|
|
1.IntroductionChronic wound infection with multidrug resistant microorganisms can be a life-threatening disease, with serious complications such as the removal of tissue/organ and mortality.1–5 Improper use of antibiotics is one of the main reasons for multidrug resistance, which applies a selective pressure and triggers evolutionary mechanisms of survival for microorganisms, resulting in the emergence of new resistance pathways. Yet in terms of treatment, it has become harder to find or develop new antibiotics in recent decades. Silver preparations or iodine-containing solutions may be used as antimicrobial agents to eradicate infections; however, they still have some disadvantages such as being toxic to healthy cells and they are not as effective as antibiotics to completely eradicate infections. They are mostly suitable for topical applications, thus they have limited effect for the treatment of deeper infections. Another antibacterial treatment is surgical removal of the infected part of the tissue from the body. This method creates new wounds, which delays the healing process and cannot completely eliminate infected tissues.6,7 Many strains of Staphylococcus aureus are responsible for nosocomial or superficial skin infections. The multidrug resistant forms, including the methicillin resistant strain, are among the most difficult to treat, causing thousands of deaths each year.8–12 Thus, development of new and effective treatment modalities is an urgent and important issue to overcome not only infection related mortality but also the related economic burden to patients and hospitals. Photodynamic therapy (PDT) could be a promising approach with which to solve this global health problem. PDT had been used to destroy some microorganisms in the early 1900s. After the discovery of penicillin in 1928, the scientific world headed toward using antibiotics, and PDT was disregarded as an antibacterial tool.2,13,14 Later, it was investigated to treat some oncological and ophthalmological diseases. Today, it is successfully used to treat various cancer types, age-related macular degeneration, acne problems, etc.9,10,15,16–22 Recently, researchers have started to investigate PDT as an alternative antibacterial tool.23–25 So far, investigations have resulted in promising outcomes. The mechanism of action of PDT involves light in the range of the visible or near-infrared spectrum and a suitable substance, such as a chemical, drug, or dye, which is called a photosensitizer, absorbing a specific wavelength.1,13,16,26,27 There are two possible mechanisms for PDT at the molecular level. When the photosensitizer absorbs light, energy transfer occurs between the photons and photosensitizer and molecules of the photosensitizer levels up to the excited state. Although they go back to the ground state, energy may be transferred to organic substrates and radical ions are produced to react with oxygen molecules. At the end, cytotoxic species are generated to destroy target tissues or cells. This is called the type I mechanism of PDT. In the type II mechanism, absorbed energy by the photosensitizer may be transferred to molecular oxygen available in the environment. This results in the production of reactive oxygen intermediates such as singlet oxygens, hydrogen peroxides, or hydroxyl radicals as cytotoxic products.2,10,26,28,29 Suitable wavelengths for PDT are in the visible and near-infrared regions of the spectrum. Wavelengths between 632.5 and 650 nm are mostly preferred in PDT studies.29 Appropriate photosensitizers for this range are toluidine blue, chlorine(e6) conjugates, methylene blue, and porphyrin derivatives. These photosensitizers are advantageous for antibacterial PDT because of their cationic nature, which can interact easily with the anionic surface of bacterial cells.30–33 On the other hand, there are few antibacterial PDT studies using wavelengths in the near-infrared spectrum. Yet these studies investigated the efficiency of PDT using only in vitro conditions.34 The appropriate photosensitizer for the near-infrared spectrum is indocyanine green (ICG). It has a high absorption capacity around 800 nm. In fact it is a dye approved by the Food and Drug Administration (FDA) and mainly used for medical imaging to check and monitor retina, liver and blood vessels and their functions. It is also widely used in ophthalmological treatments to destroy leaky blood vessels in the retina. It has become a good alternative to treat acne vulgaris, too. Its toxicity level is very low; therefore, it is very suitable for medical purposes.35–38 However, its anionic nature decreases its attractiveness as an antibacterial agent. Nevertheless, the properties of the wavelengths in near-infrared spectrum, which are used together with this photosensitizer, may make this combination more advantageous for applications on biological tissues. Wavelengths around 780 to 810 nm can penetrate deep inside the tissue, nearly 6 mm, which is nearly twice the depth that visible light can travel through the tissue.39 This feature may provide an opportunity for eliminating deeper infections. There are several successful studies indicating that ICG-PDT could be used to destroy cancerous tissue and a couple of in vitro antibacterial studies using ICG and near-infrared light together;40–44 however, no in vivo antibacterial study has been reported so far. Omar et al. investigated antibacterial PDT with ICG and near-infrared laser light on S. aureus, Streptococcus pyogenes, and Pseudomonas aeruginosa in vitro. This study was quite successful in the development of the effective antibacterial effect of destroying bacteria completely.34 Later, our group reported a study on lethal photosensitization of ICG and 809-nm diode laser on other strains of S. aureus and P. aeruginosa in vitro. This study showed that much lower energy doses and ICG concentration could be efficient to destroy these pathogens completely other than the energy dose and ICG concentrations that Omar et al. reported in their study.34,45 These successful data obtained from in vitro studies led our group to assess the lethal photosensitization effect of ICG and near-infrared light on a rat infected wound model. Here, we report for the first time the efficiency of PDT with ICG and an 808 nm wavelength on an abrasion wound model infected with a resistant strain of S. aureus. 2.Materials and Methods2.1.Bacterial StrainsA multidrug resistant strain of S. aureus was used to infect wounds. It was a clinical isolate obtained from Gazi University, Department of Microbiology, Ankara, Turkey. A single colony was used to inoculate tryptic soy broth and cultured overnight at 37°C. Bacterial suspension was then centrifuged, the supernatant was discarded and the pellet was dissolved in phosphate-buffered saline (PBS) to approximately to . This suspension was used to infect wounds on animals. 2.2.Photosensitizer and Light SourceICG (Pulsion Medical Systems AG, Munich, Germany) solution was freshly prepared in PBS before each experiment and kept in the dark to protect it from photobleaching. All the experiments were also performed in the dark. An 808-nm diode laser was used as the light source. It is a continuous-mode laser with a maximum output power of 2 W. Laser light was delivered to the target tissue with a optical fiber that was coupled to the original fiber of the laser. To illuminate an area of , a collimator was attached to the end of the optical fiber. The distance between the tip of optical fiber and the target tissue was fixed and the power of the laser was controlled before each experiment with a power meter (Newport 1918-C, California). 2.3.Animals and Abrasion Wound ModelRandomly selected Wistar albino female rats, 2- to 3-months old, weighing 170 to 220 g were used. They were obtained from Vivarium, Center for Life Sciences and Technologies Research at Bogazici University. All experiments were approved by Institutional Ethics Committee for the Local Use of Animals in Experiments of Bogazici University. Animals were anesthetized by intraperitoneal (i.p.) injection of ketamine and xylazine mixture ( ketamine, xylazine) before wound creation and laser application. The dorsal skin of the animals was shaved by an electric razor, and then the skin was cleaned by 70% (v/v) alcohol. To create abrasion wounds, 21-gauge needles were used to scratch an area of approximately on the upper layer of the epidermis. After creating the wounds, of bacterial suspension was added to the scratched area of the wound with the help of the tip of a pipette. There was a 30 min of waiting period for diffusion of bacteria into the wound. 2.4.In Vivo ExperimentsIn this study, four groups were formed to investigate and compare the effect of ICG-PDT application. Experimental group comprises “PDT-applied wounds,” which received both laser light application and ICG. As positive controls, “laser-applied wounds,” which only received laser light and “ICG-applied wounds,” which only received ICG were created. In the “control group,” wounds received neither laser nor ICG as a negative control. Three wounds were created on each animal; one of them was assigned for negative control and the other two were assigned for PDT or positive controls. In the PDT groups, ICG solution was added to the wounds after inoculation of bacteria. Immediately after the addition of ICG solution, irradiation of the wound by laser was started. First, of ICG solution was added and an additional of ICG was added to the wound at 3-min intervals until the total volume of () was reached during laser application. Laser irradiation lasted for 15 min in each application. The output power was 500 mW, therefore, the laser energy dose transferred to the wound was . In the laser groups, wounds were irradiated with of laser energy without any ICG after the inoculation of bacteria. In an ICG group, specific ICG concentrations were added to the wounds without any laser irradiation and the ICG administration was the same as in the PDT groups. Following these applications, the wounds were removed using sterile scissors and forceps. Tissue samples were cut at the boundaries of the wounds and these samples were put in 5 ml of PBS. After the weights of the samples were calculated, they were compressed in a buffer solution to release viable bacteria from the tissue by using a sterile pestle. Viable bacteria in these solutions were calculated using a serial dilution method. The aliquots were serially diluted in PBS solution by dilution factor and spread on tryptic soy agar plates and incubated overnight at 37°C. Colonies counted on these plates were then multiplied by a dilution factor to calculate the amount of bacteria within the corresponding tissue sample. Then colony-forming units (CFU) per gram were calculated for each wound depending on the weight of the tissue sample extracted from the animals: . 2.5.Wound Healing and Histological AnalysisIn order to observe the wound healing period, 2-day, 4-day, 7-day and 11-day groups were formed with five animals per group with a single wound per animal. The optimum combination of ICG concentration and energy dose were used to treat the infected wound on each animal and then these animals were followed for 2, 4, 7, and 11 days. On the treatment day, the initial size of the wounds was precisely measured by Vernier calipers and the size of the wounds was measured every day until they were sacrificed. Wounds were removed after sacrificing for further histological analysis. Due to ethical considerations, animals with untreated wounds were not allowed to live during the healing process, and were sacrificed immediately after the first day. They were just used to compare the immediate response of the experimental group and positive controls in terms of bactericidal and/or thermal effects. Besides the PDT-treated wounds, the wounds of five animals were treated with an antibacterial cream with 2% mupirocin to form a control group for comparing the antibacterial effect of the conventional treatment with the effect of ICG-PDT during the healing process. These animals were followed for 14 days after infection and treatment and how quickly they were healed after treatment was assessed by measuring the wound sizes with Vernier calipers. Removed tissue samples were fixed in 10% PBS-formalin solution for 2 to 3 days. After fixation, samples were processed in Tissue Processor (Leica TP 1020). These samples were embedded in paraffin blocks and sectioned to thickness by microtome (Leica RM 2255). These sections were stained with hematoxylin–eosin. Stained slides were assessed under a light microscope (Nikon Eclipse 80i, Japan) to observe the epithelial lining, re-epithelialization, inflammation, and collagen formation. 2.6.Temperature MeasurementsTemperature change was monitored by a K-type thermocouple, which has a response time of 0.1 s and measures changes of 0.1°C. The tip of the thermocouple was inserted into the wounds created during measurements. First, the temperature of the wound was measured before laser irradiation. Then the temperature increase was measured in the presence and absence of ICG immediately after illumination. 2.7.Statistical AnalysisAll the viable cell count data were normalized by dividing it with its corresponding data in the control wound. These normalized data were analyzed with one-way analysis of variance and then two-tailed Student test for statistical significance. values lower than 0.05 were considered as significantly different. 3.Results3.1.Antibacterial Effect of PDT on Infected Abrasion WoundsThe laser energy dose () was applied together with 500, 1000, and of ICG on infected abrasion wounds. As shown in Fig. 1, a significant reduction in cell viability was observed in the PDT groups. The reduction was around 90% and corresponds to 1 to 2 logarithmic decrease in CFU/gram. In the laser group, viable cell count after irradiation was nearly the same as in the control group. In ICG groups, a decrease was observed in the bacterial cell count when compared with the control group. However, the data in this group were not significantly different from the data in the control and laser groups. As expected, PDT groups were significantly different from all other control groups. But PDT groups were not significantly different from each other, showing that a decrease in cell viability did not depend on the ICG concentrations applied in this study. Fig. 1Bacterial cell viability on abrasion wounds after laser, indocyanine green (ICG), and photodynamic therapy (PDT) applications. Laser output power was 500 mWatt, irradiation time was 15 min, ICG concentrations used were 500, 1000, and . Asterisk represents the statistical difference with respect to control (). number of wounds in each group. 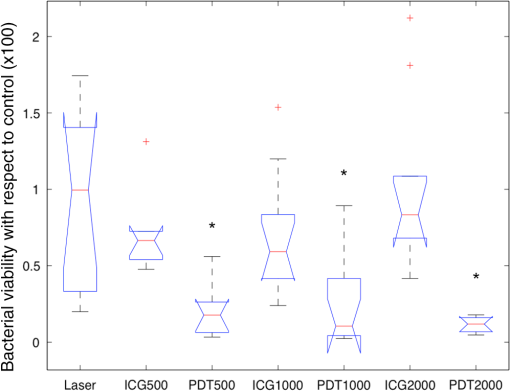 3.2.Wound HealingFigure 2 shows the percentage reduction in the size of the PDT and 2% mupirocin-treated wounds during 14 days. PDT parameters chosen for the healing period were of energy dose and of ICG. In the first 2 days, the area of PDT-treated wounds decreased by nearly 40%. Then the healing process slowed down for 1 to 2 days, and then accelerated again. After the fifth day, the size of the wounds decreased more than 50%. At the 11th day, wounds were barely visible and their sizes approached zero. Fig. 2The percentage reduction in size of PDT-treated wounds and 2% mupirocin-treated wounds. number of wounds/animals in each group. 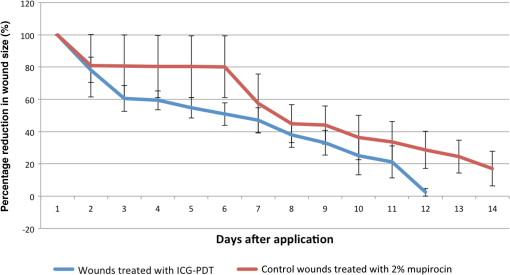 The area of 2% mupirocin-treated wounds decreased only 20% in the first 6 days. Then the healing process of these wounds increased and nearly reached to the healing process of the PDT-treated wounds, however, then it slowed down again and approximately 75% of the wound healing was observed on the 11th day. 2% mupirocin-treated wounds were still visible after 14 days. The healing process of the PDT-treated wounds was much faster than the 2% mupirocin-treated wounds, as clearly seen in Fig. 2. 3.3.Histological AnalysisAs shown in Fig. 3(a), a disrupted epithelial lining can be clearly observed on the newly opened wound. The integrity of the epidermis was destroyed because of the scratches with the needles. In order to assess whether any thermal damage occurred due to laser irradiation, tissue samples were removed after PDT application. Figure 3(b) shows this tissue sample in which the epithelial lining was disrupted as in the tissue sample shown in Fig. 3(a). Even though a high concentration of ICG was used, no thermal destruction was observed in the tissue. In Fig. 3(c), the wound sample removed at day 2 was shown. Tissue was covered with a thick scab. Beneath the scab, the epithelial lining, which became thicker, was observed. The integrity of the epidermis was recovered but was not uniform. The numbers of the fibroblasts increased and were concentrated at the edge of the wound. At day 4, it was observed that the scab of the wound almost completely disappeared, but some remnants were still present. The epithelial lining became thicker, therefore, the integrity of the epidermis was provided. Fibroblast cells at the edge of the wound were still high in number [Fig. 3(d)]. At day 7, the scab on the wound disappeared completely. The epithelial lining started to become thinner than it was at day 4. The integrity of the epidermis was still preserved and the number of fibroblast cells decreased. The wound healing process was almost completed [Fig. 3(e)]. At day 11, the scar of the wound was nearly invisible. The wound healing process was completed in 11 days as shown in Fig. 3(f). The epithelial lining reached its normal thickness, the integrity of the epidermis was uniform and fewer fibroblasts were observed. Fig. 3Histological image of (a) wound that was newly opened and had not yet received ICG or laser; (b) wound that was immediately removed after PDT application; (c) PDT-treated wound that was removed at second day after application; (d) PDT-treated wound that was removed at fourth day after application; (e) PDT-treated wound that was removed at seventh day after application; (f) PDT-treated wound that was removed at 11th day after application. Hematoxylin–eosin staining; original magnification (mag): (a, b, c, d, e, f) . 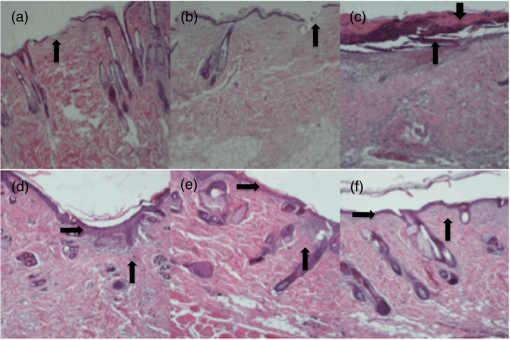 Figure 4 shows the wound morphologies during the healing process. The sample in Fig. 4(a) is a newly opened wound and the sample in Fig. 4(b) is a PDT-treated sample. The PDT-treated sample had a scab on it at the second day [Fig. 4(c)]. The scab on the wound diminished at the fourth day [Fig. 4(d)]. The scab totally disappeared, there was only a small redness, and the wound size remarkably decreased at seven days [Fig. 4(e)]. There were not any scabs or redness, and only small scars were in the place of the wound at the 11th day [Fig. 4(f)]. Fig. 4Wound appearance of (a) a sample that was newly opened and had not yet received ICG or laser; (b) sample that was immediately removed after PDT application; (c) PDT-treated sample that was removed at second day after application; (d) PDT-treated sample that was removed at fourth day after application; (e) PDT-treated sample that was removed at seventh day after application; (f) PDT-treated sample that was removed at 11th day after application. 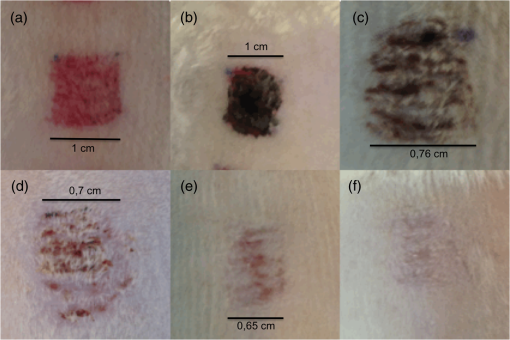 3.4.Temperature MeasurementsThe temperature of the wound before laser irradiation was measured as . In the absence of ICG, of energy dose caused only a 1.32°C temperature change after 15-min illumination. In the presence of ICG, the temperature change was 7.68°C after same duration of illumination (Table 1). ICG caused more than a 6°C change. During laser illumination, the maximum temperature reached was 39.53°C. It was still below the critical point of 45°C at which hyperthermia begins.46 Table 1Temperature change after the laser application in the presence of ICG or without ICG.
4.DiscussionPDT is regarded as a promising new antibacterial method and there are several studies concerning PDT using visible light and different photosensitizers. Researchers have generally focused on investigating more successful photosensitizers, which have a higher affinity to bacterial cells in order to obtain a better bactericidal effect.5,47–52 In this study, the near-infrared spectrum was chosen to take advantage of its deeper penetration capability through biological tissue.39 The suitable photosensitizer for 808 nm is ICG and it has some disadvantages when applied on bacterial cells. It has an anionic chemical structure and a relatively big size, which affects the interaction of this molecule with bacteria and its diffusion through the cell wall.40–44,45,53 In addition, it has been reported that ICG molecules have the capability to bind plasma proteins in 3 to 4 min.54 This situation causes ICG molecules to lose the ability to absorb enough light and subsequently to produce efficient reactive oxygen species for elimination of the bacteria. For this reason, an abrasion wound model, which has pretty low bleeding, was used to eliminate the possibility of binding to the plasma proteins in this study. In addition, ICG solution was applied to the wound with intervals of 3 min until the total volume of () was reached during laser application. Refreshing the photosensitizer on the wound every 3 min was thought to decrease the possibility of plasma protein binding and increase the possibility of light absorption and subsequent reactive oxygen production. Optimum parameters to treat abrasion wounds infected with S. aureus strain could be established. PDT application with a laser energy dose of and ICG concentrations of 500, 1000, and by applying ICG as described above resulted in a significant reduction of the bacterial cell count. More than 90% of the bacterial burden was destroyed. Since the effects of these concentrations were not significantly different from each other, was chosen to observe the effect of PDT during the healing process to diminish a possible negative effect of higher ICG concentrations. The healing process of these wounds was examined and it was observed that these wounds healed in a shorter time period than expected. When these wounds were investigated histologically, recovery could be examined in detail. It was clearly seen that there was no thermal damage depending on the laser application. Success of the treatment was clearly observed on the images of histological specimens. There was not any observed thermal damage immediately after laser irradiation or inflammatory reaction during the healing process. The organism has recovered from infection after treatment in a very short span of time. The healing period of a superficial wound is known to be between 15 and 21 days. Dai et al. also studied an abrasion wound model infected with S. aureus on mice. They showed that 90% of a of wound area was healed in 11 days.55 In our study, nearly 100% of a of wound area was healed in 11 days. 808-nm of light and ICG achieved a faster healing process on the same infected wound model. When we compared this result with the result of the treatment with an antibacterial cream (2% mupirocin), it was clearly seen that ICG-PDT treatment was more successful in eradicating infection and accelerating the healing process of the wounds. This antibacterial cream is commonly used to treat superficial wound infections and destroy several types of bacteria, including multidrug resistant strains of S. aureus. This result confirmed our hypothesis about the advantages of the antibacterial effect of ICG-PDT and its more efficient healing effect due to near-infrared light. Depending on the laser energy dose and/or power, irradiation with near-infrared light may cause thermal damage in the tissue. Increasing the tissue temperature beyond 45°C causes irreversible tissue damage, i.e., coagulation, carbonization of the healthy tissue.46,56 This could prevent or prolong the healing process of the biological tissue. Illumination with an output power of 500 mW for 15 min in the presence of ICG caused an increase of approximately 8°C on average and the total tissue temperature was still below the critical point for irreversible tissue damage. Histological analyses also confirmed these results showing that there was not any thermal damage in the target or neighboring tissue. 5.ConclusionIt was shown that PDT with ICG and an 808-nm laser light was successful in rapid eradication of viable bacterial cells, as well as providing an accelerated healing process compared to conventional antibacterial treatments. This method was also known to be advantageous since it does not cause any drug-resistance like antibiotics and easily destroys antibiotic-resistant strains of gram-positive bacteria. Besides, this treatment has minimum side effects, and is not toxic/harmful to healthy, normal cells. Still further studies are needed to improve this modality to be successful in the treatment of other types of infected wound models and destroy any kind of bacteria efficiently. After eliminating the disadvantages depending on anionic nature of ICG, i.e., doping ICG in nanoparticles and improving the efficiency of reactive oxygen species, PDT with near-infrared light and ICG would be a powerful therapy to treat chronic wound infections with a shorter healing period and minimum side effects, such as thermal damage. AcknowledgmentsWe thank the Department of Microbiology, Gazi University (Ankara, Turkey) for providing the S. aureus 1755 strain. We also thank Arzu Temizyürek, Ersin Eruz, and other staff in Vivarium, Center for Life Sciences and Technologies Research (Bogazici University) for their help in animal experiments. This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK 111E255). Transparency declarations: none to declare. ReferencesT. Dai, Y.-Y. Huang and M. R. Hamblin,
“Photodynamic therapy for localized infections—state of the art,”
Photodiagn. Photodyn. Ther., 6
(3–4), 170
–188
(2009). http://dx.doi.org/10.1016/j.pdpdt.2009.10.008 PPTHBF 1572-1000 Google Scholar
T. Maisch et al.,
“Antibacterial photodynamic therapy in dermatology,”
Photochem. Photobiol. Sci., 3
(10), 907
–917
(2004). http://dx.doi.org/10.1039/b407622b PPSHCB 1474-905X Google Scholar
G. Jori et al.,
“Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications,”
Lasers Surg. Med., 38
(5), 468
–481
(2006). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
G. Jori and G. Roncucci,
“Photodynamic therapy in microbial infections,”
Adv. Clin. Exp. Med., 15
(3), 421
–426
(2006). Google Scholar
O. Simonetti et al.,
“Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection,”
Br. J. Dermatol., 164
(5), 987
–995
(2011). http://dx.doi.org/10.1111/j.1365-2133.2011.10232.x BJDEAZ 1365-2133 Google Scholar
M. N. Khan and A. H. Naqvi,
“Antiseptics, iodine, povidone iodine and traumatic wound cleansing,”
J. Tissue Viability, 16
(4), 6
–10
(2006). http://dx.doi.org/10.1016/S0965-206X(06)64002-3 0965-206X Google Scholar
R. Sibbald et al.,
“Preparing the wound bed-debridement, bacterial balance, and moisture balance,”
Ostomy Wound Manage., 46
(11), 24
–28
(2000). 0889-5899 Google Scholar
E. Klein, D. Smith and R. Laxminarayan,
“Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005,”
Emerg. Infect. Dis., 13
(12), 1840
–1846
(2007). http://dx.doi.org/10.3201/eid1312.070629 1080-6059 Google Scholar
Z. Huang,
“A review of progress in clinical photodynamic therapy,”
Technol. Cancer Res. Treat., 4
(3), 283
–293
(2005). http://dx.doi.org/10.1177/153303460500400308 TCRTBS 1533-0346 Google Scholar
G. B. Kharkwal et al.,
“Photodynamic therapy for infections: clinical applications,”
Lasers Surg. Med., 43
(7), 755
–767
(2011). http://dx.doi.org/10.1002/lsm.21080 LSMEDI 0196-8092 Google Scholar
M. R. Hamblin et al.,
“Optical monitoring and treatment of potentially lethal wound infections in vivo,”
J. Infect. Dis., 187
(11), 1717
–1725
(2003). http://dx.doi.org/10.1086/jid.2003.187.issue-11 JIDIAQ 0022-1899 Google Scholar
J. Lin et al.,
“Toluidine blue-mediated photodynamic therapy of oral wound infections in rats,”
Lasers Med. Sci., 25
(2), 233
–238
(2010). http://dx.doi.org/10.1007/s10103-009-0700-5 LMSCEZ 1435-604X Google Scholar
O. E. Akilov et al.,
“Photodynamic therapy against intracellular pathogens: problems and potentials,”
Med. Laser Appl., 21
(4), 251
–260
(2006). http://dx.doi.org/10.1016/j.mla.2006.07.002 1615-1615 Google Scholar
K. O’Riordan, O. Akilov and T. Hasan,
“The potential for photodynamic therapy in the treatment of localized infections,”
Photodiagn. Photodyn. Ther., 2
(4), 247
–262
(2005). http://dx.doi.org/10.1016/S1572-1000(05)00099-2 PPTHBF 1572-1000 Google Scholar
T. N. Demidova et al.,
“Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria,”
J. Photochem. Photobiol. B, 81
(1), 15
–25
(2005). http://dx.doi.org/10.1016/j.jphotobiol.2005.05.007 JPPBEG 1011-1344 Google Scholar
D. Kessel,
“Photodynamic therapy: from the beginning,”
Photodiagn. Photodyn. Ther., 1
(1), 3
–7
(2004). http://dx.doi.org/10.1016/S1572-1000(04)00003-1 PPTHBF 1572-1000 Google Scholar
T. J. Dougherty et al.,
“Photodynamic therapy,”
J. Natl. Cancer Inst., 90
(12), 889
–905
(1998). http://dx.doi.org/10.1093/jnci/90.12.889 JNCIEQ 0027-8874 Google Scholar
D. Dolmans, D. Fukumara and R. Jain,
“Photodynamic therapy for cancer,”
Nat. Rev. Cancer, 3
(5), 380
–387
(2003). http://dx.doi.org/10.1038/nrc1071 NRCAC4 1474-175X Google Scholar
S. B. Brown, E. A. Brown and I. Walker,
“The present and future role of photodynamic therapy in cancer treatment,”
Lancet Oncol., 5 497
–508
(2004). http://dx.doi.org/10.1016/S1470-2045(04)01529-3 LOANBN 1470-2045 Google Scholar
Y. Itoh et al.,
“Photodynamic therapy of acne vulgaris with topical delta-aminolaevulinic acid and incoherent light in Japanese patients,”
Br. J. Dermatol., 144
(3), 575
–579
(2001). http://dx.doi.org/10.1046/j.1365-2133.2001.04086.x BJDEAZ 1365-2133 Google Scholar
K. Nouri and C. J. Ballard,
“Laser therapy for acne,”
Clin. Dermatol., 24
(1), 26
–32
(2006). http://dx.doi.org/10.1016/j.clindermatol.2005.10.020 0738-081X Google Scholar
J. M. Steinbauer et al.,
“Photodynamic therapy in dermatology,”
J. Ger. Soc. Dermatol., 8
(6), 454
–464
(2010). http://dx.doi.org/10.1111/j.1610-0387.2010.07343.x 1610-0379 Google Scholar
M. Wainwright,
“Photodynamic antimicrobial chemotherapy (PACT),”
J. Antimicrob. Chemother., 42 13
–28
(1998). http://dx.doi.org/10.1093/jac/42.1.13 JACHDX 0305-7453 Google Scholar
N. S. Soukos et al.,
“Targeted antimicrobial photochemotherapy,”
Antimicrob. Agents Chemother., 42
(10), 2595
–2601
(1998). 0066-4804 Google Scholar
M. R. Hamblin et al.,
“Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging,”
Photochem. Photobiol., 75
(1), 51
–57
(2002). http://dx.doi.org/10.1562/0031-8655(2002)075<0051:RCOWIB>2.0.CO;2 PHCBAP 0031-8655 Google Scholar
M. R. Hamblin and T. Hasan,
“Photodynamic therapy: a new antimicrobial approach to infectious disease?,”
Photochem. Photobiol. Sci., 3
(5), 436
–450
(2004). http://dx.doi.org/10.1039/b311900a PPSHCB 1474-905X Google Scholar
B. C. Wilson and M. S. Patterson,
“The physics, biophysics and technology of photodynamic therapy,”
Phys. Med. Biol., 53
(9), 61
–109
(2008). http://dx.doi.org/10.1088/0031-9155/53/9/R01 PHMBA7 0031-9155 Google Scholar
X.-J. Fu, Y. Fang and M. Yao,
“Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection,”
Biomed Res. Int., 2013 159157
(2013). http://dx.doi.org/10.1155/2013/159157 BRIIDT 2314-6133 Google Scholar
M. A. Calin and S. V. Parasca,
“Light sources for photodynamic inactivation of bacteria,”
Lasers Med. Sci., 24
(3), 453
–460
(2009). http://dx.doi.org/10.1007/s10103-008-0588-5 LMSCEZ 1435-604X Google Scholar
S. Banfi et al.,
“Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria,”
J. Photochem. Photobiol. B, 85
(1), 28
–38
(2006). http://dx.doi.org/10.1016/j.jphotobiol.2006.04.003 JPPBEG 1011-1344 Google Scholar
S. P. Tseng et al.,
“Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa,”
Lasers Surg. Med., 41
(5), 391
–397
(2009). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
X. Ragàs et al.,
“Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies,”
Lasers Surg. Med., 42
(5), 384
–390
(2010). http://dx.doi.org/10.1002/lsm.v42:5 LSMEDI 0196-8092 Google Scholar
H. M. Tang, M. R. Hamblin and C. M. N. Yow,
“A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens,”
J. Infect. Chemother., 13
(2), 87
–91
(2007). http://dx.doi.org/10.1007/s10156-006-0501-8 JICHFN 1341-321X Google Scholar
G. S. Omar, M. Wilson and S. P. Nair,
“Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light,”
BMC Microbiol., 8 111
(2008). http://dx.doi.org/10.1186/1471-2180-8-111 BMMIBC 1471-2180 Google Scholar
T. Nahimisa,
“Indocyanine green test and its development,”
Tokai J. Exp. Clin. Med., 7 419
–423
(1982). TJEMDR 0385-0005 Google Scholar
S. Fickweiler et al.,
“Indocyanine green: intracellular uptake and phototherapeutic effects in vitro,”
J. Photochem. Photobiol. B, 38
(2–3), 178
–183
(1997). http://dx.doi.org/10.1016/S1011-1344(96)07453-2 JPPBEG 1011-1344 Google Scholar
I. Fox and E. Wood,
“Indocyanine green: physical and physiologic properties,”
Proc. Staff Meet. Mayo Clin., 35 732
–744
(1960). Google Scholar
I. Fox et al.,
“New dyes for continuous recording of dilution curves in whole blood independent of variations in blood oxygen saturation,”
Proc. Staff Meet. Mayo Clin., 32
(18), 478
–484
(1956). Google Scholar
A. N. Bashkatov et al.,
“Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm,”
J. Phys. D, 38
(15), 2543
–2555
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/004 JPAPBE 0022-3727 Google Scholar
C. Abels et al.,
“Indocyanine green (ICG) and laser irradiation induce photooxidation,”
Arch. Dermatol. Res., 292
(8), 404
–411
(2000). http://dx.doi.org/10.1007/s004030000147 ADREDL 0340-3696 Google Scholar
W. Bäumler et al.,
“Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light,”
Br. J. Cancer, 80
(3–4), 360
–363
(1999). http://dx.doi.org/10.1038/sj.bjc.6690363 BJCAAI 0007-0920 Google Scholar
W. W. Tseng et al.,
“Infrared laser activation of indocyanine green inhibits growth in human pancreatic cancer,”
Pancreas, 27
(3), e42
–e45
(2003). http://dx.doi.org/10.1097/00006676-200310000-00018 PANCE4 0885-3177 Google Scholar
E. Crescenzi et al.,
“Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells,”
Mol. Cancer Ther., 3
(5), 537
–544
(2004). MCTOCF 1535-7163 Google Scholar
E. A. Genina et al.,
“Low-intensity indocyanine-green laser phototherapy of acne vulgaris: pilot study,”
J. Biomed. Opt., 9
(4), 828
–834
(2004). http://dx.doi.org/10.1117/1.1756596 JBOPFO 1083-3668 Google Scholar
N. Topaloglu, M. Gulsoy and S. Yuksel,
“Antimicrobial photodynamic therapy of resistant bacterial strains by indocyanine green and 809-nm diode laser,”
Photomed. Laser Surg., 31
(4), 155
–162
(2013). http://dx.doi.org/10.1089/pho.2012.3430 PLDHA8 1549-5418 Google Scholar
M. Niemz, Laser—Tissue Interactions: Fundamentals and Applications, 77
–80 3rd ed.Springer-Verlag, Berlin Heidelberg
(2007). Google Scholar
M. C. E. Hashimoto et al.,
“Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection: an in vivo study,”
Photochem. Photobiol., 88
(3), 590
–595
(2012). http://dx.doi.org/10.1111/php.2012.88.issue-3 PHCBAP 0031-8655 Google Scholar
J.-H. Park et al.,
“In vitro and in vivo antimicrobial effect of photodynamic therapy using a highly pure chlorin e6 against Staphylococcus aureus Xen29,”
Biol. Pharm. Bull., 35
(4), 509
–514
(2012). http://dx.doi.org/10.1248/bpb.35.509 BPBLEO 0918-6158 Google Scholar
E. Kugelberg et al.,
“Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes,”
Antimicrob. Agents Chemother., 49
(8), 3435
–3441
(2005). http://dx.doi.org/10.1128/AAC.49.8.3435-3441.2005 0066-4804 Google Scholar
P. Carvalho et al.,
“In vivo killing of Staphylococcus aureus by toluidine blue-mediated photosensitization in an animal model wounds,”
ConScientiae Saude, 7
(4), 423
–429
(2008). http://dx.doi.org/10.5585/conssaude.v7i4.1415 1677-1028 Google Scholar
P. S. Zolfaghari et al.,
“In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent,”
BMC Microbiol., 9
(27), 1
–8
(2009). http://dx.doi.org/10.1186/1471-2180-9-27 BMMIBC 1471-2180 Google Scholar
S. Sharma et al.,
“Drug discovery of antimicrobial photosensitizers using animal models,”
Curr. Pharm. Des., 17
(13), 1303
–1319
(2011). http://dx.doi.org/10.2174/138161211795703735 CPDEFP 1381-6128 Google Scholar
A.-M. Mamoon et al.,
“In vitro efficiency and mechanistic role of indocyanine green as photodynamic therapy agent for human melanoma,”
Photodiagn. Photodyn. Ther., 6
(2), 105
–116
(2009). http://dx.doi.org/10.1016/j.pdpdt.2009.05.002 PPTHBF 1572-1000 Google Scholar
A. Kirchherr, A. Briel and K. Mader,
“Stabilization of indocyanine green by encapsulation within micellar systems,”
Mol. Pharm., 6
(2), 480
–491
(2009). http://dx.doi.org/10.1021/mp8001649 1543-8384 Google Scholar
T. Dai et al.,
“Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model,”
Lasers Surg. Med., 42
(1), 38
–44
(2010). http://dx.doi.org/10.1002/lsm.v42:1 LSMEDI 0196-8092 Google Scholar
A. R. Moritz and F. C. Henriques,
“Studies of thermal injury,”
Am. J. Pathol., 23 695
–720
(1947). AJPAA4 0002-9440 Google Scholar
BiographyNermin Topaloglu obtained a BSc degree in molecular biology and genetics (Boğaziçi University, Turkey, 2005) and an MS degree in biomedical engineering (Boğaziçi University, Turkey, 2005). She finished her PhD in the Institute of Biomedical Engineering, Boğaziçi University, Turkey, in 2014. She works on laser-tissue interactions and medical laser applications. Her current research projects include antibacterial photodynamic therapy and biostimulation. Melike Güney is a PhD student at Boğaziçi University. Her major research interests are photodynamic therapy, photodynamic inactivation, and laser-tissue interaction mechanisms. Sahru Yuksel received her PhD degree from the University of Maryland, College Park (2003). After her postdoctoral work at the University of California Los Angeles, she joined the Department of Molecular Biology and Genetics, Bogazici University, as a faculty (2005–2014). She is currently working as a visiting professor in the Chulalongkorn University, Faculty of Medicine, Department of Microbiology. Her current research interests include autoinflammatory disorders, antimicrobial peptides, innate immune response, and photodynamic therapy. Murat Gülsoy is a professor with the Institute of Biomedical Engineering at Boğaziçi University. His group is working on laser applications in medicine in the Biophotonics–Medical Lasers Laboratory. His current research studies include photodynamic therapy for cancer treatment and antimicrobial usage, and developing surgical applications (e.g., laser tissue welding, laser ablation of soft tissue, and corneal welding). |

