|
|
1.IntroductionNew optical methods in biomedical research, providing a wide range of information concerning the studied biological object, include optical coherence tomography,1–3 surface-enhanced Raman scattering,4,5 surface plasmon resonance,6–8 and coherent anti-Stokes Raman scattering spectroscopy.9 Yet another widely used method is elastic light scattering (ELS) from cells and tissues. ELS patterns depend on many factors such as the size and shape of the object, relative refractive index between the object and the surrounding medium, as well as the wavelength of light. To understand the behavior and connections behind light scattering from particles and measured light scattering distributions, several theoretical approaches have been advanced. They have highlighted the ratio between the size of the object and the used wavelength. Although geometric approximation and Rayleigh scattering regimes can be easily distinguished, the number of objects falling outside the scope of these theories makes quantitative analysis challenging. Particularly in the intermediate range, where particle size and wavelength are comparable, light scattering by spherical objects can be described by Mie theory.10 Moreover, different computational methods have been developed for a theoretical analysis of light scattering in that range.11–14 Continuous progress is being made in theoretical research in this field: Theoretical reviews are published, computational and simulation problems are elucidated,15,16 and a database of theoretical work is updated periodically.17,18 After the introduction of the light scattering method, much research work has been devoted to determining the size of spherical particles and measuring the angular light scattering of aerosols.19,20 Other applications include mineral and soot aerosols, volcanic ashes, snow and ice crystals, dust, and a range of particles of different shapes in astrophysical environments.21 Also single-cell applications and analysis have attracted a lot of attention. Because intracellular structures have a refractive index distribution and they affect light scattering, extensive work has been conducted to understand the origin of ELS in the identification of cells, to analyze intracellular structures, and to develop models for light scattering from cells.22–24 Forward-scattered light is primarily dependent on the size and refractive index of the cell under study,25 as well as its shape and morphology,25 whereas scattering at larger angles (50 deg to 130 deg) depends on the cell’s internal structure.26–28 Typically, the diameter of a mammalian cell is 10 to , while the diameter of the nucleus is in the range of 3 to .29 Several attempts have been made to measure the full-phase function in biological cells30–33 in order to provide a basis for detailed characterization. ELS also forms the foundation for commercial flow cytometry systems, measuring light scattering at different angles.34,35 ELS can be used to characterize biological thin samples and to differentiate the origin of subcellular structures.36,37 ELS can also be utilized to calculate the anisotropy parameter of cells, cell suspensions, and tissues, which provides valuable information for developing different types of optical noninvasive methods of investigation and can also be used to confirm theoretical predictions.24,38 ELS can be used to study the connection between cell structure and light scattering patterns, which is very important for optical diagnostic methods.28,36,38,39 In addition, instead of measuring angular distribution, elastic scattering spectroscopy (ESS) enables characterizing the sizes of subcellular components from backscattering measurements of cells and tissues.37,39–41 Many directions of research based on light scattering can be considered as ELS. A range of such methods has been presented and widely discussed in reviews and books (e.g., see Refs. 10, 36, 38, 39, and 42). Additionally, new interesting directions and specific methods are being developed, some of which have yet to appear in review papers. The development of new directions and methods often combines with the development of new technologies and the improvement of present techniques and devices with new modern approaches to known problems. This paper points out some of these new methods and approaches in order to draw researchers’ attention to them. In this way, they may serve as an initial impetus for other researchers. In addition to covering basic concepts, we shall focus on explaining and discussing different measurement principles and methods of analysis that can be used to measure ELS from single cells and subcellular structures. We are keen to present the application of ELS to single-cell scattering and to present available software for ELS analysis and modeling. This paper demonstrates some approaches to ELS applications including ESS, optical tweezers in combination with a goniometer, flow cytometry (based on ELS), and light-scattering measurement principles such as Fourier transform light scattering (FTLS), in conjunction with a microscope. Recent results of the authors will also be discussed, and examples of published data will be shown. Overall, this review aims to provide a comprehensive and easily accessible resource on ELS in a narrow and little noted application domain. 2.Theoretical AspectsELS studies were begun in the second half of the 19th century,43–45 and the scattering problem was theoretically described at the beginning of the 20th century.46–48 Extensive literature already exists on the history and theory of ELS from single particles.49–56 Due to new methods and current technical progress in computation, ELS methods are being developed in a number of directions, and new applications are constantly being found and investigated. Together with research papers, review papers and books are being published on some rapidly developing application areas of elastic scattering10,38,39,42 including flow cytometry57–60 and light scattering spectroscopy.61 Theoretical background and analysis of methods related to ELS in the biomedical context, particularly at the single-cell level, are described in some books.36,61 Cell behavior in different conditions, including interaction with nanoparticles, has been described and new models have been developed for various shapes of cells with an internal structure.59 These models include finite-difference time-domain (FDTD) modeling of clusters of nanoparticles in cell cytoplasm or randomly distributed nanoparticles on the surface of the cell nucleus,59 mononuclear cells with an inhomogeneous core,59 and a neutrophil model with nucleus.62 In addition, light scattering from cells and isolated nuclei has been modeled and measured.63 Models have also been presented for multicellular spheroids and their scattering has been compared with that of single cells.64 At the single-cell level, ELS measurements must fulfill certain conditions for single scattering. They are: (1) multiple scattering can be assumed to be negligible, (2) the scatterer will only be exposed to radiation from the original laser beam, and (3) light scattering from the particle will not be subjected to further scatter by another particle.36 In the single particle case, the incident plane wave will hit the particle and scatter as a spherical wave. When illuminating a particle or cell with a plane wave, the relation between the incident and scattered fields will be given by Eq. (1)36 (see also Refs. 49, 51, and 65): where are the complex functions of and with amplitude and phase and can be represented in a matrix form: is the incident field, whereas is related to the scattered field. The letters and denote parallel and perpendicular polarizations of the field, respectively. is the angle between the incident and scattered directions, whereas denotes the azimuthal angle of scatter. Information on light scattering from a single scatterer can be obtained by a calculation of the elements in a Müller matrix [S].36,66 This matrix provides information on cell morphology,66 but it can also reveal details about the polarization of light propagating in a multiple scattering medium.67 Because an analytical solution of the problem is challenging and has been calculated for spheres,49,50 numerical approaches have been developed for ELS from arbitrarily shaped particles. At the moment, the T-matrix11,12 and FDTD13 methods, developed by Waterman and Yee, respectively, are commonly used to model light scattering from single cells and cell organelles (e.g., Ref. 24). Much progress in this field has also been made by faster and more advanced calculation methods.68–73 References related to T-matrix calculations are systematically updated in a T-matrix database of references, making it the most commonly used method for ELS calculations today.17,18ELS distribution is affected by several factors such as polarization of light, refractive index (), particle size and shape, and the wavelength of the light. The size parameter defines the ratio between particle size and wavelength: where is the particle diameter and is the wavelength. This relation is important in ESS.40,74 Other important factors for describing light scattering at the single particle and cell level are the relative refractive index ( is the refractive index of the scatterer, and is the refractive index of the surrounding medium) and the scattering cross-section. The scattering cross-section per particle can be presented as Eq. (3),65,75 which is suitable for experimental evaluations: where is the intensity of the incident light, is the angular distribution of the intensity of light scattered by the particle, and is the scattering angle. However, it is worth remembering that Eq. (3) assumes the particles to be spherical. For a dielectric sphere (Mie theory) with parameters close to a red blood cell (RBC) irradiated by visible or NIR light (, , ), the scattering cross-section can be approximated as76 where represents the size parameters [Eq. (2)], is the relative refractive index of the particle, and is the scattering anisotropy factor, defined as the mean cosine of the scattering angle ,38 where is the phase function that describes the scattering properties of the particle and is the probability density function for scattering in some direction of a photon travelling in another direction. is a nondimensional phase function, whose integral over the solid angle is equal to 1.49,50 ELS measurements, conducted in cell suspensions and at the single-cell level, enable an estimation of the anisotropy parameter . It is an important parameter, describing the directivity of a scattering event and is used as an input parameter when simulating light propagation in tissues and cells.38,77 It has also been shown that the light transport depends on the exact form of the angular scattering probability distribution.78The Internet offers a variety of programs for calculating the characteristics of ELS particles (cells) with different parameters. A number of these programs are available on special sites,79–87 which offer the possibility to calculate ELS and other relevant parameters such as scattering, absorption, and attenuation cross-section,81 from homogeneous spheres,79 coated spheres,81 and multilayer spheres.80 Some papers describe the possibility of direct online simulation, calculation, and analysis.15,16,85–87 This is a highly welcome development as it saves valuable research time for scientifically interesting problems. Software at different levels of sophistication is provided for free use.87–90 3.Available TechniquesSeveral techniques have been developed to detect ELS from cells and tissues. In this paper, we describe some methods enabling the measurement of ELS at the single-cell level. One commonly utilized device in ELS is the goniometer, which has been used in a wide range of applications to measure ELS in diluted cell concentrations,91,92 cell suspensions,28,37,93,94 and single cells33,95 using either a cylindrical cuvette, as in Fig. 1, or a slab cuvette. Other methods combine scattering light intensity detection methods with a microscope, which also enables measuring dynamic light scattering (DLS) from cells. These techniques include FTLS, which allows simultaneous measurement of ELS and DLS properties.96 This approach lends itself to monitoring changes in cell membrane dynamics. Fig. 1Basic components of a typical goniometric setup with a cylindrical cuvette. L—light source, C—cuvette, and D—detector/detection optics. 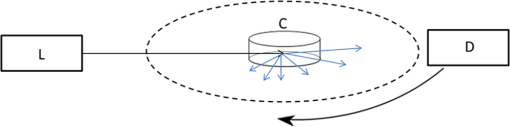 3.1.Elastic Scattering SpectroscopyESS measures backscattered light from a sample illuminated by a white light source (e.g., xenon arc lamp40). Relative scattering intensity, , is measured at a known scattering angle. Strongly dependent on the size parameter, relative scattering intensity is a linear combination of the spectra of different subcellular components.40 Relative intensity is sensitive to particle size97 and ESS provides information about the size of subcellular structures.41,97,98 A lot of effort has gone into trying to understand the theoretical basis of these phenomena and to experimentally confirm theoretical results.40,74,99 A recent application involves monitoring apoptosis on the basis of morphological changes in cell cultures.41,98 3.2.Detection of Angular Dependence of ScatteringA typical goniometric setup for the ELS measurement of single cells and cell suspensions consists of a light source, cuvette, detector, and rotating arm (Fig. 1). A stepper motor is used to rotate the detector around the sample at specific steps. These setups typically use an HeNe laser as a light source.28,33,100,101 Other approaches include multiple lasers at different wavelengths,66 a short arc Xe lamp with interference filters,102 a laser diode,103 or a coherent white-light supercontinuum laser.104 When using a phase-sensitive detection method, a chopper is implemented between the laser and the cuvette to modulate the beam.101 Cleaning and limiting beam size may also require the use of additional focusing optics and apertures.99,103 Moreover, neutral density filters are used to extend the dynamic range of the system, because the detector’s dynamic range is limited and forward-scattered light tends to be very strong.28,33 On the detection side, a typical setup includes mechanical apertures to limit the field-of-view, an optical lens to collect scattered photons, and a sensitive detecting element attached to a motorized rotating stage.28,33,100 Various detectors have been used in ELS measurements (different cuvettes and configurations). Brunsting and Mullaney105 measured light scattering with a photometer using a high-speed film as the detector, Arnfield et al. used a radiometer,106 and several other researchers have relied on photodiodes.92,93,107 Doornbos et al.33 used an avalanche photodiode. When the field-of-view is limited by a pinhole, it is also possible to use a power meter.91 Also photomultiplier tubes (PMTs) have been commonly used in recent studies (e.g., see Refs. 28, 100, and 108109.110.–111). In typically used cylindrical cuvettes (see Fig. 1), a detector rotates around the sample and measures light scattered from it.101 Scattering that originates from the background suspension can be decreased by reducing the path length of light in the suspension, for example, by using a smaller cuvette. When measuring ELS from a single object, special attention must be paid to sample purity and filtering of all unwanted dust and particles from the background medium.33,100 It is typical for goniometric measurements to take several minutes or even several tens of minutes. As a result of the long measurement time for each scattering angle, time-dependent information can easily be averaged.33 To reduce the measurement time, Watson et al.95 developed a system that employs an elliptical reflector instead of a stepper-motor rotation stage. In this setup, a fast rotating aperture disk (up to 2000 revolutions per minute) was used in front of the detector to specify the receiving angle at a specific time. This enables measuring ELS from trapped cells with high temporal resolution (in the range of a few tens of milliseconds). Also optical fibers can be used to receive light at different angles and to guide light into the detector. Wyatt,112 as well as Holthoff et al.,108 describe a multiangle measurement system using several optical fibers simultaneously. Foschum and Kienle103 measured ELS with a detector in a fixed place. These setup configurations can potentially be used in conjunction with an optical trap to select a single particle or cell for measurements. Nephelometry is a method to measure scattering phase functions of particles in a suspension. Originally, the method was developed to measure scattering phase functions of aerosol particles using an elliptical reflector.113 Thereafter, a setup using off-axis parabolas, a mirror on a motorized stage, and confocal imaging was developed to measure the angular light scattering from particle suspensions and to define the size of the particles.114,115 Other possible instrumentation approaches to measure ELS from cells include slab cuvettes with different thicknesses for cells, tissues, and tissue phantoms.37,91,92 In most cases, sample thickness is reduced and cell samples are diluted to reach the single-scattering regime.91,92 The shape of the cuvette may limit the scattering angles detectable by the instrument. Additional computing may be necessary to interpret the results.92 A novel technique, using a white light source, enables a new type of multiwavelength investigation of single particles and cells, and the principle was recently demonstrated using a slab cuvette and reflection-mode configuration.104 This instrument enables measuring hyperspectral, polarimetric, and angular light-scattering and was used to measure the properties of an in vitro model, namely multicellular tumor spheroids. 3.3.Optical Tweezer-Assisted Elastic Light Scattering StudiesOptical tweezers offer a noncontact method for capturing single particles and cells in a trap.116 They enable interaction force measurements and manipulation of micro-objects. Wright et al.117 demonstrated the usage of infrared optical tweezers for trapping a single cell in a microscope and measuring the diffraction profiles of a trapping laser diffracted from the cell by using a photodiode array. Optical tweezers can keep a particle or a cell in one place [Fig. 2(a)], while scattering patterns are measured around it by a goniometric detector in a plane orthogonal to the trapping beam [Fig. 2(b)].33,95,100 It is worth noting that the orthogonal measurements allow viewing an image of the scattering distribution around a trapped cell on a video camera (from the bottom or top direction of the cuvette).95,100 This image can be used as an aid to system adjustment as well as for analytic purposes to determine fast, dynamic changes in light scattering (see Fig. 4). Optical tweezers come in a variety of constructions. They can be formed using a high numerical-aperture water-immersion objective (),100 two counter-propagating beams with low numerical-aperture objectives (),33 or in an inverted microscope using a low numerical-aperture lens ().95 When the trapping laser and light-scattering measurement laser (HeNe) are orthogonal to each other, long working-distance objectives must be used to trap particles to prevent them from affecting and distorting the wave shape of the HeNe laser. Another important consideration is that the cells must be lifted far up from the bottom of the cuvette to minimize reflections from it.100,109 Fig. 2Schematic of an optically trapped red blood cell (RBC) (a) (modified from Ref. 110) and a combination of a double-beam optical trap and goniometric setup (b).  It is possible to use several traps to fix the position and orientation of the cell under study.100,111 Further, cylindrical lenses can be used to modify the intensity distribution of trapping lasers, which allows trapping several cells during ELS measurements.109 Special attention must be paid to the stability of the traps, the power of the trapping laser, possible heating of the sample, and potential cell damage. In most cases, an IR laser with a moderate trapping power (a few tens of milliwatts) is suitable for this type of experiment. More recently, supercontinuum white light has been used to trap and characterize a single particle.118 The researchers behind this feat also studied optical scattering spectroscopy of a single spherical scatterer, illuminated with a tightly focused supercontinuum light.119 A wide spectrum of different wavelengths enables droplet size determination by observing the spectrum of the on-axis backscattered light. In contrast to monochromatic trapping, the broad spectrum of supercontinuum light covers several resonances of the first excited Mie coefficients.120 ELS distributions depend on the size of scattering structures.121,122 Thus, to develop characterization, it is important to measure ELS in a wide angular range. As different cells have been characterized by flow cytometry and in cell suspensions, single-cell measurements with optical tweezer-assisted systems offer the advantage of more detailed analysis. Doornbos et al.33 measured ELS of a lymphocyte in the angular range of 20 deg to 60 deg. However, they experienced problems with cell stability in the trap during the measurements. Watson et al. conducted more detailed measurements on ELS and the behavior and dynamic changes of trapped cells. Figure 3 shows the measured scattering diagram from a lymphocyte and a granulocyte in a wide angular range. As seen, ELS has the ability to reveal differences between cells. An experimentally determined phase function contains information obtained from different particles in cells and can identify morphological differences. Detailed information about cells can be used for sensitive label-free analysis and cell sorting. Their group studied the effect of cell motility and rotation in the trap and found that the trapped cells oscillate a little on the millisecond time scale.95 Fig. 3Light-scattering diagrams of a normal blood lymphocyte (lower curve) and two different granulocytes (two upper curves). [Reprinted from Ref. 95, Copyright (2004), with permission from Elsevier.] 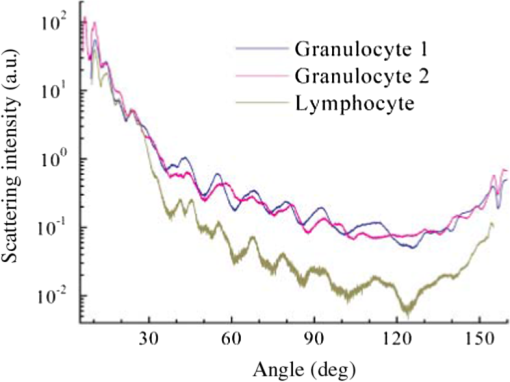 Although optical tweezers offer a clear advantage in terms of separating single cells, there are also some problems associated with scattering measurements involving a cell in an optical trap. In single-cell measurements, possible problems with optical tweezers include cells sticking to the glass cuvette, changes in cell orientation (scattering intensity fluctuations), and convection flows.33,95,100 Sticking can be overcome by coating the cuvette with an appropriate material (pluronic33 and agarose95), while changes in cell orientation and convection flows can be overcome by decreasing the size of the cuvette and increasing the power of the trap. Double-beam optical tweezers enable controlling and fixing RBC orientation during ELS measurements [Figs. 2(b) and 4].100,109 Fig. 4Trapped RBCs in different orientations, light scattering image in the orthogonal direction, and corresponding ELS distribution. Face-on incidence: (b), (c), and (e) and rim-on incidence: (a) and (g). (i) shows the scattering distributions from cells in (a) and (b), (j) scattering distributions from cells in (c) and (e), and (k) scattering distribution from cells in (g). (d), (f), and (h) show the camera images of light scattering distributions from trapped cells in (c), (e), and (g), respectively, when sample lightning has been turned off and the HeNe laser has been turned on. The arrow shows the direction of the incident laser light. (Modified from Refs. 100 and 109.) 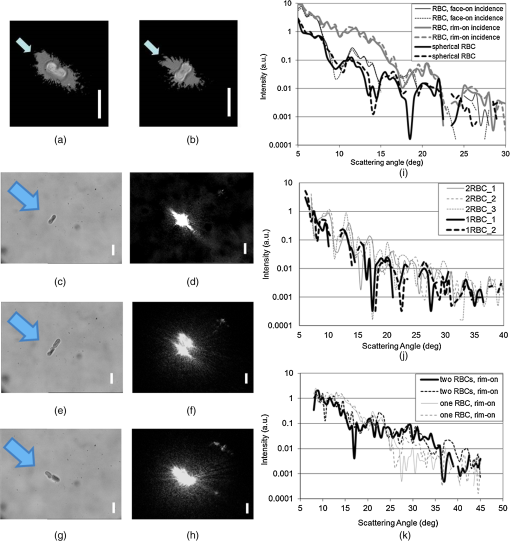 As isovolumetric sphering of RBCs is typically used in flow cytometry measurements,123 by which the theoretical models of single cells can be easily compared with those of homogeneous spheres. Also spherical models with coatings have been developed to mimic light scattering from cells and to analyze light scattering.23,124 However, they do a fairly poor job of representing real RBCs. Several theoretical attempts have been made to model ELS in RBCs, including a spheroid model,68–70,125–129 and they show that the scattering is affected by the thickness, orientation, and shape of the cell under study.126,127 Figure 4 also shows that the shape of the RBC affects light scattering. Therefore, it is important to analyze differences in the scattering patterns of spheres or spherical RBCs. These differences were experimentally demonstrated in our previous work.100 Theoretical models for ELS from RBCs based on equivalent sphere or oblate spheroid approximations are not optimal.125 The latter type, for example, is only applicable to face-on incidence in a limited angular range (0 deg to 4 deg). Essentially, the ELS technique is based on the assumption that a single-scattering regime exists in the sample and involves calculating the size and refractive index of single particles and cells. When two cells are too close to each other, dependent scattering may occur. As a result, scattering from different particles is no longer independent, as the distance and orientation of the cells in relation to the incoming laser beam induce the interference in the scattered and incoming beams. Figure 4 shows that the optical tweezers with point and elliptical traps enable adjusting the number and orientation of RBCs in measurements.109 Our previous work111 showed that two-point traps allow us to adjust the place of polystyrene spheres and RBCs and to measure dependent scattering between real cells in a phosphate-buffered saline (PBS). Thus, it is now possible to investigate dependent scattering problems experimentally, which was earlier possible only in theory.126,128 Modeling light scattering from multiple RBCs is more complicated than modeling based on single cells, since multiple scattering effects need to be considered. Results by He et al. show that, although the lateral distance of cells in face-on orientation may change, scattering probability distributions remain almost unaffected. Simulations with several cells in face-on orientation along the direction of the incident beam showed that multiple scattering becomes more pronounced and can no longer be neglected.128 3.4.Fourier Transform Light ScatteringThe FTLS method can be incorporated into a computer-controlled microscope to detect ELS. A general requirement for this method is accurate phase retrieval of the scattered field, which can be accomplished with a common path interferometer. Moreover, the illumination light beam must have full spatial coherence.96,130 Far-field scattering patterns are calculated from electric field measurements using a two-dimensional (2-D) Fourier transform.131,132 Capable of measuring both ELS and DLS, the FTLS method offers a powerful tool for characterizing biological cells. FTLS can reveal fine details in the ELS signal obtained from different cells. Ding et al.96 demonstrated that this method is capable of differentiating between several cell types including RBCs, myoblasts (C2C12), and neurons. Further, Park et al. have shown that FTLS can be used to identify and distinguish intraerythrocytic stages of Plasmodium falciparum malaria (Fig. 5). In addition, FTLS can show differences in normal and ATP-depleted RBCs and reveal correlations between ATP levels and the mechanical properties of RBCs,131 as well as measuring the Hb-content of single cells using the spectroscopic approach.132 In order to enhance the benefits of the FTLS method, single cell studies can be extended to cover blood smears. Lim et al. have developed faster analysis of ELS signals with FTLS method. A Born approximation allows calculating several parameters including the diameter and thickness as well as the depth and width of the dimple region from blood smears, enabling high-throughput analysis.133 FTLS method can also reveal changes in the shape of the object under study by measuring the anisotropic nature of the scattering signal. A case in point is the ELS distribution of normal and sickle cells.134 Recent developments of this technique include demonstrating light scattering measurements and characterizing single rod-shaped bacteria, which open new application perspectives for applications such as microfluidic sorting.135 Fig. 5(a) Amplitude and (b) phase map of a healthy RBC. (c) A retrieved light scattering pattern of the same cell. Light-intensity scattering patterns of (d) healthy RBCs, (e) ring, (f) trophozoite, and (g) schizont stages of Pf-RBCs. (h) -Values of scattering patterns of different intraerythrocytic stages of Pf-RBCs are compared with healthy RBCs. (Reprinted with permission from Ref. 131.) 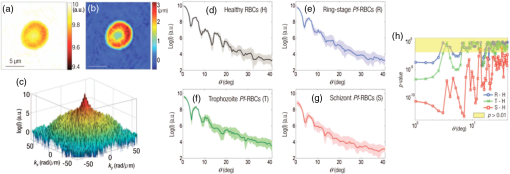 Using a white light source allows measuring the spectroscopic angular scattering properties of microscopic objects. Jung et al. demonstrated the usage of swept-source FTLS with polystyrene spheres. This method lends itself to investigating diseases such as malaria and sickle cell anemia.136 3.5.Microscopic MethodsDue to the standardized sample preparation protocols and sample holders, microscopic instruments and their modifications have obtained increasing interest. Brock et al.137 used confocal imaging and automated image processing to reconstruct a three-dimensional (3-D) model of NALM-6 cells from a stack of 2-D images. Further, they used the FDTD method to calculate angle-resolved light-scattering distributions and Müller matrix elements for the reconstructed 3-D cell and compared the ELS distributions of the reconstructed cells with those of homogeneous and coated-sphere models. They showed that the coated-sphere model is appropriate for B-cells only in the forward direction (0 deg to 20 deg), and that this region can be used to estimate the phase function. At higher angles, however, the coated-sphere model proved useless.137 As an extension of typical confocal microscopy, Itzkan et al.138 developed a confocal light absorption and scattering spectroscopic microscope which combines a confocal microscope with light-scattering spectroscopy. This microscope allows measuring the size, shape, refractive index, and location of particles smaller than the diffraction limit without exogenous labeling. Hence, it is suitable for observing submicrometer intracellular structures within living cells.138 More recently, Richter et al.139 demonstrated measurement of backscattered angular light distributions from cells using an inverted microscope and additional laser light illumination. A laser diode at the wavelength of 470 nm was used on the illumination side, while the detector was a PMT. They used ELS for cell differentiation before and after apoptosis, which induces shrinking of cells and alteration of cell shape. Richter et al. found an increase in backscattered light as a function of time in response to apoptosis induced by staurosporine. Light scattering was found to be a good complementary tool to microscopic imaging for label-free detection of apoptosis and may represent a first step toward label-free in vivo diagnostics.139 3.6.Flow CytometryFlow cytometry with different modifications is a well-known technique for light scattering-based cell characterization and has been reviewed in a number of books and review publications.57–60 It has the capacity to identify cells with high flow rates, and the analysis is based on determining their size and refractive index from the recorded scattering distributions. It also allows presenting the achieved results in cytograms and histograms.140 Flow cytometers use, among others, laser diodes and several detectors fixed at different angles to determine the strength of the light-scattering signals,140,141 whereas scattering flow cytometry enables the measurement of ELS in a wider angular range.34,35,60,142 They also use a flow cell/chamber to introduce the sample to the measurement range and are capable of measuring at high flow speeds (up to ).124 Flow cytometry has proven its position as a standard high-throughput ELS measurement method. Among clinical applications of light scattering is RBC characterization, based on hematologic parameters such as mean cell volume, mean cell hemoglobin concentration, mean cell hemoglobin mass, as well as red cell volume distribution.143,144 ELS from a single RBC enables measuring cell volume and hemoglobin.140,145 Extensive research has been conducted into measuring and analyzing ELS from lymphocytes and other white blood cells124 with flow cytometry. As a theoretical understanding of light–matter interactions and flow cytometry measurements provides a wealth of information, an exact model is necessary for the characterization of different cells. The two-layer model (coated sphere) has been found as an appropriate model to describe scattering from lymphocytes, and it can be used to solve inverse light-scattering problems.124 Earlier work with flow cytometry has shown that, by monitoring orthogonal light scattering, it is possible to distinguish cytotoxic lymphocytes from normal ones.146 An extension of normal flow cytometry, known as scanning flow cytometry,60 enables characterizing different types of white blood cells such as T lymphocytes, neutrophils, granulocytes, and monocytes.124 Using advanced optical models of white blood cells (coated sphere or multilayered sphere) and solving the inverse light-scattering problem allow us to differentiate between different classes of white blood cells.124 Recently, Konokhova et al.147 demonstrated the use of the scanning flow cytometer to measure angle-resolved light-scattering patterns of individual blood microparticles. By fitting experimental curves with the homogeneous sphere model, they were able to determine the size and refractive index of particles, thus demonstrating the possibility of label-free identification of blood microparticles, i.e., their separation from the nonspherical constituents of platelet-rich plasma. This submicron size range is an important and topical research area that requires new characterization methods. A new method has recently been developed to measure 2-D light-scattering images from single cells in a flow cytometry instrument. It takes advantage of 2-D imaging sensors to record the angular distribution of coherently scattered light from flowing particles and cells.148–150 This method is being developed to facilitate label-free cell classification and extraction of 3-D morphological features.150 Neukammer et al. used two wavelengths to separate different blood cells. They used an optical trap to position and orient single particles and particle clusters to investigate differential light scattering and demonstrated the analysis of optically trapped microspheres with light scattering from a 2-D image.149 4.Applications and Future PerspectiveMultimodal instruments allow the simultaneous application of complementary methods of studying an object. Smith and Berger151 developed an instrument that enables measuring ELS in combination with Raman signals to characterize different cells, lymphocytes, and granulocytes. This permits measuring the morphology and chemistry of cells without labeling them with, for example, fluorescence dyes. It is worth noting that when using a tightly focused laser beam for illumination, Mie theory needs to be extended to account for the non-negligible cone angle of the Gaussian beam.151 Refractive index matching (optical clearing) forms the basis for developing methods to modify optical properties, to improve light penetration, and to reduce scattering.152–154 Optical clearing has been studied at the cellular level in different contexts. Mourant et al.94 studied light scattering from cell suspensions. Due to the different refractive indices of cell and tissue components as well as PBS, the scattering properties of cells can be modeled using different background suspensions with various refractive indices. Mourant and her team found that the scattering from particles in contact with the medium decreased when the medium’s refractive index increased. This mimics the situation when cells are close to each other, as in a tissue. Popescu et al.155 monitored single RBCs during hemolysis with Hilbert phase microscopy. They established that as hemoglobin flushes out, refractive indices will be matched. In both research papers described above,94,155 the key physical principle involved matching the refractive indices of the cell surface and the background medium, thereby changing the optical properties of the cell (, ). Local hemolysis has also been studied theoretically.156 We recently studied the effect of optical clearing (matching of refractive indices) at the single-cell and single-particle levels.110,157 Optical clearing strives to manipulate optical properties such that light can penetrate deeper into tissue. It relies on refractive index matching, which increases and decreases scattering. Optical clearing also affects the scattering cross-section of cells. When an RBC was fixed in a trap and 5% glucose was used as clearing agent, the -value increased from 0.877 to 0.944.110 In this line of research, the following issues are highly important and must be taken into account. If only part of the ELS signal is measured, it will lead to problems in fitting the theoretical curve and estimating the value of . In addition, optical clearing with a high concentration of clearing solution (glycerol and glucose) changes trapping efficiency and the dynamical behavior of a cell in a trap, which needs to be considered.110 Current progress in wide areas of nanotechnology has made nanotoxicity an important issue. Flow cytometry and ELS have been used to characterize the cellular intake of nanoparticles.158 Despite their hazardous potential, nanoparticles have a range of applications in therapy and image contrast enhancement.159 There is thus an increasing need to quantify and analyze the amount of nanoparticles within cells. Fast development of measurement methods and analysis has opened a new horizon for nonlabeled imaging and quantification of the chemical structure of single cells.132,160,161 Further, the implementation of separate optical elements into mobile phones enables microscopic imaging of RBCs in the field outside of research laboratories.162 Microscopic images enable measuring cell size, cell size distribution, and cell number, making ELS-based diagnostics feasible for biomedical applications.163–166 5.ConclusionThis paper has reviewed a collection of theoretical and experimental papers related to ELS at the single-cell level. ELS complements other optical techniques and, being label-free, it deserves a place in this fast developing area. A combination of high experimental sensitivity and theoretical understanding is the key to future applications. Moreover, the development of light sources enables multispectral measurements and manipulation at the single-cell and particle level. Optical tweezers have proven a powerful tool for manipulating cell orientation and enabling measurement of ELS from trapped cells. Moreover, microscopic methods and FTLS have opened new possibilities for cellular level diagnostics, particularly as new, portable ELS-based diagnostics methods are finding their way into practical use. AcknowledgmentsThe authors wish to extend their thanks to the Finnish Funding Agency for Innovation (Fidipro project 40111/11) and to the Academy of Finland (277748) for financial support. They also wish to acknowledge the value of personal discussions with Prof. Valery Tuchin and Dr. Zuomin Zhao. ReferencesOptical Coherence Tomography Technology and Applications, Biological and Medical Physics, Biomedical Engineering, 1346 Springer, Berlin, New York
(2008). Google Scholar
R. Bernardes and J. Cunha-Vaz, Optical Coherence Tomography. A Clinical and Technical Update, Biological and Medical Physics, Biomedical Engineering XV, 255 Springer, Berlin, Heidelberg
(2012). Google Scholar
R. Wessels et al.,
“Optical biopsy of epithelial cancers by optical coherence tomography (OCT),”
Lasers Med. Sci., 29 1297
–1305
(2014). http://dx.doi.org/10.1007%2Fs10103-013-1291-8 LMSCEZ 1435-604X Google Scholar
Z. A. Nima et al.,
“Applications of surface-enhanced Raman scattering in advanced bio-medical technologies and diagnostics,”
Drug Metab. Rev., 46
(2), 155
–175
(2014). http://dx.doi.org/10.3109/03602532.2013.873451 DMTRAR 1687-9457 Google Scholar
S. Schlucker,
“Surface-enhanced Raman spectroscopy: concepts and chemical applications,”
Angew. Chem. Int. Ed., 53 4756
–4795
(2014). http://dx.doi.org/10.1002/anie.201205748 ACIEF5 1433-7851 Google Scholar
Y. Yanase et al.,
“Application of SPR imaging sensor for detection of individual living cell reactions and clinical diagnosis of type I allergy,”
Allergol. Int., 62 163
–169
(2013). http://dx.doi.org/10.2332/allergolint.12-RA-0505 ALINFR 1323-8930 Google Scholar
C. T. Campbell and G. Kim,
“SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics,”
Biomaterials, 28 2380
–2392
(2007). http://dx.doi.org/10.1016/j.biomaterials.2007.01.047 BIMADU 0142-9612 Google Scholar
Y. Yanase et al.,
“Surface plasmon resonance for cell-based clinical diagnosis,”
Sensors, 14 4948
–4959
(2014). http://dx.doi.org/10.3390/s140304948 SNSRES 0746-9462 Google Scholar
H. Tu and S. A. Boppart,
“Coherent anti-Stokes Raman scattering microscopy: overcoming technical barriers for clinical translation,”
J. Biophotonics, 7
(1–2), 9
–22
(2014). http://dx.doi.org/10.1002/jbio.v7.1/2 JBOIBX 1864-063X Google Scholar
Biomedical Photonics Handbook, CRC Press, Boca Raton, London, New York, Washington, DC
(2003). Google Scholar
P. C. Waterman,
“Matrix formulation of electro-magnetic scattering,”
Proc. IEEE, 53 805
(1965). http://dx.doi.org/10.1109/PROC.1965.4058 IEEPAD 0018-9219 Google Scholar
P. C. Waterman,
“Symmetry, unitarity, and geometry in electro-magnetic scattering,”
Phys. Rev. D, 3 825
(1971). http://dx.doi.org/10.1103/PhysRevD.3.825 PRVDAQ 1550-7998 Google Scholar
S. K. Yee,
“Numerical solution of initial boundary value problems involving Maxwell’s equations in isotropic media,”
IEEE Trans. Antennas Propag., 14 302
(1966). http://dx.doi.org/10.1109/TAP.1966.1138693 IETPAK 0018-926X Google Scholar
Light Scattering by Nonspherical Particles, Elsevier (Academic Press), London
(1999). Google Scholar
T. Wriedt,
“Light scattering theory and programs: discussion of latest advances and open problems,”
J. Quant. Spectrosc. Radiat. Transfer, 113
(18), 2465
–2469
(2012). http://dx.doi.org/10.1016/j.jqsrt.2012.03.036 JQSRAE 0022-4073 Google Scholar
T. Wriedt,
“Light scattering theory and programs: open problems and questions,”
AAPP Phys. Math. Nat. Sci., 89
(Suppl. No.1),
(2011). http://dx.doi.org/10.1478/C1V89S1P014 Google Scholar
M. I. Mishchenko et al.,
“Comprehensive T-matrix reference database: a 2011–2013 update,”
J. Quant. Spectrosc. Radiat. Transfer, 123 145
–152
(2013). http://dx.doi.org/10.1016/j.jqsrt.2013.01.024 JQSRAE 0022-4073 Google Scholar
M. I. Mishchenko et al.,
“Comprehensive T-matrix reference database: a 2013–2014 update,”
J. Quant. Spectrosc. Radiat. Transfer, 146 349
–354
(2014). http://dx.doi.org/10.1016/j.jqsrt.2014.03.022 JQSRAE 0022-4073 Google Scholar
M. Kerker and M. I. Hampton,
“The use of unfiltered light in determining particle radius by the polarization ratio of scattered the light,”
J. Opt. Soc. Am., 43
(5), 370
–371
(1953). http://dx.doi.org/10.1364/JOSA.43.000370 JOSAAH 0030-3941 Google Scholar
D. L. Jaggard et al.,
“Light scattering from particles of regular and irregular shape,”
Atmos. Environ., 15
(12), 2511
–2519
(1981). http://dx.doi.org/10.1016/0004-6981(81)90066-4 AENVEQ 0004-6981 Google Scholar
O. Munoz and J. W. Hovenier,
“Laboratory measurements of single light scattering by ensembles of randomly oriented small irregular particles in air. A review,”
J. Quant. Spectrosc. Radiat. Transfer, 112 1646
–1657
(2011). http://dx.doi.org/10.1016/j.jqsrt.2011.02.005 JQSRAE 0022-4073 Google Scholar
P. J. Wyatt,
“Differential light scattering: a physical method for identifying living bacterial cells,”
Appl. Opt., 7 1879
(1968). http://dx.doi.org/10.1364/AO.7.001879 APOPAI 0003-6935 Google Scholar
A. Brunsting and P. F. Mullaney,
“Light scattering from coated spheres: model for biological cells,”
Appl. Opt., 11
(3), 675
–680
(1972). http://dx.doi.org/10.1364/AO.11.000675 APOPAI 0003-6935 Google Scholar
A. Dunn and R. Richards-Kortum,
“Three-dimensional computation of light scattering from cells,”
Sel. Top. Quantum Electron., 2
(4), 898
–905
(1996). http://dx.doi.org/10.1109/2944.577313 IJSQEN 1077-260X Google Scholar
P. F. Mullaney et al.,
“Cell sizing: a light scattering photometer for rapid volume determination,”
Rev. Sci. Instrum., 40 1029
–1032
(1969). http://dx.doi.org/10.1063/1.1684143 RSINAK 0034-6748 Google Scholar
M. Kerker et al.,
“Light scattering and fluorescence by small particles having internal structure,”
J. Histochem. Cytochem., 27 250
–263
(1979). http://dx.doi.org/10.1177/27.1.438501 JHCYAS 0022-1554 Google Scholar
M. Kerker,
“Elastic and in elastic light scattering in flow cytometry (Paul Mullaney Memorial Lecture),”
Cytometry, 4 1
–10
(1983). http://dx.doi.org/10.1002/(ISSN)1097-0320 CYTODQ 0196-4763 Google Scholar
J. R. Mourant et al.,
“Mechanisms of light scattering form biological cells relevant to noninvasive optical-tissue diagnostics,”
Appl. Opt., 37
(16), 3586
–3593
(1998). http://dx.doi.org/10.1364/AO.37.003586 APOPAI 0003-6935 Google Scholar
A. Alberts et al., Molecular Biology of the Cell, 18
–19 Garland, New York
(1994). Google Scholar
G. C. Salzman et al.,
“Cell classification by laser light scattering: identification and separation of unstained leukocytes,”
Acta Cytol., 19 374
–377
(1975). ACYTAN 0001-5547 Google Scholar
M. R. Loken, R. G. Sweet and L. A. Herzenberg,
“Cell discrimination by multiangle light scattering,”
J. Histochem. Cytochem., 24 284
–291
(1976). http://dx.doi.org/10.1177/24.1.1254923 JHCYAS 0022-1554 Google Scholar
M. Bartholdi et al.,
“Differential light scattering photometer for rapid analysis of single particles in flow,”
Appl. Opt., 19 1573
–1581
(1980). http://dx.doi.org/10.1364/AO.19.001573 APOPAI 0003-6935 Google Scholar
R. M. P. Doornbos et al.,
“Elastic light-scattering measurements of single biological cells in an optical trap,”
Appl. Opt., 35 729
–734
(1996). http://dx.doi.org/10.1364/AO.35.000729 APOPAI 0003-6935 Google Scholar
K. A. Sem’yanov et al.,
“Calibration-free method to determine the size and hemoglobin concentration of individual red blood cells from light scattering,”
Appl. Opt., 39
(31), 5884
–5889
(2000). http://dx.doi.org/10.1364/AO.39.005884 APOPAI 0003-6935 Google Scholar
M. A. Yurkin et al.,
“Experimental and theoretical study of light scattering by individual mature red blood cells by use of scanning flow cytometry and a discrete dipole approximation,”
Appl. Opt., 44
(25), 5249
–5256
(2005). http://dx.doi.org/10.1364/AO.44.005249 APOPAI 0003-6935 Google Scholar
N. N. Boustany and N. V. Thakor,
“Light scatter spectroscopy and imaging of cellular and subcellular events,”
Biomedical Photonics Handbook, CRC Press, Boca Raton, London, New York, Washington, DC
(2003). Google Scholar
T. T. Wu and J. Y. Qu,
“Assessment of the relative contribution of cellular components to the acetowhitening effect in cell cultures and suspensions using elastic light-scattering spectroscopy,”
Appl. Opt., 46
(21), 4834
–4842
(2007). http://dx.doi.org/10.1364/AO.46.004834 APOPAI 0003-6935 Google Scholar
V. V. Tuchin, Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 840 2nd ed.SPIE Press, Bellingham, Washington
(2007). Google Scholar
N. N. Boustany, S. A. Boppart and V. Backman,
“Microscopic imaging and spectroscopy with scattered light,”
Annu. Rev. Biomed. Eng., 12 285
–314
(2010). http://dx.doi.org/10.1146/annurev-bioeng-061008-124811 ARBEF7 1523-9829 Google Scholar
H. Fang et al.,
“Noninvasive sizing of subcellular organelles with light scattering spectroscopy,”
Sel. Top. Quantum Electron., 9
(2), 267
–276
(2003). http://dx.doi.org/10.1109/JSTQE.2003.812515 IJSQEN 1077-260X Google Scholar
C. S. Mulvey et al.,
“Wavelength-dependent backscattering measurements for quantitative monitoring of apoptosis, Part 2: early spectral changes during apoptosis are linked to apoptotic volume decrease,”
J. Biomed. Opt., 16
(11), 117002
(2011). http://dx.doi.org/10.1117/1.3644911 JBOPFO 1083-3668 Google Scholar
Handbook of Optical Biomedical Diagnostics, SPIE Press, Bellingham, Washington
(2002). Google Scholar
L. Rayleigh,
“On the light from the sky, its polarization and colour,”
Philos. Mag., XLI 107274
–120279
(1871). PMHABF 1478-6435 Google Scholar
L. Rayleigh,
“On the scattering of light by small particles,”
Philos. Mag., XLI 447
–454
(1871). PMHABF 1478-6435 Google Scholar
L. Rayleigh,
“On the transmission of light through an atmosphere containing small particles in suspension, and on the origin of the blue of the sky,”
Philos. Mag., XLVII 375
–384
(1899). http://dx.doi.org/10.1080/14786449908621276 PMHABF 1478-6435 Google Scholar
G. Mie,
“Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen,”
Ann. Phys., 330
(3), 377
–445
(1908). http://dx.doi.org/10.1002/(ISSN)1521-3889 ANPYA2 0003-3804 Google Scholar
G. Mie,
“Contributions to the optics of turbid media, particularly of colloidal metal solutions,”
R. Aircr. Establ., Libr. Transl., 1873 1
–72
(1976). Google Scholar
A. E. H. Love,
“The scattering of electric waves by a dielectric sphere,”
Proc. London Math. Soc., 30
(678), 308
–321
(1899). PLMTAL 0024-6115 Google Scholar
C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, New York
(1983). Google Scholar
H. C. van de Hulst, Light Scattering by Small Particles, John Wiley & and Sons Inc., New York
(1957). Google Scholar
R. G. Newton, Scattering Theory of Waves and Particles, 2nd ed.Springer-Verlag, New York
(1982, 2002). Google Scholar
T. Wriedt,
“A review of elastic light scattering theories,”
Part. Part. Syst. Charact., 15 67
–74
(1998). http://dx.doi.org/10.1002/(ISSN)1521-4117 PPCHEZ 0934-0866 Google Scholar
N. A. Logan,
“Early history of the Mie solution,”
J. Opt. Soc. Am., 52 342
(1962). http://dx.doi.org/10.1364/JOSA.52.0342_1 JOSAAH 0030-3941 Google Scholar
N. A. Logan,
“Survey of some early studies of the scattering of plane waves by a sphere,”
Proc. IEEE, 53
(8), 773
–785
(1965). http://dx.doi.org/10.1109/PROC.1965.4055 IEEPAD 0018-9219 Google Scholar
A. L. Aden and M. Kerker,
“Scattering of electromagnetic waves from two concentric spheres,”
J. Appl. Phys., 22 1242
(1951). http://dx.doi.org/10.1063/1.1699834 JAPIAU 0021-8979 Google Scholar
M. I. Mishchenko, L. D. Travis and A. A. Lacis, Scattering, Absorption, and Emission of Light by Small Particles, Cambridge University Press, Cambridge
(2002). Google Scholar
Flow Cytometry: Principles and Applications, Humana Press, Totowa, New Jersey
(2007). Google Scholar
Flow Cytometry: Principles, Methodology and Applications (Cell Biology Research Progress), Nova Science Publishers, New York
(2013). Google Scholar
Advanced Optical Flow Cytometry: Methods and Disease Diagnoses, Wiley-VCH, Weinheim
(2011). Google Scholar
Scanning Flow Cytometry, Cytometry and Biokinetics Laboratory Institute of Chemical Kinetics and Combustion, Novosibirsk, Russia,
(2015) http://www.kinetics.nsc.ru/llpc/cyto/sfc01.html February ). 2015). Google Scholar
L. T. Perelman and V. Backman,
“Light scattering spectroscopy of epithelial tissues: principles and applications,”
Handbook of Optical Biomedical Diagnostics, SPIE Press, Bellingham, Washington
(2002). Google Scholar
D. Y. Orlova et al.,
“Light scattering by neutrophils: model, simulation, and experiment,”
J. Biomed. Opt., 13 054057
(2008). http://dx.doi.org/10.1117/1.2992140 JBOPFO 1083-3668 Google Scholar
J. R. Mourant et al.,
“Polarized angular dependent spectroscopy of epithelial cells and epithelial cell nuclei to determine the size scale of scattering structures,”
J. Biomed. Opt., 7
(3), 378
–387
(2002). http://dx.doi.org/10.1117/1.1483317 JBOPFO 1083-3668 Google Scholar
J. R. Mourant et al.,
“Angular dependent light scattering from multicellular spheroids,”
J. Biomed. Opt., 7
(1), 93
–99
(2002). http://dx.doi.org/10.1117/1.1427053 JBOPFO 1083-3668 Google Scholar
V. V. Tuchin, L. Wang and D. A. Zimnyakov, Optical Polarization in Biomedical Applications, Springer-Verlag, Berlin, Heidelberg
(2006). Google Scholar
H. Ding et al.,
“Angle-resolved Mueller matrix study of light scattering by B-cells at three wavelengths of 442, 633, and 850 nm,”
J. Biomed. Opt., 12
(3), 034032
(2007). http://dx.doi.org/10.1117/1.2749730 JBOPFO 1083-3668 Google Scholar
S. Bartel and A. H. Hielscher,
“Monte Carlo simulations of the diffuse backscattering Mueller matrix for highly scattering media,”
Appl. Opt., 39
(10), 1580
–1588
(2000). http://dx.doi.org/10.1364/AO.39.001580 APOPAI 0003-6935 Google Scholar
E. Eremina, Y. Eremin and T. Wriedt,
“Analysis of light scattering by erythrocyte based on discrete sources method,”
Opt. Commun., 244 15
–23
(2005). http://dx.doi.org/10.1016/j.optcom.2004.09.037 OPCOB8 0030-4018 Google Scholar
E. Eremina et al.,
“Different shape models for erythrocyte: light scattering analysis based on the discrete sources method,”
J. Quant. Spectrosc. Radiat. Transfer, 102 3
–10
(2006). http://dx.doi.org/10.1016/j.jqsrt.2006.02.067 JQSRAE 0022-4073 Google Scholar
D. Petrov, Y. Shkuratov and G. Videen,
“Light scattering by arbitrary shaped particles with rough surfaces: Sh-matrices approach,”
J. Quant. Spectrosc. Radiat. Transfer, 113 2406
–2418
(2012). http://dx.doi.org/10.1016/j.jqsrt.2012.04.016 JQSRAE 0022-4073 Google Scholar
L. Nagdimunov, L. Kolokolova and D. Mackowski,
“Characterization and remote sensing of biological particles using circular polarization,”
J. Quant. Spectrosc. Radiat. Transfer, 131 59
–65
(2013). http://dx.doi.org/10.1016/j.jqsrt.2013.04.018 JQSRAE 0022-4073 Google Scholar
L. Bi and P. Yang,
“Modeling of light scattering by biconcave and deformed red blood cells with the invariant imbedding T-matrix method,”
J. Biomed. Opt., 18
(5), 055001
(2013). http://dx.doi.org/10.1117/1.JBO.18.5.055001 JBOPFO 1083-3668 Google Scholar
M. I. Mishchenko, L. D. Travis and D. W. Mackowski,
“T-matrix computations of light scattering by nonspherical particles: a review,”
J. Quant. Spectrosc. Radiat. Transfer, 55
(5), 535
–575
(1996). http://dx.doi.org/10.1016/0022-4073(96)00002-7 JQSRAE 0022-4073 Google Scholar
M. Canpolat and J. R. Mourant,
“Particle size analysis of turbid media with a single optical fiber in contact with the medium to deliver and detect white light,”
Appl. Opt., 40
(22), 3792
–3799
(2001). http://dx.doi.org/10.1364/AO.40.003792 APOPAI 0003-6935 Google Scholar
V. V. Tuchin and G. B. Altshuler,
“Dental and oral tissue optics,”
Fundamentals and Applications of Biophotonics in Dentistry, Vol. 4 Series on Biomaterials and Bioengineering, 327 Imperial College Press, London
(2007). Google Scholar
R. Graaff et al.,
“Reduced light scattering properties for mixtures of spherical particles: a simple approximation derived from Mie calculations,”
Appl. Opt., 31
(10), 1370
–1376
(1992). http://dx.doi.org/10.1364/AO.31.001370 APOPAI 0003-6935 Google Scholar
S. L. Jacques,
“Optical properties of biological tissues: a review,”
Phys. Med. Biol., 58 R37
–R61
(2013). http://dx.doi.org/10.1088/0031-9155/58/11/R37 PHMBA7 0031-9155 Google Scholar
J. R. Mourant et al.,
“Influence of the scattering phase function on light transport measurements in turbid media performed with small source-detector separations,”
Opt. Lett., 21
(7), 546
–548
(1996). http://dx.doi.org/10.1364/OL.21.000546 OPLEDP 0146-9592 Google Scholar
“Light Scattering Information Portal for the light scattering community,”
(2015) http://www.scattport.org/ February ). 2015). Google Scholar
Guangran Kevin Zhu,
“Matlab Central: Sphere scattering,”
(2015) http://www.mathworks.com/matlabcentral/fileexchange/31119-sphere-scattering February ). 2015). Google Scholar
Miroslaw Jonasz,
“MJC Optical Technology: Light scattering calculator: coated sphere,”
(2015) http://www.mjcopticaltech.com/Products/LscCoatSphHelp.htm#Introduction February ). 2015). Google Scholar
O. Pena-Rodriquez, P. P. G. Perez and U. Pal,
“MieLab: a software tool to perform calculations on the scattering of electromagnetic waves by multilayered spheres,”
Int. J. Spectrosc., 2011 583743
(2011). http://dx.doi.org/10.1155/2011/583743 Google Scholar
Scott Prahl,
“Oregon Medical Laser Center; Mie Scattering,”
(2015) http://omlc.org/software/mie/ February ). 2015). Google Scholar
Bernhard Michel,
“MieCalc–freely configurable program for light scattering calculations (Mie theory),”
(2015) http://www.lightscattering.de/MieCalc/eindex.html February ). 2015). Google Scholar
J. Hellmers and T. Wriedt,
“Classification of software for the simulation of light scattering and realization within an internet information portal,”
J. Univers. Comput. Sci., 16
(9), 1176
–1189
(2010). http://dx.doi.org/10.3217/jucs-016-09-1176 0948-695X Google Scholar
J. Hellmers et al.,
“ScattPort light scattering internet information portal: present state and further development,”
Electromagnetic and Light Scattering XII,, 74
–77 University of Helsinki, Helsinki
(2010). Google Scholar
Laven Philip,
“MiePlot: A computer program for scattering of light from a sphere using Mie theory & the Debye series,”
(2015) http://www.philiplaven.com/mieplot.htm February ). 2015). Google Scholar
J. Hellmers et al.,
“Customizable web service interface for light scattering simulation programs,”
J. Quant. Spectrosc. Radiat. Transfer, 113 2243
–2250
(2012). http://dx.doi.org/10.1016/j.jqsrt.2012.07.006 JQSRAE 0022-4073 Google Scholar
J. Leinonen,
“High-level interface to T-matrix scattering calculations: architecture, capabilities and limitations,”
Opt. Express, 22
(2), 1655
–1660
(2014). http://dx.doi.org/10.1364/OE.22.001655 OPEXFF 1094-4087 Google Scholar
M. A. Yurkin and A. G. Hoekstra,
“The discrete-dipole-approximation code ADDA: capabilities and known limitations,”
J. Quant. Spectrosc. Radiat. Transfer, 112 2234
–2247
(2011). http://dx.doi.org/10.1016/j.jqsrt.2011.01.031 JQSRAE 0022-4073 Google Scholar
M. Hammer et al.,
“Single scattering by red blood cells,”
Appl. Opt., 37
(31), 7410
–7418
(1998). http://dx.doi.org/10.1364/AO.37.007410 APOPAI 0003-6935 Google Scholar
J. M. Steinke and A. P. Shepherd,
“Comparison of Mie theory and the light scattering of red blood cells,”
Appl. Opt., 27
(19), 4027
–4033
(1988). http://dx.doi.org/10.1364/AO.27.004027 APOPAI 0003-6935 Google Scholar
R. Drezek, A. Dunn and R. Richards-Kortum,
“Light scattering from cells: finite-difference time-domain simulations and goniometric measurements,”
Appl. Opt., 38
(16), 3651
–3661
(1999). http://dx.doi.org/10.1364/AO.38.003651 APOPAI 0003-6935 Google Scholar
J. R. Mourant et al.,
“Light scattering from cells: the contribution of the nucleus and the effects of proliferative status,”
J. Biomed. Opt., 5
(2), 131
–137
(2000). http://dx.doi.org/10.1117/1.429979 JBOPFO 1083-3668 Google Scholar
D. Watson et al.,
“Elastic light scattering from single cells: orientational dynamics in optical trap,”
Biophys. J., 87
(2), 1298
–1306
(2004). http://dx.doi.org/10.1529/biophysj.104.042135 BIOJAU 0006-3495 Google Scholar
H. Ding et al.,
“Fourier transform light scattering of biological structure and dynamics,”
IEEE J. Sel. Top. Quantum Electron., 16
(4), 909
–918
(2010). http://dx.doi.org/10.1109/JSTQE.2009.2034752 IJSQEN 1077-260X Google Scholar
J. R. Mourant, T. M. Johnson and J. P. Freyer,
“Characterizing mammalian cells and cell phantoms by polarized backscattering fiber-optic measurements,”
Appl. Opt., 40
(28), 5114
–5123
(2001). http://dx.doi.org/10.1364/AO.40.005114 APOPAI 0003-6935 Google Scholar
C. S. Mulvey, I. J. Bigio and C. A. Sherwood,
“Wavelength-dependent backscattering measurements for quantitative real-time monitoring of apoptosis in living cells,”
J. Biomed. Opt., 14
(6), 064013
(2009). http://dx.doi.org/10.1117/1.3259363 JBOPFO 1083-3668 Google Scholar
A. Amelink and H. J. C. M Sterenborg,
“Measurement of the local optical properties of turbid media by differential path-length spectroscopy,”
Appl. Opt., 43
(15), 3048
–3054
(2004). http://dx.doi.org/10.1364/AO.43.003048 APOPAI 0003-6935 Google Scholar
M. Kinnunen et al.,
“Effect of the size and shape of a red blood cell on elastic light scattering properties at the single-cell level,”
Biomed. Opt. Express, 2 1803
–1814
(2011). http://dx.doi.org/10.1364/BOE.2.001803 BOEICL 2156-7085 Google Scholar
M. Kinnunen et al.,
“Low-intensity light detection methods for selected biophotonic applications,”
Proc. SPIE, 9421 94210D
(2014). http://dx.doi.org/10.1117/12.2084915 PSISDG 0277-786X Google Scholar
R. A. Bolt and F. F. M. de Mul,
“Goniometric instrument for light scattering measurement of biological tissues and phantoms,”
Rev. Sci. Instrum., 73
(5), 2211
–2213
(2002). http://dx.doi.org/10.1063/1.1472466 RSINAK 0034-6748 Google Scholar
F. Foschum and A. Kienle,
“Optimized goniometer for determination of the scattering phase function of suspended particles: simulations and measurements,”
J. Biomed. Opt., 18
(8), 085002
(2013). http://dx.doi.org/10.1117/1.JBO.18.8.085002 JBOPFO 1083-3668 Google Scholar
R. Ceolato et al.,
“Light-scattering by aggregates of tumor cells: spectral, polarimetric, and angular measurements,”
J. Quant. Spectrosc. Radiat. Transfer, 146 207
–213
(2014). http://dx.doi.org/10.1016/j.jqsrt.2014.04.027 JQSRAE 0022-4073 Google Scholar
A. Brunsting and P. F. Mullaney,
“Differential light scattering from spherical mammalian cells,”
Biophys. J., 14 439
–453
(1974). http://dx.doi.org/10.1016/S0006-3495(74)85925-4 BIOJAU 0006-3495 Google Scholar
M. R. Arnfield, J. Tulip and M. S. McPhee,
“Optical propagation in tissue with anisotropic scattering,”
IEEE Trans. Biomed. Eng., 35
(5), 372
–381
(1988). http://dx.doi.org/10.1109/10.1396 IEBEAX 0018-9294 Google Scholar
J. D. Wilson et al.,
“Light scattering from intact cells reports oxidative-stress-induced mitochondrial swelling,”
Biophys. J., 88 2929
–2938
(2005). http://dx.doi.org/10.1529/biophysj.104.054528 BIOJAU 0006-3495 Google Scholar
H. Holthoff et al.,
“Coagulation rate measurement of colloidal particles by simultaneous static and dynamic light scattering,”
Langmuir, 12 5541
–5549
(1996). http://dx.doi.org/10.1021/la960326e LANGD5 0743-7463 Google Scholar
A. Kauppila et al.,
“Elastic light scattering measurements from multiple red blood cells in elliptical optical tweezers,”
Proc. SPIE, 8097 80970K
(2011). http://dx.doi.org/10.1117/12.893383 PSISDG 0277-786X Google Scholar
M. Kinnunen et al.,
“Optical clearing at a cellular level,”
J. Biomed. Opt., 19
(7), 071409
(2014). http://dx.doi.org/10.1117/1.JBO.19.7.071409 JBOPFO 1083-3668 Google Scholar
M. Kinnunen et al.,
“Measurement of elastic light scattering from two optically trapped microspheres and red blood cells in a transparent medium,”
Opt. Lett., 36
(18), 3554
–3556
(2011). http://dx.doi.org/10.1364/OL.36.003554 OPLEDP 0146-9592 Google Scholar
P. J. Wyatt,
“Light scattering and the absolute characterization of macromolecules,”
Anal. Chim. Acta, 272 1
–40
(1993). http://dx.doi.org/10.1016/0003-2670(93)80373-S ACACAM 0003-2670 Google Scholar
W. Kaller,
“A new polar nephelometer for measurement of atmospheric aerosols,”
J. Quant. Spectrosc. Radiat. Transfer, 87 107
–117
(2004). http://dx.doi.org/10.1016/j.jqsrt.2003.12.018 JQSRAE 0022-4073 Google Scholar
J.-L. Castagner and I. J. Bigio,
“Polar nephelometer based on rotational confocal imaging setup,”
Appl. Opt., 45
(10), 2232
–2239
(2006). http://dx.doi.org/10.1364/AO.45.002232 APOPAI 0003-6935 Google Scholar
J.-L. Castagner and I. J. Bigio,
“Particle sizing with a fast polar nephelometer,”
Appl. Opt., 46
(4), 527
–532
(2007). http://dx.doi.org/10.1364/AO.46.000527 APOPAI 0003-6935 Google Scholar
A. Ashkin et al.,
“Observation of a single-beam gradient force optical trap for dielectric particles,”
Opt. Lett., 11 288
–290
(1986). http://dx.doi.org/10.1364/OL.11.000288 OPLEDP 0146-9592 Google Scholar
W. H. Wright et al.,
“Measurement of light scattering from cells using an inverted infrared optical trap,”
Proc. SPIE, 1427 279
–287
(1991). http://dx.doi.org/10.1117/12.44113 PSISDG 0277-786X Google Scholar
P. Li, K. Shi and Z. Liu,
“Manipulation and spectroscopy of a single particle by use of white-light optical tweezers,”
Opt. Lett., 30
(2), 156
–158
(2005). http://dx.doi.org/10.1364/OL.30.000156 OPLEDP 0146-9592 Google Scholar
P. Li, K. Shi and Z. Liu,
“Optical scattering spectroscopy by using tightly focused supercontinuum,”
Opt. Express, 13
(22), 9039
–9044
(2005). http://dx.doi.org/10.1364/OPEX.13.009039 OPEXFF 1094-4087 Google Scholar
M. Guillon, K. Dholakia and D. McGloin,
“Optical trapping and spectral analysis of aerosols with a supercontinuum laser source,”
Opt. Express, 16
(11), 7655
–7664
(2008). http://dx.doi.org/10.1364/OE.16.007655 OPEXFF 1094-4087 Google Scholar
J. D. Wilson and T. H. Foster,
“Mie theory interpretations of light scattering from intact cells,”
Opt. Lett., 30
(18), 2442
–2444
(2005). http://dx.doi.org/10.1364/OL.30.002442 OPLEDP 0146-9592 Google Scholar
J. D. Wilson,
“Measurements and interpretations of light scattering from intact biological cells,”
231 University of Rochester,
(2007). Google Scholar
Y. R. Kim and L. Ornstein,
“Isovolumetric sphering of erythrocytes for more accurate and precise cell volume measurement by flow cytometry,”
Cytometry, 3
(6), 419
–427
(1983). http://dx.doi.org/10.1002/(ISSN)1097-0320 CYTODQ 0196-4763 Google Scholar
V. P. Maltsev, A. G. Hoekstra and M. A. Yurkin,
“Optics of white blood cells: optical models, simulations, and experiments,”
Advanced Optical Flow Cytometry: Methods and Disease Diagnoses, Wiley-VCH, Weinheim
(2011). Google Scholar
S. V. Tsinopoulos and D. Polyzos,
“Scattering of He-Ne laser light by an average-sized red blood cell,”
Appl. Opt., 38 5499
–5510
(1999). http://dx.doi.org/10.1364/AO.38.005499 APOPAI 0003-6935 Google Scholar
A. Karlsson et al.,
“Numerical simulations of light scattering by red blood cells,”
IEEE Trans. Biomed. Eng., 52 13
–18
(2005). http://dx.doi.org/10.1109/TBME.2004.839634 IEBEAX 0018-9294 Google Scholar
A. M. K. Nilsson et al.,
“T-matrix computations of light scattering by red blood cells,”
Appl. Opt., 37 2735
–2748
(1998). http://dx.doi.org/10.1364/AO.37.002735 APOPAI 0003-6935 Google Scholar
J. He et al.,
“Light scattering by multiple red blood cells,”
J. Opt. Soc. Am. A, 21 1953
–1961
(2004). http://dx.doi.org/10.1364/JOSAA.21.001953 JOAOD6 0740-3232 Google Scholar
T. Wriedt et al.,
“Light scattering by single erythrocyte: comparison of different methods,”
J. Quant. Spectrosc. Radiat. Transfer, 100 444
–456
(2006). http://dx.doi.org/10.1016/j.jqsrt.2005.11.057 JQSRAE 0022-4073 Google Scholar
H. Ding et al.,
“Fourier transform light scattering of inhomogeneous and dynamic structures,”
Phys. Rev. Lett., 101 238102
(2008). http://dx.doi.org/10.1103/PhysRevLett.101.238102 PRLTAO 0031-9007 Google Scholar
Y. Park et al.,
“Static and dynamic light scattering of healthy and malaria-parasite invaded red blood cells,”
J. Biomed. Opt., 15
(2), 020506
(2010). http://dx.doi.org/10.1117/1.3369966 JBOPFO 1083-3668 Google Scholar
Y. Park et al.,
“Spectroscopic phase microscopy for quantifying hemoglobin concentrations in intact red blood cells,”
Opt. Lett., 34
(23), 3668
–3670
(2009). http://dx.doi.org/10.1364/OL.34.003668 OPLEDP 0146-9592 Google Scholar
J. Lim et al.,
“Born approximation model for light scattering by red blood cells,”
Biomed. Opt. Express, 2
(10), 2784
–2791
(2011). http://dx.doi.org/10.1364/BOE.2.002784 BOEICL 2156-7085 Google Scholar
Y. Kim et al.,
“Anisotropic light scattering of individual sickle red blood cells,”
J. Biomed. Opt., 17
(4), 040501
(2012). http://dx.doi.org/10.1117/1.JBO.17.4.040501 JBOPFO 1083-3668 Google Scholar
Y. Jo et al.,
“Angle-resolved light scattering of individual rod-shaped bacteria based on Fourier transform light scattering,”
Sci. Rep., 4 5090
(2014). http://dx.doi.org/10.1038/srep05090 SRCEC3 2045-2322 Google Scholar
J. Jung and Y. Park,
“Spectro-angular light scattering measurements of individual microscopic objects,”
Opt. Express, 22
(4), 4108
–4114
(2014). http://dx.doi.org/10.1364/OE.22.004108 OPEXFF 1094-4087 Google Scholar
R. S. Brock et al.,
“Effect of detailed cell structure on light scattering distribution: FDTD study of a B-cell with 3D structure constructed from confocal images,”
J. Quant. Spectrosc. Radiat. Transfer, 102 25
–36
(2006). http://dx.doi.org/10.1016/j.jqsrt.2006.02.075 JQSRAE 0022-4073 Google Scholar
I. Itzkan et al.,
“Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels,”
Proc. Natl. Acad. Sci. U. S. A., 104
(44), 17255
–17260
(2007). http://dx.doi.org/10.1073/pnas.0708669104 PNASA6 0027-8424 Google Scholar
V. Richter et al.,
“Light scattering microscopy with angular resolution and its possible application to apoptosis,”
J. Microsc., 257
(1), 1
–7
((2015). http://dx.doi.org/10.1111/jmi.12180 JMICAR 0022-2720 Google Scholar
D. H. Tycko et al.,
“Flow-cytometric light scattering measurement of red blood cell volume and hemoglobin concentration,”
Appl. Opt., 24
(9), 1355
–1365
(1985). http://dx.doi.org/10.1364/AO.24.001355 APOPAI 0003-6935 Google Scholar
M. Brown and C. Wittwer,
“Flow cytometry: principles and clinical applications in hematology,”
Clin. Chem., 46
(8), 1221
–1229
(2000). CLCHAU 0009-9147 Google Scholar
V. P. Maltsev,
“Scanning flow cytometry for individual particle analysis,”
Rev. Sci. Instrum., 71
(1), 243
–255
(2000). http://dx.doi.org/10.1063/1.1150190 RSINAK 0034-6748 Google Scholar
M. M. Wintrobe et al., Clinical Hematology, 18 Lea & Febiger, Philadelphia, Pennsylvania
(1981). Google Scholar
J. D. Bessman and R. K. Johnson,
“Erythrocyte volume distribution in normal and abnormal subjects,”
Blood, 46 369
(1975). BLOOAW 0006-4971 Google Scholar
N. Mohandas et al.,
“Accurate and independent measurement of volume and hemoglobin concentration of individual red cells by laser light scattering,”
Blood, 68
(2), 506
–513
(1986). BLOOAW 0006-4971 Google Scholar
L. W. Terstappen et al.,
“Discrimination of human cytotoxic lymphocytes from regulatory and B-lymphocytes by orthogonal light scattering,”
J. Immunol. Methods, 95 211
–216
(1986). http://dx.doi.org/10.1016/0022-1759(86)90408-4 JIMMBG 0022-1759 Google Scholar
A. I. Konokhova et al.,
“Light-scattering flow cytometry for identification and characterization of blood microparticles,”
J. Biomed. Opt., 17
(5), 057006
(2012). http://dx.doi.org/10.1117/1.JBO.17.5.057006 JBOPFO 1083-3668 Google Scholar
S. Holler et al.,
“Two-dimensional angular optical scattering for the characterization of airborne microparticles,”
Opt. Lett., 23 1489
–1491
(1998). http://dx.doi.org/10.1364/OL.23.001489 OPLEDP 0146-9592 Google Scholar
J. Neukammer et al.,
“Angular distribution of light scattered by single biological cells and oriented particle agglomerates,”
Appl. Opt., 42
(31), 6388
–6397
(2003). http://dx.doi.org/10.1364/AO.42.006388 APOPAI 0003-6935 Google Scholar
X.-H. Hu and J. Q. Lu,
“Label-free cell classification with diffraction imaging flow cytometer,”
Advanced Optical Flow Cytometry: Methods and Disease Diagnoses, Wiley-VCH, Weinheim
(2011). Google Scholar
Z. J. Smith and A. J. Berger,
“Validation of an integrated Raman- and angular scattering microscopy system on heterogeneous bead mixtures and single human immune cells,”
Appl. Opt., 48
(10), D109
–D120
(2009). http://dx.doi.org/10.1364/AO.48.00D109 APOPAI 0003-6935 Google Scholar
E. A. Genina, A. N. Bashkatov and V. V. Tuchin,
“Tissue optical immersion clearing,”
Expert Rev. Med. Devices, 7
(6), 825
–842
(2010). http://dx.doi.org/10.1586/erd.10.50 1743-4440 Google Scholar
V. V. Tuchin, Optical Clearing of Tissues and Blood, 256 SPIE Press, Bellingham, Washington
(2006). Google Scholar
D. Zhu et al.,
“Recent progress in tissue optical clearing,”
Laser Photonics Rev., 7
(5), 732
–757
(2013). http://dx.doi.org/10.1002/lpor.2013.7.issue-5 1863-8880 Google Scholar
G. Popescu et al.,
“Erythrocyte structure and dynamics quantified by Hilbert phase microscopy,”
J. Biomed. Opt., 10
(6), 060503
(2005). http://dx.doi.org/10.1117/1.2149847 JBOPFO 1083-3668 Google Scholar
V. V. Tuchin et al.,
“Theoretical study of immersion optical clearing of blood in vessels at local hemolysis,”
Opt. Express, 12 2966
–2971
(2004). http://dx.doi.org/10.1364/OPEX.12.002966 OPEXFF 1094-4087 Google Scholar
M. Kinnunen et al.,
“Optical tweezers-assisted measurements of elastic light scattering,”
Proc. SPIE, 9031 90310A
(2014). http://dx.doi.org/10.1117/12.2051443 PSISDG 0277-786X Google Scholar
H. Suzuki, T. Toyooka and Y. Ibuki,
“Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis,”
Environ. Sci. Technol., 41 3018
–3024
(2007). http://dx.doi.org/10.1021/es0625632 ESTHAG 0013-936X Google Scholar
L. Dykman and N. Khlebtsov,
“Gold nanoparticles in biomedical applications: recent advances and perspectives,”
Chem. Soc. Rev., 41
(6), 2256
–2282
(2012). http://dx.doi.org/10.1039/c1cs15166e CSRVBR 0306-0012 Google Scholar
K. Kim et al.,
“High-resolution three-dimensional imaging of red blood cells parasitized by Plasmodium falciparum and in situ hemozoin crystals using optical diffraction tomography,”
J. Biomed. Opt., 19
(1), 011005
(2014). http://dx.doi.org/10.1117/1.JBO.19.1.011005 JBOPFO 1083-3668 Google Scholar
T. Kim et al.,
“White-light diffraction tomography of unlabelled live cells,”
Nat. Photonics, 8 256
–263
(2014). http://dx.doi.org/10.1038/nphoton.2013.350 1749-4885 Google Scholar
A. Skandarajah et al.,
“Quantitative imaging with a mobile phone microscope,”
PLoS One, 9
(5), e96906
(2014). http://dx.doi.org/10.1371/journal.pone.0096906 1932-6203 Google Scholar
Z. J. Smith, K. Chu and S. Wachsmann-Hogiu,
“Nanometer-scale sizing accuracy of particle suspensions on an unmodified cell phone using elastic light scattering,”
PLoS One, 7
(10), e46030
(2012). http://dx.doi.org/10.1371/journal.pone.0046030 1932-6203 Google Scholar
Z. J. Smith et al.,
“Cell-phone-based platform for biomedical device development and education applications,”
PLoS One, 6
(3), e17150
(2011). http://dx.doi.org/10.1371/journal.pone.0017150 1932-6203 Google Scholar
Q. Wei et al.,
“Detection and spatial mapping of mercury contamination in water samples using a smart-phone,”
ACS Nano, 8
(2), 1121
–1129
(2014). http://dx.doi.org/10.1021/nn406571t 1936-0851 Google Scholar
H. Zhu et al.,
“Cost-effective and rapid blood analysis on a cell-phone,”
Lab Chip., 13
(7), 1282
–1288
(2013). http://dx.doi.org/10.1039/c3lc41408f LCAHAM 1473-0197 Google Scholar
BiographyMatti Kinnunen received his MSc (Tech) and DSc (Tech.) degrees in electrical engineering from the University of Oulu, Oulu, Finland, in 2002 and 2006, respectively. He is currently working as a senior research fellow at the University of Oulu. His research interests include light–matter interactions in tissues and at the single-cell level, sensors, and measurement techniques, as well as optical noninvasive measurement techniques for biomedical applications. Artashes Karmenyan has his PhD degree in physics-mathematics from Yerevan State University (YSU), Armenia. He was head of the Spectroscopy Laboratory in “Laserayin Technika” Institute, YSU, then joined the Laboratory of Laser Methods for Tumor Diagnosis and Therapy, Cancer Research Center, Moscow, Russia. Since 2001 he has worked in Taiwan, in National Yang-Ming University and National Dong-Hwa University, in the fields of laser spectroscopy (Raman, fluorescence, time-resolved), biomedical applications, and noninvasive optical techniques for mammalian embryos research and reconstruction. |

