google Scholar
A. BaroneU. Covani,
“Effect of Er:YAG laser on diseased root surfaces: an in vivo study,”
J. Periodontol., 76 1386
–1390
(2005). http://dx.doi.org/10.1902/jop.2005.76.8.1386 JOPRAJ 0022-3492 Google Scholar
D. Simoneet al.,
“Ultramorphology of the root surface subsequent to hand-ultrasonic simultaneous instrumentation during non-surgical periodontal treatments: an in vitro study,”
J. Appl. Oral Sci., 19 74
–81
(2011). http://dx.doi.org/10.1590/S1678-77572011000100015 JAOSBM 1678-7757 Google Scholar
A. Aokiet al.,
“Lasers in nonsurgical periodontal therapy,”
Periodontol. 2000, 36 59
–97
(2004). http://dx.doi.org/10.1111/prd.2004.36.issue-1 0906-6713 Google Scholar
R. Amidet al.,
“Effect of hand, ultrasonic scaler and erbium-doped yttrium aluminum garnet (Er:YAG) laser on the morphology of root surfaces with periodontitis: a comparative in vitro scanning electron microscopy study,”
J. Lasers Med. Sci., 3 122
–126
(2012). Google Scholar
V. MaciulskieneS. Kelbauskiene,
“A pilot study of Er,Cr:YSGG laser therapy used as an adjunct to scaling and root planing in patients with early and moderate periodontitis,”
Stomatologija, 9 21
–26
(2007). Google Scholar
R. CrespiA. BaroneU. Covani,
“Er:YAG laser scaling of diseased root surfaces: a histologic study,”
J. Periodontol., 76 1386
–1390
(2005). http://dx.doi.org/10.1902/jop.2005.76.8.1386 JOPRAJ 0022-3492 Google Scholar
F. Schwarzet al.,
“In vivo and in vitro effects of an Er:YAG laser, a GaAlAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study,”
Lasers Surg. Med., 32 359
–366
(2003). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
Y. Kimuraet al.,
“Effects of erbium, chromium:YSGG laser irradiation on root surface: morphological and atomic analytical studies,”
J. Clin. Laser Med. Surg., 19 69
–72
(2001). http://dx.doi.org/10.1089/PLT.2001.19.issue-2 JCLSEO 1044-5471 Google Scholar
H. Watanabeet al.,
“Clinical assessments of the erbium:YAG laser for soft tissue surgery and scaling,”
J. Clin. Laser Med. Surg., 14 67
–75
(1996). http://dx.doi.org/10.1089/clm.1996.14.67 JCLSEO 1044-5471 Google Scholar
F. Schwarzet al.,
“Periodontal treatment with an Er:YAG laser compared to scaling and root planing. A controlled clinical study,”
J. Periodontol., 72 361
–367
(2001). http://dx.doi.org/10.1902/jop.2001.72.3.361 JOPRAJ 0022-3492 Google Scholar
S. Hakkiet al.,
“Effects of root planing procedures with hand instrument or erbium, chromium:yttrium-scandium–gallium-garnet laser irradiation on the root surfaces: a comparative scanning electron microscopy study,”
Lasers Med. Sci., 25 345
–353
(2010). http://dx.doi.org/10.1007/s10103-009-0643-x LMSCEZ 1435-604X Google Scholar
M. Folwacznyet al.,
“Removal of bacterial endotoxin from root surface with Er:YAG laser,”
Am. J. Dent., 16 3
–5
(2003). Google Scholar
I. IshikawaA. AokiA. A. Takasaki,
“Clinical application of erbium:YAG laser in periodontology,”
J. Int. Acad. Periodontol., 10 22
–30
(2008). JAPEFN 1466-2094 Google Scholar
H. E. Schroeder, Formation and Inhibition of Dental Calculus, 94
–122 Hans Huber, Berne
(1969). Google Scholar
L. ZachG. Cohen,
“Pulp response to externally applied heat,”
Oral Surg. Oral Med. Oral Pathol., 19 515
–530
(1965). http://dx.doi.org/10.1016/0030-4220(65)90015-0 OSOMAE 0030-4220 Google Scholar
Y. Kimuraet al.,
“Effects of erbium, chromium:YSGG laser irradiation on canine mandibular bone,”
J. Periodontol., 72 1178
–1182
(2001). http://dx.doi.org/10.1902/jop.2000.72.9.1178 JOPRAJ 0022-3492 Google Scholar
F. Schwarzet al.,
“Periodontal treatment with an Er:YAG laser or scaling and root planing. A 2 year follow up split mouth study,”
J. Periodontol., 74 590
–596
(2003). http://dx.doi.org/10.1902/jop.2003.74.5.590 JOPRAJ 0022-3492 Google Scholar
L. H. Theodoroet al.,
“Effect of Er:YAG and diode laser irradiation on the root surface: morphological and thermal analysis,”
J. Periodontol., 74 838
–843
(2003). http://dx.doi.org/10.1902/jop.2003.74.6.838 JOPRAJ 0022-3492 Google Scholar
A. Aokiet al.,
“In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser,”
J. Periodontol., 65 1097
–1106
(1994). http://dx.doi.org/10.1902/jop.1994.65.12.1097 JOPRAJ 0022-3492 Google Scholar
M. Kreisleret al.,
“Effect of diode laser irradiation on root surfaces in vitro,”
J. Clin. Laser Med. Surg., 20 63
–69
(2002). http://dx.doi.org/10.1089/PLT.2002.20.issue-2 JCLSEO 1044-5471 Google Scholar
M. KreislerH. Al-HajB. D’Hoedt,
“Intrapulpal temperature changes during root surface irradiation with an 809-nm GaAlAs laser,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 93 730
–735
(2002). http://dx.doi.org/10.1067/moe.2002.124766 1079-2104 Google Scholar
G. J. P. L. OliveiraJ. E. C. SampaioR. A. C. Marcantonio,
“Effects of Er,Cr:YSGG laser irradiation on root surfaces for adhesion of blood components and morphology,”
Photomed. Laser Surg., 28 751
–756
(2010). http://dx.doi.org/10.1089/pho.2009.2681 PLDHA8 1549-5418 Google Scholar
A. Etemadiet al.,
“Comparing efficiency and root surface morphology after scaling with Er:YAG and Er,Cr:YSGG lasers,”
Int. J. Periodontics Restorative Dent., 33 140
–144
(2013). http://dx.doi.org/10.11607/prd.1765 0198-7569 Google Scholar
R. HmudW. A. KahlerL. J. Walsh,
“Temperature changes accompanying near infrared diode laser endodontic treatment of wet canals,”
J. Endod., 36 908
–911
(2010). http://dx.doi.org/10.1016/j.joen.2010.01.007 0099-2399 Google Scholar
S. S. Hakkiet al.,
“Comparison of Er,Cr:YSGG laser and hand instrumentation on the attachment of periodontal ligament fibroblasts to periodontally diseased root surfaces: an in vitro study,”
J. Periodontol., 81 1216
–1225
(2010). http://dx.doi.org/10.1902/jop.2010.090715 JOPRAJ 0022-3492 Google Scholar
G. PolimeniA. V. XiropaidisU. M. Wikesjö,
“Biology and principles of periodontal wound healing/regeneration,”
Periodontol 2000, 41 30
–47
(2006). http://dx.doi.org/10.1111/prd.2006.41.issue-1 0906-6713 Google Scholar
A. Cekiciet al.,
“Evaluation of blood cell attachment on Er:YAG laser applied root surface using scanning electron microscopy,”
Int. J. Med. Sci., 10 560
–566
(2013). http://dx.doi.org/10.7150/ijms.5233 IJMSGZ 1449-1907 Google Scholar
B. GaspircU. Skaleric,
“Morphology, chemical structure and diffusion processes of root surface after Er:YAG and Nd:YAG laser irradiation,”
J. Clin. Periodontol., 28 508
–516
(2001). http://dx.doi.org/10.1034/j.1600-051x.2001.028006508.x JCPEDZ 0303-6979 Google Scholar
K. M. Sasakiet al.,
“Compositional analysis of root cementum and dentin after Er:YAG laser irradiation compared with lased and intact roots using Fourier transformed infrared spectroscopy,”
J. Periodontal Res., 37 50
–59
(2002). http://dx.doi.org/10.1034/j.1600-0765.2002.00657.x JPDRAY 0022-3484 Google Scholar
R. ChanthabouryT. Irinakis,
“The use of lasers for periodontal debridement: marketing tool or proven therapy?,”
J. Can. Dent. Assoc., 71 653
–658
(2005). JCDAAS 0008-3372 Google Scholar
L. H. Theodoroet al.,
“Comparative analysis of root surface smear layer removal by different etching modalities or erbium:yttrium-aluminum–garnet laser irradiation. A scanning electron microscopy study,”
Lasers Med. Sci., 25 485
–491
(2010). http://dx.doi.org/10.1007/s10103-009-0665-4 LMSCEZ 1435-604X Google Scholar
A. Aokiet al.,
“In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling,”
J. Periodontal Res., 35 266
–277
(2000). http://dx.doi.org/10.1034/j.1600-0765.2000.035005266.x JPDRAY 0022-3484 Google Scholar
BiographyRene Franzen studied physics at the University of Düsseldorf and received his PhD in theoretical medicine from the Medical Faculty at RWTH Aachen University in 2004. In addition to a research position at the University Hospital of RWTH, he also works at the Aachen Dental Laser Centre and has authored and co-authored more than 70 publications in national and international journals. Norbert Gutknecht studied medicine and dentistry. After receiving his PhD in 1993 he became associate professor at the Department of Operative Dentistry at Aachen University Medical and Dental Faculty in 1998 and subsequently full professor in 2003. He is director of the Aachen Dental Laser Centre, president of the German Society for Laser Dentistry, and CEO of the World Federation for Laser Dentistry (WFLD). He has authored more than 155 publications in international journals. |