|
|
|
Cilia-driven fluid flow clears mucus, bacteria, and particulates from the lungs.1 Despite its importance, it is a poorly understood aspect of respiratory physiology. Severe dysfunction of cilia in conditions such as primary ciliary dyskinesia (PCD) is associated with impaired respiratory mucus clearance and recurrent pulmonary infections. It is unknown, however, if subtle variations in cilia-driven mucus clearance underlie clinically significant changes in respiratory diseases severity, such as in asthma. Prior measurements of ciliary flow have shown that flow speed can vary considerably between different specimens within an experimental population.2,3 Additionally, within a single specimen, flow has been previously described to vary as a function of distance from the cilia.4,5 Thus, when quantifying subtle variations in ciliary performance, it can be helpful to make cross-sectional measurements localized near a ciliated surface. Given these considerations, there has been increasing interest in applying optical coherence tomography (OCT), a modality that offers both - to scale resolution and depth-resolved, cross-sectional imaging, toward studying ciliary physiology.5–7 We previously demonstrated that OCT-based particle tracking velocimetry (OCT-PTV) could be used to estimate the velocity flow field in the Xenopus animal model system.5 In this letter, we build on these results and show that OCT-PTV can be used to quantify subtle changes induced by physical, chemical, and genetic perturbations. We quantify changes in ciliary flow due to changes in the viscosity of the fluidic environment, disruption of the serotonin signaling pathway, and diminished molecular expression of two important ciliary proteins, dnah9 and kif3a. Additionally, we use OCT-PTV to characterize the developmental process of a ciliated surface. Xenopus embryos (tadpoles), including X. laevis and X. tropicalis species, express cilia on their epithelial surface during development. The cilia themselves provide a time-varying backscattered signal that can be detected by OCT. Using speckle variance processing, we identified ciliated patches of the epithelium [Fig. 1(a) and Video 1], consistent with the methods described in Ref. 6. The ciliated patches in turn drive a microfluidic flow that can also be imaged using OCT. After immobilizing the embryo with benzocaine and seeding microspheres in the fluid, we used OCT-PTV to estimate the microfluidic vector flow field, a spatial map showing the direction and magnitude of flow velocity at each location relative to a ciliated surface [Fig. 1(b)]. Of note, although chemical anesthetics have the potential to alter flow, benzocaine was previously described to have no discernable effect on ciliary performance,8 and we observed no visible effects. Fig. 1(a) Optical coherence tomography (OCT) speckle variance identifies ciliated epithelial cells (Video 1, MOV, 1.05 MB) [URL: http://dx.doi.org/10.1117/1.JBO.20.3.030502.1]. (b) Flow field generated by OCT-based particle tracking velocimetry. 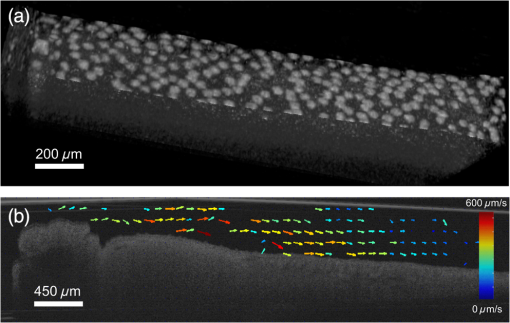 Flow field estimation using OCT-PTV provides a two-dimensional (, ), two-component (, ) description of steady-state flow. Flow near the surface of the embryos is consistently directed head to tail, but flow more than several hundreds of microns from the surface varied in magnitude and directionality depending on the exact positioning of the embryo in the well. As such, we extracted only the component of flow spatially near the surface and directed along it a metric we denote as the average tangential flow speed. The tangential flow speed was calculated by manually drawing a line tangent to the surface of the embryo, extracting the flow field measurements above the line and projecting each velocity vector along the tangent vector. These tangential flow measurements over the length of the embryos were then averaged to give the average tangential flow speed. In order to verify that average tangential flow speed could be used to quantify changes in ciliary flow, we first tested the effects of a simple physical perturbation, an increase in viscosity. We increased the viscosity of the physiologic solution [ modified Ringer’s (MR) solution] surrounding X. tropicalis embryos by adding high molecular weight dextrans (Sigma 95771, MW 2,000,000) to final concentrations of 1.3% and 2.3%. These concentrations lead to an expected viscosity of 2.0 and 2.95 cP ( and ) as estimated from Ref. 9. As the viscosity of the solution surrounding each embryo was doubled and tripled [Fig. 2(a)], the relative tangential flow speed was decreased by an average factor of and (standard error of mean), respectively. Thus, we were able to quantify changes in ciliary flow speed due to a simple physical perturbation. Fig. 2Quantification of physical, chemical, and biological perturbations of ciliary function in (a and b) X. tropicalis and (c) X. laevis. All error bars showing standard error of mean (SEM). (a) Effects of increased viscosity on average tangential flow speed (), with relative viscosity of equal to that of water. (b) Rescue of diminished ciliary flow from PCPA using serotonin (). (c) Dose-dependent knockdown of dnah9 ciliary motor protein () leads to intermediate defects in flow while knockdown of kif3a (1 pmol , 2 pmol , 4 pmol ) only leads to statistically significant decrease at highest dose. 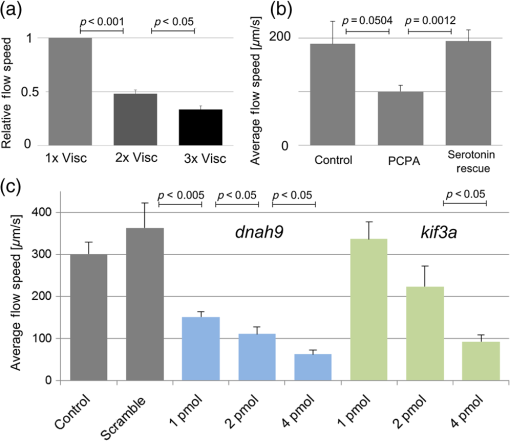 We next investigated the effects of pharmacological intervention on ciliary flow. It has been shown in Xenopus embryos that depletion of serotonin, an important signaling molecule, can decrease flow rates while repletion can restore flow.3 Following the pharmacological protocol in Ref. 3, we incubated X. tropicalis embryos with (1) control MR, (2) para-chlorophenylalanine (PCPA) (4-chloro-dl-phenylalanine methyl ester hydrochloride), an agent which depletes endogenous serotonin stores, and (3) PCPA plus 1 mM serotonin hydrochloride for repletion. Indeed, we observed that decreased flow in the PCPA-only group was rescued by the addition of serotonin [Fig. 2(b)]. We next sought to characterize novel genetic-based phenotypes. PCD can be caused by disruption in a number of genes including dynein and kinesin motor proteins.10 Common to all these genetic abnormalities is that ciliary dysfunction is defined by complete disruption of the gene, an all-or-nothing phenotype. Another plausible mechanism of disease, however, is the decreased expression of the same genes. Thus, we investigated the effects of intermediate, but not complete, knockdown of dynein axonemal heavy chain 9, dnah9, a dynein motor protein responsible for generation of movement and force in cilia, as well as kinesin family member 3a, kif3a, a kinesin motor protein involved in ciliogenesis. In order to assess the effects of intermediate levels of ciliary gene expression on the flow phenotype, we used morpholino oligonucleotides, a type of antisense technology that can decrease protein expression by preventing mRNA splicing or translation.11 Due to the existence of previously characterized morpholinos in X. laevis, we chose to investigate knockdown in X. laevis, a comparable system to X. tropicalis with a higher baseline flow speed. Using the dnah9 splice-blocking morpholino as previously described in Ref. 8, we injected varying doses into single-cell zygotes, ranging from 1 to 4 picomoles (pmol) per embryo. We coinjected an Alexa488 (Invitrogen) tracer into the embryos to verify proper delivery after 24 h. As shown in Fig. 2(c), increased morpholino dosing diminished average tangential speed in a dose-dependent manner when compared with both the uninjected controls, as well as a negative control injected with 4 pmol of a scramble morpholino sequence. Thus, we observed intermediate decreases in ciliary flow due to intermediate decreases in gene expression. This result highlights how subtle variations in ciliary function can be modulated by plausible molecular mechanisms, and how quantitative imaging can enable the detection of these intermediate phenotypes. We also investigated the effects of disrupting the kinesin motor protein kif3a [Fig. 2(c)]. Under-expression of kif3a has been associated with a more severe asthma phenotype.12 Using sequence information available on Xenbase,13 we designed a morpholino to bind to the first splice site of kif3a in X. laevis (sequence 5′-AGAGCCTCTCCTTACCGGCATTGTT-3′). Noting that an 8 pmol dosage leads to nonspecific embryo toxicity, we injected single-cell zygotes with 1 to 4 pmol. We found that, in contrast to dnah9, there was not a significant difference between the 1 and 2 pmol groups of kif3a (the power of comparison was 0.81). Only the highest dose of 4 pmol leads to a statistically significant decrease in flow ( for 2 to 4 pmol comparison, as calculated by the Mann–Whitney test). Indeed, not all genes would necessarily be expected to generate intermediate phenotypes, but some may lead to binary phenotypes after knockdown past a given threshold. More studies increasing the number of dosing levels as well as quantifying protein expression would further elucidate this hypothesis. Lastly, we used OCT to investigate how a ciliated surface transitions from the absence of flow to the presence of flow. Like other organs, ciliated surfaces undergo a developmental process. The development of coordinated ciliary flow in Xenopus embryos is known to occur over approximately 24 h and to involve the interplay between tissue patterning and hydrodynamic signaling.14 In spite of having been extensively studied from a molecular perspective, however, to our knowledge, the flow speed during this period has not yet been described in a longitudinal and quantitative manner. Thus, we quantified the average tangential speed during the onset of flow during 24 to 50 h, corresponding approximately to Nieuwkoop–Faber (NF) developmental stages 21 to 34 in X. laevis.15 We imaged nine embryos over this period, with measurements taken every 2 to 4 h [Fig. 3(a), Video 2]. For each measurement, the embryo was immobilized with benzocaine and suctioning for the duration of imaging (), and then immediately washed and placed in a reservoir of clean MR. Aggregating the data, we generated a normative curve [Fig. 3(b)] showing the average flow plus or minus one standard deviation (STD). Embryos began the process with an average flow speed of (STD) at NF stage 21, and increased to by approximately stage 34. Notably, 2/9 embryos failed to develop a sustained flow during this period. Fig. 3(a) Longitudinal measurements of developmental flow on nine different embryos (Video 2, MOV, 312 KB) [URL: http://dx.doi.org/10.1117/1.JBO.20.3.030502.2] and (b) aggregate data showing deviation during 24 to 50 h postfertilization, or approximately Nieuwkoop–Faber (NF) stages 21 to 34. 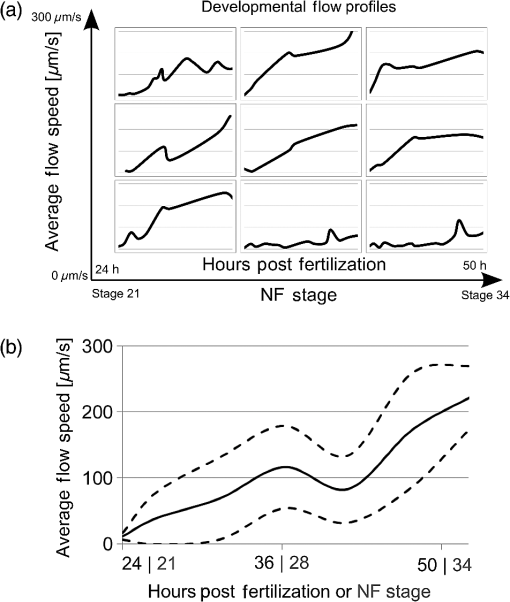 In conclusion, the physiology of cilia-driven fluid flow is important for respiratory disease, but it is currently unknown whether small perturbations can cause intermediate defects in ciliary flow. Here, we have used OCT to show that genetic perturbations can cause intermediate flow phenotypes. We were also able to characterize flow in a developmental context. OCT-PTV is well-suited toward quantifying subtle changes because it can be used to interrogate flow near a ciliated surface with high spatial resolution. Based on our results, we believe that OCT-PTV will continue to find an important role in quantitatively investigating microfluidic ciliary physiology. AcknowledgmentsThis work was supported by March of Dimes Basil O’Connor Starter Scholar Research Award and NIH1R01HL118419-01. B.K.H. was additionally supported by NIH MSTP TG T32GM07205. M.K.K. was supported by 1R21HL120783 and 1R01HD081379. ReferencesB. Huang and M. Choma,
“Microscale imaging of cilia-driven fluid flow,”
Cell. Mol. Life Sci., 72
(6), 1095
–1113
(2015). http://dx.doi.org/10.1007/s00018-014-1784-z CMLSFI 1420-9071 Google Scholar
L. N. Vandenberg, J. M. Lemire and M. Levin,
“Serotonin has early, cilia-independent roles in Xenopus left-right patterning,”
Dis. Model Mech., 6
(1), 261
–268
(2012). http://dx.doi.org/10.1242/dmm.010256 1754-8411 Google Scholar
P. Walentek et al.,
“A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles,”
Development, 141
(7), 1526
–1533
(2014). http://dx.doi.org/10.1242/dev.102343 1011-6370 Google Scholar
W. Supatto, S. E. Fraser and J. Vermot,
“An all-optical approach for probing microscopic flows in living embryos,”
Biophys. J., 95
(4), L29
–L31
(2008). http://dx.doi.org/10.1529/biophysj.108.137786 BIOJAU 0006-3495 Google Scholar
S. Jonas et al.,
“Microfluidic characterization of cilia-driven fluid flow using optical coherence tomography-based particle tracking velocimetry,”
Biomed. Opt. Express, 2
(7), 2022
–2034
(2011). http://dx.doi.org/10.1364/BOE.2.002022 BOEICL 2156-7085 Google Scholar
A. L. Oldenburg et al.,
“Monitoring airway mucus flow and ciliary activity with optical coherence tomography,”
Biomed. Opt. Express, 3
(9), 1978
–1992
(2012). http://dx.doi.org/10.1364/BOE.3.001978 BOEICL 2156-7085 Google Scholar
L. B. Liu et al.,
“Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography,”
PLoS One, 8
(1), e54473
(2013). http://dx.doi.org/10.1371/journal.pone.0054473 1932-6203 Google Scholar
P. Vick et al.,
“Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis,”
Dev. Biol., 331
(2), 281
–291
(2009). http://dx.doi.org/10.1016/j.ydbio.2009.05.547 DEBIAO 0012-1606 Google Scholar
L. Gheber, A. Korngreen and Z. Priel,
“Effect of viscosity on metachrony in mucus propelling cilia,”
Cell Motil. Cytoskeleton, 39
(1), 9
–20
(1998). http://dx.doi.org/10.1002/(ISSN)1097-0169 CMCYEO 0886-1544 Google Scholar
M. Fliegauf, T. Benzing and H. Omran,
“When cilia go bad: cilia defects and ciliopathies,”
Nat. Rev. Mol. Cell Biol., 8
(11), 880
–893
(2007). http://dx.doi.org/10.1038/nrm2278 NRMCBP 1471-0072 Google Scholar
M. K. Khokha et al.,
“Techniques and probes for the study of Xenopus tropicalis development,”
Dev. Dyn., 225
(4), 499
–510
(2002). http://dx.doi.org/10.1002/(ISSN)1097-0177 DEDYEI 1097-0177 Google Scholar
M. B. Kovacic et al.,
“Identification of KIF3A as a novel candidate gene for childhood asthma using RNA expression and population allelic frequencies differences,”
PLoS One, 6
(8), e23714
(2011). http://dx.doi.org/10.1371/journal.pone.0023714 1932-6203 Google Scholar
J. B. Bowes et al.,
“Xenbase: a Xenopus biology and genomics resource,”
Nucleic Acids Res., 36 D761
–767
(2007). http://dx.doi.org/10.1093/nar/gkm826 NARHAD 0305-1048 Google Scholar
B. Mitchell et al.,
“The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin,”
Curr. Biol., 19
(11), 924
–929
(2009). http://dx.doi.org/10.1016/j.cub.2009.04.018 CUBLE2 0960-9822 Google Scholar
P. D. Nieuwkoop and J. Faber, Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development From the Fertilized Egg Till the End of Metamorphosis, Garland Pub., New York
(1994). Google Scholar
|

