|
|
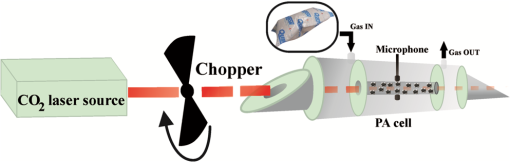
1.IntroductionSchizophrenia (SCZ) is a common psychiatric disorder, marked by gross distortion from reality and disturbances in thinking, feeling, and behavior. It has a life-time prevalence of of the world’s population.1 It is believed that increased oxidative stress may be relevant to the pathophysiology of SCZ, but most of the results regarding this subject are contrasting.2–5 Behavior disorder in the absence of mental health and social problems is best managed with psychological therapies, but the success rate is variable. Some individuals may, therefore, end up being treated with antipsychotic medications along with other approaches, despite the lack of a clear evidence base for drug use in this area,6 with the exception of Risperidone, which, in small doses, has been found to be beneficial for a subgroup of patients with behavior disorders.7–11 Levomepromazine (Methotrimeprazine) is a phenothiazine that was first introduced (in treatments) in 1956. It is structurally similar to Chlorpromazine and Clozapine.12 The contraindications, cautions, and side effects of Levomepromazine listed in the British National Formulary13 are essentially the same as those for other typical antipsychotics, such as Haloperidol and Chlorpromazine. It is also known to cause hypothermia14 and postural hypotension in ambulant patients over the age of 50 years. Exhaled breath analysis is extremely attractive, because it is not only convenient and totally noninvasive, but also exhibits good patient tolerance, having no undesirable side effects.15–20 Real-time breath testing by simply exhaling into a sample bag would be especially useful, because the data could be immediately available to the clinician, allowing swift treatment decisions and reducing the number of visits to the clinic. Human breath is mainly composed of nitrogen, oxygen, carbon dioxide (), water vapors, and inert gases. In addition, thousands of volatile organic compounds (VOCs) are exhaled at very low concentrations (estimated as parts per trillion or parts per billion by volume of the exhaled breath).21 Part of these substances are of endogenous origin and could be characteristic for metabolic processes in the human body, while several hundred others are exogenic, that is, passing through the human body.22 These VOCs are transported with the blood to the alveoli of the lung, from where they are exhaled as breath biomarkers (measurable odorants). Consequently, many established methods for breath analysis have been performed including GC-MS analyses, chemiluminescence, or many chemical techniques which do not meet all the requirements, and only in some cases have researchers and clinicians succeeded in identifying VOCs that are specific to certain diseases. laser photoacoustic spectroscopy (LPAS) is a relatively accurate and reliable method for detecting breath biomarkers from the exhaled breath of SCZ patients, which could represent an effective and convenient screening method for this intellectual disability. Ideally, a sensing tool has to meet important features such as high sensitivity and selectivity, high accuracy and precision, large dynamic range, multicomponent capability, none or only minor sample preparation, good temporal resolution, versatility, reliability, ease of use, and robustness. Spectroscopic systems include differential optical absorption spectroscopy, Fourier-transform infrared spectrometers, and light detection and ranging systems. Although there is no ideal instrument that would fulfill all the requirements mentioned above, the sensing techniques based on LPAS principles offer some important advantages in breath monitoring, such as continuous, sensitive (down to ppb – or even sub-ppb concentrations), specific, and near real-time monitoring of numerous biomarkers. The success of the photoacoustic based trace gas sensing techniques crucially depends on the availability and the performance of the tunable laser source (accessible wavelengths, tuning characteristics, typical power range) and of the detection scheme employed. Lasers offer the advantage of high spectral power density owing to their intrinsic narrow linewidth in the range of megahertz. Since the laser linewidth is usually much smaller than the molecular absorption linewidth (gigahertz region at atmospheric pressure), it is not an important issue in most measurements. The most widely used sources are CO and lasers, lead salt diode lasers, quantum cascade lasers, and nonlinear optical devices like optical parametric oscillators and difference frequency generation. Because the spectrum of laser overlaps, at room temperature and normal atmospheric pressure, the absorption spectra of numerous gases (VOCs), a good choice is to use a frequency-stabilized laser and a photoacoustic cell (PA cell) in performing the patients’ exhaled breath measurements.23,24 The kind and number of detectable substances is related to the spectral overlapping of the laser emission with the absorption bands of the trace gas molecules. The number of detectable compounds is first limited by the laser wavelength range that should overlap the absorption spectrum of each individual gaseous compound and secondly by the fact that the laser source ( laser) enables only discrete wavelength tuning. On the other hand, a partial overlapping of the individual absorption spectra of several compounds existing in the sample could happen, making it difficult to distinguish between them. This issue could be overcome by looking for a specific wavelength placed at a reasonable distance in the spectrum at which one of the compounds has a strong absorption, while the other one is transparent and vice versa. A generally applicable method to limit the gases’ interference is to separate gases, by gas chromatographic methods, selective trapping inside a cold trap, or by a specific chemical reaction (e.g., by and water). In this context, we utilized the LPAS method to compare ethylene and ammonia exhalations from individuals having a healthy physiological state with ethylene and ammonia exhalations from SCZ patients having a pathological state (before and after the treatment with Levomepromazine), thereby allowing for the identification of SCZ-related breath biomarkers in exhaled air. 2.Biomarkers in Exhaled Breath2.1.Breath Ethylene in HumansThe relation among ethylene (), free radicals, and SCZ disease can be explained by the oxidative stress. In a normal healthy human body, the generation of pro-oxidants in the form of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is stored by the antioxidant defense.25 When it gets exposed to psychiatric disorder, adverse physicochemical, environmental, or pathological agents, atmospheric pollutants, radiation, toxic chemicals, or overnutrition, the antioxidant defense is shifted and replaced with pro-oxidants with a role in the initiation of oxidative damage/stress.26 Oxidative stress has been associated with the pathophysiology of SCZ. In contrast with other organs in the body, brain tissues exhibit high vulnerability to oxidative stress because of their high oxygen consumption, high content of polyunsaturated fatty acids (PUFA), and low level of antioxidant defenses in addition to a high metal content, which can catalyze the formation of ROS/RNS. Under physiological conditions, the potential for free radical mediated damage is counteracted by the antioxidant defense system, which is composed of a series of enzymatic and nonenzymatic components. The critical antioxidant enzymes include superoxide dismutase, catalase, and glutathione peroxidase. In SCZ, the antioxidant defense is considered to be weak and oxidative stress to be present. Superoxide dismutase converts free radicals into hydrogen peroxide, which is then decomposed into water and oxygen by catalase, thereby preventing the formation of hydroxy radicals that initiate lipid peroxidation (LP).27 LP is a free radical mediated process and the initiation of a peroxidative sequence is due to the attack by any species, which can abstract a hydrogen atom from a methylene group (), together with an electron on the carbon atom (). The resultant carbon radical is stabilized by molecular rearrangement to produce a conjugated diene to give a lipid peroxyl radical (). These radicals can further abstract hydrogen atoms from other lipid molecules to form lipid hydroperoxides (LOOH) and at the same time propagate LP further. The process of LP ends with many products including malondialdehyde, 4-hydroxynonenal, and a variety of hydrocarbons, including pentane, ethane, and ethylene.25–29 Most previous studies in SCZ have been invasive,30,31 requiring samples of blood or cerebrospinal fluid or indirect measures of antioxidant enzyme levels have been used. A new way to measure LP noninvasively in humans is to measure free radical damage by analyzing early products of oxidation like exhaled hydrocarbons. Breath analysis is an emerging methodology that, being noninvasive and rapid, is ideally suited to clinical monitoring. A recent study32 has correlated the systemic oxidative stress with changes in brain metabolism defining ethane () as a terminal product of the oxidation of omega-3 PUFA. Ethylene is a product of the LP of linoleic acid and can assess free radical damage.33,34 Given the correlation of breath ethylene with brain metabolism,27,31,32 measuring the breath concentration of this compound may represent a useful means to examine oxidative stress in SCZ. 2.2.Breath Ammonia in HumansAmmonia is disposed primarily by the formation of urea in the liver but can also be produced by all tissues during the metabolism of different compounds.35–37 Elevated blood (breath) ammonia causes pathophysiologic changes (hyperammonemia) in the central nervous system. Hyperammonemia is not a true disease, but it is a sign that specific abnormalities may be present that cause blood ammonia to become elevated.35,38,39 The kidneys generate ammonia from glutamine (which is then excreted into the urine as ), or from the hydrolysis of glutamine (by intestinal glutaminase).37 Ammonia is also formed from urea and absorbed from the intestine then is removed by the liver (severe impairment of metabolic liver function will produce increased blood ammonia).37 Formation of urea in the liver is quantitatively the most important disposal route for ammonia. Urea travels in the blood from the liver to the kidneys, where it passes into the glomerular filtrate. As small molecules, ammonia can penetrate the blood–lung barrier and appear in exhaled breath. Higher concentrations of ammonia in the blood can cause ammonia intoxication and cell damage (for example, somnolence, tremors, slurring of speech, Helicobacter pylori infection, and so on).35–38 Generally speaking, exhaled breath analysis (called breath test) can be represented as follows: production of the biomarker during a particular biochemical reaction or a complex metabolic process; diffusion of biomarker through tissues and input into hematic flow; possible intermediate accumulation (buffering); possible trapping of biomarker by utilization and assimilation systems or natural chemical transformation; transport to the lungs; transmembrane diffusion to the air space of lungs; diffusion of biomarkers and their mixing with inhaled air in the alveolar space of lungs; release of biomarkers in the breathing air; collection of a breath sample; and assessment of the biomarkers in the breath sample. 3.Experimental Section3.1.SubjectsSCZ patients were recruited and informed consent was obtained from staff at the C.S.C.C.H.S. Center, Calarasi, Romania (all gave their informed consent to participate in this research, which was approved by the institutional review boards of both institutions). The trial protocol was reviewed by ward consultants at the C.S.C.C.H.S. Center, and patients matched for age, gender, and smoking status. The diagnosis of SCZ was made based on the criteria of SCZ disorders as evaluated in the Complex Evaluation Service of the C.S.C.C.H.S. Center. A total of 15 subjects (6 males and 9 females, age range from 20 to 23 years, : ) who had been previously diagnosed as suffering from SCZ and 19 subjects without any history of psychiatric illness or other diseases and nonsmokers were selected as a control group (15 males and 4 females, age range from 25 to 33, : ) and included in the study. The control subjects were non- or ex-smokers, nonalcoholic, nonrenal, nondiabetic, and free from psychiatry disorders, somatic diseases, or brain tumors, and had never been treated with antidepressant or antipsychotic medications. The SCZ group comprising 15 patients were nonsmokers, nonalcoholic, nonrenal, nondiabetic, and on a range of drug therapies with an antipsychotic and anxiolitic treatment: Levomepromazine. Some studies12,39,40 indicate that Levomepromazine may be useful in a small number of patients with severe aggression; the drug appears to be efficacious not only in controlling aggression but also lethargy, stereotypy, irritability, and hyperactivity symptoms. Prior to the analysis of breath, the subjects were asked to avoid for at least 6 h, before or at any time during the breath sample collection, alcohol and coffee, food or beverages, and to refrain from exercise in the morning. On the day prior to the test, products such as onions, leeks, eggs, and garlic should be avoided. 3.2.Breath CollectionTo collect a clean breath air sample, we used aluminized multipatient collection bags (750 mL aluminum-coated bags), designed to collect multiple samples from patients and hold a sample for a maximum of 6 h. The alveolar breath sampling procedure was performed in accordance with previous studies.41–44 Briefly, after an approximately normal inspiration, the subject places the mouthpiece in his mouth, forming a tight seal around it with the lips. A normal expiration is then made through the mouth in order to empty the lungs of as much air as required to provide the breath sample. When an adequate sample is collected, the subject stops exhaling and the samples of exhaled gas from the schizophrenic subjects can be transferred into the PA cell. To remove any residual contaminants, all of these bags were thoroughly cleaned by flushing with nitrogen gas (purity 99.9999%) and subsequently evacuated for breath sample collection. All of the collected samples were analyzed within 3 h after sampling over a period of three months. 3.3.CO2 LPAS AnalysesThe LPAS used for the gas content measurement and presented in this report is schematically shown in Fig. 1 and is also described in other works.23–45 In brief, LPAS utilizes a line-tunable laser and a PA cell, where the gas is analyzed. The experimental setup consists of a homebuilt, line-tunable and frequency stabilized laser. This laser, emitting radiation in the 9.2 to region on 73 different vibrational-rotational lines, has a maximum power of 6.5 W on the 10P(20) line.23,24,41 Our laser beam was modulated by a high-quality, low-vibration noise and variable-speed (4 to 4000 Hz) mechanical chopper model DigiRad C-980 or C-995 (30 aperture blade), operated at the appropriate resonant frequency of the cell (564 Hz). We used a dual-phase, digital lock-in amplifier Stanford Research Systems model SR 830 with the following characteristics: full scale sensitivity, 2 nV to 1 V; input noise, at 1 kHz; dynamic reserve, ; frequency range, 1 mHz to 102 kHz; time constants, to 30 s, or up to 30,000 s. The PA cell has a total volume of , and is made of stainless steel and Teflon to reduce the outgassing problems. The PA cell consists of an acoustic resonator tube, windows, gas inlets and outlets, microphones, and an acoustic filter to suppress the window noise. The PA cell windows are made of ZnSe and positioned at the Brewster angle to their mounts. The resonant conditions are obtained as longitudinal standing waves in an open tube (excited in its first longitudinal mode). To achieve an optimum signal, we chose a long absorption path length of 300 mm and an inner diameter of the pipe of 7 mm. The fundamental longitudinal wave, therefore, has a nominal wavelength of 600 mm and a resonance frequency of 564 Hz. The two buffer volumes placed near the Brewster windows have a length of 75 mm and a diameter of 57 mm. The inner wall of the stainless steel resonator tube is highly polished. It is centered inside the outer stainless steel tube with Teflon spacers. A massive spacer is positioned at one end to prevent bypassing of gas in the flow system; the other is partially open to avoid the formation of closed volumes. Gas is admitted and exhausted through two ports located near the ends of the resonator tube. The perturbation of the acoustic resonator amplitude by the gas flow noise is thus minimized. The acoustic waves generated in the PA cell are detected by four Knowles electrets miniature microphones (sensitivity each) in series, mounted flush with the wall. They are situated at the loops of the standing wave pattern at an angle of 90 deg to one another. The electrical output from these microphones is summed and the signal is selectively amplified by the lock-in amplifier.23,24 Comparing with other values reported in the literature [minimum detectable concentration of 3.846 parts per billion by volume (ppbV)], our PA system is one of the most sensitive instruments, having a responsivity of and being able to measure a minimum detectable concentration of 0.9 ppbV. We used a modular software architecture (Keithley TestPoint software) aimed at controlling the experiments, collecting data, and preprocessing information. It helps to automate the process of collecting and processing the experimental results. The software transfers powermeter readings, normalizes data, and automatically stores files. It allows the user to record parameters such as the PA cell responsivity (a constant used to normalize raw data), gas absorption coefficient, number of averaged samples at every measurement point, sample acquisition rate, and the total number of measurement points. This software interfaces the lock-in amplifier, the chopper, the laser powermeter, and the gas flowmeter. It allows the user to set or read input data and instantaneous values for the PA voltage, average laser power after chopper, and trace gas concentration.23,24 Of great significance in these determinations is the gas handling system due to its role in ensuring gas purity in the PA cell. It can be used to pump out the cell, to introduce the sample gas in the PA cell at a controlled flow rate, and monitor the total and partial pressures of gas mixtures. Also, the gas handling system can perform several functions without necessitating any disconnections.24 LPAS performs well in terms of sensitive and selective detection of trace gas and it allows near on-line measurements. The calibration measurements (concentration-dependent response) for both ammonia and ethylene (Fig. 2) were experimentally determined using commercially prepared, certified gas mixtures containing 0.96 ppmV ethylene diluted in pure nitrogen and 10 ppmV ammonia diluted in pure nitrogen.23,24 For calibration, we examined this reference mixture at a total pressure of and a temperature of 23°C, using the commonly accepted values: (for ethylene) and (for ammonia). To analyze the gas from the bags, we evacuated the extra gas and then we flushed the system with pure nitrogen at atmospheric pressure for few minutes; then the exhaled air sample can be transferred to the cell using a controlled flow rate. Because ammonia is a highly adsorbing compound and the results of successive measurements are often altered by the molecules previously adsorbed on the pathway and cell wall, an intensive cycle of washing was performed between samples in order to have a maximum increase of 10% for the background PA signal (to ensure the quality of each measurement). It has to be underlined that the measured PA signal is due mainly to the absorption of ammonia and ethylene, but some traces of , , ethanol, etc., influenced the measurements (overall contribution is ). The response to all absorbing species at a given laser wavelength (PA signal) decreased considerably when we inserted a KOH trap (with a volume ), proving that amounts of and vapors in the breath can significantly alter the results, thus making their removal compulsory.47 An important parameter in the measurements is the responsivity () of the PA cell, which depends on the pressure of the gas inside the cell. Taking into account the fact that the initial pressure in the sample bags filled by the healthy humans and by the subjects with different disorders differs from one case to other, it is necessary to know the pressure dependence of the PA cell responsivity (Fig. 3). The exhaled air sample was transferred to the PA cell at 600 standard cubic centimeters per minute, and the total pressure of the gas in the PA cell was measured, then applying the correction factor for the responsivity according to the calibration curve from Fig. 3. The responsivity of the PA cell was determined by using a calibrated mixture (Linde Gas) of 0.96 ppmV () diluted in nitrogen 6.0 (purity 99.9999%) and of 10 ppmV () diluted in nitrogen 5.0 (purity 99.999%).24 The pressure dependence of the responsivity was always measured at the center of the laser line by using a frequency stabilized laser (instability ). The absorption coefficients of ethylene and ammonia at different laser wavelengths were precisely measured previously23,24,48,49 and the laser was kept tuned at the 10P (14) line () where ethylene exhibit a strong peak, corresponding to an absorption coefficient of and at 9R(30) laser line (), where the ammonia absorption coefficient has the maximum value of . 4.Results and Discussion4.1.ResultsIn this study, ethylene and ammonia concentrations from breath samples were measured before/after the treatment with Levomepromazine in SCZ patients, and the results were compared with healthy controls using LPAS. Figure 4 shows the average concentrations of breath ethylene for SCZ patients, before and 30 min after ingestion of Levomepromazine treatment compared to the ethylene concentrations of a healthy group control. Fig. 4Breath ethylene biomarker in 15 schizophrenia (SCZ) patients and 19 age-matching control people. 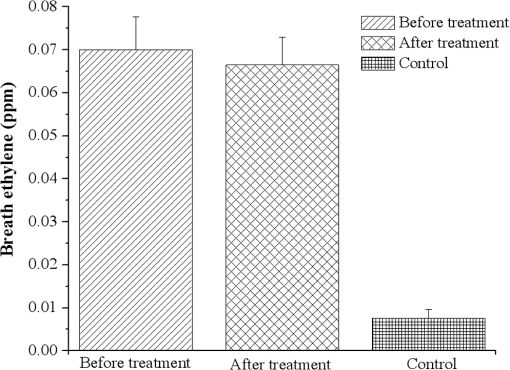 As an observation of our primary result of interest, we see that the mean ethylene level of SCZ patients is higher (0.07 ppm) compared to the mean ethylene level of healthy subjects (0.008 ppm). In addition, at 30 min after the start of the treatment with Levomepromazine, the mean ethylene level of SCZ patients is smaller (0.066 ppm) than before the treatment (but still high compared with the control subjects). Using gas chromatography and mass spectrometry, previous studies30,50 reported an increase in exhaled ethane (like ethylene, ethane is also a hydrocarbon derived from n-3 PUFA) of patients with SCZ (e.g., 5.15 ppb or 8 ppbV) compared with those of the healthy controls (e.g., 2.63 ppb or 2.5 ppbV). It is important to mention that the SCZ patients from the previous studies30,50 had not been in receipt of psychotropic medication for three weeks prior to participating in the study but had received medication for the purpose of the study. So our findings confirm previous determinations that oxidative stress is increased in SCZ and that this is unlikely to be a consequence of antipsychotic medications because the breath biomarkers after the treatment were not significantly increased. As ethylene is produced as a byproduct of oxidative stress, ammonia is produced as a byproduct of amino acids and protein ingestion. Figure 5 shows the average concentrations of breath ammonia for SCZ patients, before and 30 min after ingestion of Levomepromazine compared to the ammonia concentrations of a healthy group control. It should be pointed out that the mean ammonia level of SCZ patients is higher (2.02 ppm) compared to the mean ammonia level of healthy subjects (0.29 ppm). At 30 min after the start of the treatment with Levomepromazine, the mean ammonia level is even higher (2.2 ppm). Other possible confounding variables, such as age or sex, showed no statistically significant differences between the two groups. 4.2.DiscussionOxidative stress seems to be a key piece in the SCZ pathophysiology. When oxidants exceed the antioxidant defense, biological systems suffer oxidative stress with damage to biomolecules and functional impairment. The possible responsible factor for the differences between the concentrations of breath ethylene before and after the treatment with Levomepromazine could be explained by the difference between untreated and treated SCZ patients. Most invasive measurements of oxidative stress in patients with SCZ have been made on peripheral tissues.51–56 There is a lack of information on oxidative processes in cerebrospinal fluid and brain. It must be mentioned that traces of oxidative damage may originate from various sources in the body, and consequently, such a peripheral indicator may not necessarily reflect the conditions of the oxidative stress parameters in the brain.55 Our measurements are based on the detection of biomarkers from breath and are in good agreement with those (based on oxidative stress analysis) reported in the literature.57–66 While the majority of invasive studies have reported decreased antioxidant defense in patients with SCZ, there are also some studies where the opposite has been reported.67–74 Several factors, such as the differences in measuring techniques, differences in material tested, exposure to antipsychotic treatment, sampling of patients at different stages of the disease, lifestyle, and dietary patterns, may be responsible for this discrepancy. Our study also reviewed the efficacy of Levomepromazine in patients with SCZ, and the findings indicate that breath ethylene decreases after the treatment and breath ammonia increases after the treatment (but not significantly). So, while the oxidative stress is mildly reduced after the treatment, a mild impairment of metabolic liver function will produce increased blood (breath) ammonia. Taking into consideration that the Levomepromazine is achieved in 2 to 3 h depending on the route of administration,75 at 30 min after the administration, there is no significant chance in the chemical levels from breath of patients. The physiological basis of these findings is still speculative and future studies are needed that would clearly identify the etiologic relation between breath biomarkers and treatment with Levomepromazine. The relation between level of ammonia in the exhaled breath and SCZ could be explained by the treatment with Levomepromazine that can lead to a deficiency of amino acids which are required to detoxify toxins in the liver.76 Along with their useful effects, most medicines can cause unwanted side effects, although not everyone experiences them. Levomepromazine at SCZ patients, seems that, mildly reduced kidney function resulting an insufficient detoxification pathways with a very small accumulation of ammonia in the breath.77 The most important route for ammonia is the formation of urea in the liver; then the urea is transported to the blood from the liver to the kidneys and lastly appear in the exhaled breath of SCZ patients. From the results of this study, the ammonia breath of SCZ patients were identified in higher concentrations (at treated patients) when compared to the healthy group. Our data support a dysregulation of energy metabolism in SCZ and suggests new markers that may contribute to a better understanding of this disease. Both the feasibility and the importance of monitoring exhaled ammonia and exhaled ethylene from different subjects have been shown. 5.Conclusions and Future DirectionsThe use of related markers in exhaled breath air for SCZ analysis is theoretically reasonable; metabolic changes occur in patients with SCZ that inevitably lead to the production of certain abnormal metabolites. These metabolites are transported through the blood to the alveoli of the lungs, through alveolar gas exchange, and volatile metabolites will then be discharged into the air as components of each exhaled breath. In the current study, we analyzed the breath ethylene and breath ammonia of SCZ patients before and after the treatment with Levomepromazine, and we compared the results with the exhaled breath of normal controls. The sample bags that were utilized to collect exhaled air from the SCZ patients and healthy subjects did not release contaminants at room temperature; moreover, the bags underwent standard washing and evacuation procedures prior to use to exclude gas contamination from the external environment. From the results of this study, the ethylene and ammonia breaths of SCZ patients were identified in higher concentrations when we compared to the healthy group. The results also reveal that the ethylene levels can be considered as a measure of oxidative stress index in SCZ people. In conclusion, the data from this study support the hypothesis of the oxidant/antioxidant balance as a key component that may contribute to SCZ pathology. Based on a noninvasive sampling method, stable in biological materials and easy to measure, we conclude that LPAS analyses of breath ethylene/ammonia in alveolar air appeared to distinguish patients with SCZ from non-SCZ controls. Although LPAS is a sensitive, noninvasive, and real-time method to accurately analyze breathing gas concentrations, finding a sensitive, specific, and noninvasive biomarker of SCZ, which could be measured in alveolar air, still remains an important task. Considering that oxidative stress is a factor that can be corrected, future studies that would clearly identify the etiologic relation between antioxidant deficiencies and SCZ may provide prophylactic treatments, as well as new treatment schemes in addition to available antipsychotic schemes. Further studies placebo-controlled with a larger number of patients also need to carefully determine which antioxidants and what dosages/in what combinations will have the greatest therapeutic benefit, considering the importance of oxidative stress in many biological reactions. With improved sensitivity and specificity, LPAS analyses of alveolar air might offer a new approach to the detection of SCZ and a better understanding of the metabolic basis of the disease. AcknowledgmentsWe gratefully acknowledge the assistance provided by Mrs. Margareta Achim, the C.S.C.C.H.S. Center night supervisor, and Mrs. Daniela Arbagic, the C.S.C.C.H.S. Center director. In addition, we acknowledge the financial support of the Sectoral Operational Programme Human Resources Development 2007-2013 of the Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/132395, CNCS-UEFISCDI, project number LAPLAS 3/PN 09 39, and project number PN-II-PT-PCCA-2013-4-0608 (72/2014). ReferencesS. H. Schultz, S. W. North and C. G. Shields,
“Schizophrenia: a review,”
Am. Fam. Physician, 75
(12), 1821
–1829
(2007). AFPYAE 0002-838X Google Scholar
M. Kunz, C. S. Gama and A. C. Andreazza,
“Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia,”
Prog. Neuropsychopharmacol. Biol. Psychiatry, 32 1677
–1681
(2008). http://dx.doi.org/10.1016/j.pnpbp.2008.07.001 PNPPD7 0278-5846 Google Scholar
G. Dadheech et al.,
“Evaluation of antioxidant deficit in schizophrenia,”
Indian J. Psychiatry, 50 16
–20
(2008). http://dx.doi.org/10.4103/0019-5545.39753 IJRPAB 0019-5545 Google Scholar
S. J. Wood et al.,
“Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress,”
Ann. Acad. Med. Singapore, 38
(5), 396
–406
(2009). AAMSCG 0304-4602 Google Scholar
M. Padurariu et al.,
“Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease,”
Neurosci. Lett., 469 6
–10
(2010). http://dx.doi.org/10.1016/j.neulet.2009.11.033 NELED5 0304-3940 Google Scholar
J. Brylewski and L. Duggan,
“Antipsychotic medication for challenging behaviour in people with learning disability,”
(2015) http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000377.pub2/abstract May ). 2015). Google Scholar
A. Bokszanska et al.,
“Olanzapine and Risperidone in adults with learning disability: a clinical naturalistic study,”
Int. Clin. Psychopharmacol., 18 285
–291
(2003). http://dx.doi.org/10.1097/00004850-200309000-00005 ICLPE4 0268-1315 Google Scholar
S. A. Cohen et al.,
“Risperidone for aggression and self-injurious behaviour in adults with mental retardation,”
J. Autism Dev. Disord., 28 229
–233
(1998). http://dx.doi.org/10.1023/A:1026069421988 JADDDQ 0162-3257 Google Scholar
J. P. Horrigan and L. J. Barnhill,
“Risperidone and explosive aggressive autism,”
J. Autism Dev. Disord., 27 313
–323
(1997). http://dx.doi.org/10.1023/A:1025854532079 JADDDQ 0162-3257 Google Scholar
D. B. McAdam et al.,
“Effects of Risperidone on aberrant behaviour in persons with developmental disabilities: II. Social validity measures,”
Am. J. Ment. Retard., 107 261
–269
(2002). http://dx.doi.org/10.1352/0895-8017(2002)107<0261:EOROAB>2.0.CO;2 AJMREA 0895-8017 Google Scholar
J. R. Zarcone et al.,
“Effects of Risperidone on aberrant behaviour in persons with developmental disabilities: I. A double-blind crossover study using multiple measures,”
Am. J. Ment. Retard., 106 525
–538
(2001). http://dx.doi.org/10.1352/0895-8017(2001)106<0525:EOROAB>2.0.CO;2 AJMREA 0895-8017 Google Scholar
J. Devapriam et al.,
“Use of levomepromazine in the management of aggression in adults with intellectual disability,”
Br. J. Dev. Disabil., 54 11
–17
(2008). http://dx.doi.org/10.1179/096979508799103305 BJDDE2 0969-7950 Google Scholar
British National Formulary 52, British Medical Association and Royal Pharmaceutical Society of Great Britain, BMJ Group, London
(2006). Google Scholar
R. J. Van Marum, S. Jansen and H. H. Ponssen,
“Antipsychotic medication as a cause of deep hypothermia,”
Ned. Tijdschr. Geneeskd., 147
(25), 1201
–1204
(2003). NETJAN 0028-2162 Google Scholar
I. Kohl et al.,
“First observation of a potential non-invasive breath gas biomarker for kidney function,”
J. Breath Res., 7 017110
(2013). http://dx.doi.org/10.1088/1752-7155/7/1/017110 JBROBW 1752-7155 Google Scholar
M. R. McCurdy et al.,
“Recent advances of laser-spectroscopy-based techniques for applications in breath analysis,”
J. Breath Res., 1 014001
(2007). http://dx.doi.org/10.1088/1752-7155/1/1/014001 JBROBW 1752-7155 Google Scholar
M. A. Leja, H. Liu and H. Haick,
“Breath testing: the future for digestive cancer detection,”
Expert Rev. Gastroenterol. Hepatol., 7
(5), 389
–391
(2013). http://dx.doi.org/10.1586/17474124.2013.811033 ERGHBD 1747-4124 Google Scholar
T. H. Risby and J. D. Pleil,
“Breath analysis—past, present and future: a special issue in honour of Michael Phillips’ 70th birthday,”
J. Breath Res., 7
(1), 010201
(2013). http://dx.doi.org/10.1088/1752-7155/7/1/010201 JBROBW 1752-7155 Google Scholar
I. R. White et al.,
“Real-time multi-marker measurement of organic compounds in human breath: towards fingerprinting breath,”
J. Breath Res., 7
(1), 017112
(2013). http://dx.doi.org/10.1088/1752-7155/7/1/017112 JBROBW 1752-7155 Google Scholar
R. A. Dweik and A. Amann,
“Exhaled breath analysis: the new frontier in medical testing,”
J. Breath Res., 2
(3), 030301
(2008). http://dx.doi.org/10.1088/1752-7163/2/3/030301 JBROBW 1752-7155 Google Scholar
C. Wang et al.,
“Noninvasive detection of colorectal cancer by analysis of exhaled breath,”
Anal. Bioanal. Chem., 406
(19), 4757
–4763
(2014). http://dx.doi.org/10.1007/s00216-014-7865-x ABCNBP 1618-2642 Google Scholar
J. D. Pleil, M. A. Stiegel and T. H. Risby,
“Clinical breath analysis: discriminating between human endogenous compounds and exogenous (environmental) chemical confounders,”
J. Breath Res., 7
(1), 017107
(2013). http://dx.doi.org/10.1088/1752-7155/7/1/017107 JBROBW 1752-7155 Google Scholar
D. C. Dumitras et al.,
“Ultrasensitive laser photoacoustic system,”
Infrared Phys. Technol. J., 53
(5), 308
–314
(2010). http://dx.doi.org/10.1016/j.infrared.2010.05.001 IPTEEY 1350-4495 Google Scholar
D. C. Dumitras et al.,
“Laser photoacoustic spectroscopy: principles, instrumentation, and characterization,”
J. Optoelectron. Adv. Mater., 9
(12), 3655
–3701
(2007). 1454-4164 Google Scholar
T. P. Devasagayam et al.,
“Free radicals and antioxidants in human health: current status and future prospects,”
J. Assoc. Physicians India, 52 794
–804
(2004). JPHIAR 0004-5772 Google Scholar
Darwins, “Free radicals and antioxidants in human health,”
(2013) https://www.scribd.com/doc/164608057/free-radicals-and-antioxidants-in-human-health-pdf Google Scholar
B. K. Y. Bitanihirwe and T.-U. Woo,
“Oxidative stress in schizophrenia: an integrated approach,”
Neurosci. Biobehav. Rev., 35 878
–893
(2011). http://dx.doi.org/10.1016/j.neubiorev.2010.10.008 NBREDE 0149-7634 Google Scholar
J. A. Knight,
“Review: free radicals, antioxidants and the immune system,”
Ann. Clin. Lab. Sci., 30
(2), 145
–158
(2000). ACLSCP 0091-7370 Google Scholar
G. Kennedy et al.,
“Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms,”
Free Radic. Biol. Med., 39
(5), 584
–589
(2005). http://dx.doi.org/10.1016/j.freeradbiomed.2005.04.020 FRBMEH 0891-5849 Google Scholar
B. K. Puri, B. M. Ross and I. H. Treasaden,
“Increased levels of ethane, a non-invasive, quantitative, direct marker of n-3 lipid peroxidation, in the breath of patients with schizophrenia,”
Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 32 858
–862
(2008). http://dx.doi.org/10.1016/j.pnpbp.2008.01.001 PNPPD7 0278-5846 Google Scholar
S. Mukerjee et al.,
“Impaired antioxidant defense at the onset of psychosis,”
Schizophr. Res., 19 19
–26
(1996). http://dx.doi.org/10.1016/0920-9964(95)00048-8 SCRSEH 0920-9964 Google Scholar
B. K. Puri et al.,
“Evidence from in vivo 31-phosphorus magnetic resonance spectroscopy phoshodiesters that exhaled ethane is a biomarker of cerebral n-3 polyunsaturated fatty acid peroxidation in humans,”
BMC Psychiatry, 8
(Suppl 1),
(2008). http://dx.doi.org/10.1186/1471-244X-8-S1-S2 Google Scholar
A. Puiu et al.,
“Stress monitoring in a Guinness 10-day scuba dive,”
Laser Phys., 17
(5), 772
(2007). http://dx.doi.org/10.1134/S1054660X07050271 LAPHEJ 1054-660X Google Scholar
R. Kocielnik et al.,
“Smart technologies for long-term stress monitoring at work,”
in IEEE 26th Int. Symp. on Computer-Based Medical Systems,
53
–58
(2013). Google Scholar
R. A. Harvey and D. R. Ferrier, Lippincott’s Illustrated Reviews: Biochemistry, 5th ed.Wolters Kluwer Lippincott Williams & Wilkins Health, Philadelphia
(2011). Google Scholar
I. D. Weiner and J. W. Verlander,
“Role of and transporters in renal acid-base transport,”
Am. J. Physiol. Renal Physiol., 300
(1), F11
–F23
(2011). http://dx.doi.org/10.1152/ajprenal.00554.2010 0363-6127 Google Scholar
R. A. Harvey and D. R. Ferrier, Unit IV in Biochemistry, 245
–291 5th ed.Lippincott Williams & Wilkins, Philadelphia
(2010). Google Scholar
N. Bakouh et al.,
“ is involved in the transport induced by the functional expression of the human Rh C glycoprotein,”
J. Biol. Chem., 279 15975
–15983
(2004). http://dx.doi.org/10.1074/jbc.M308528200 JBCHA3 0021-9258 Google Scholar
M. E. Handlogten et al.,
“Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract,”
Am. J. Physiol. Gastrointest. Liver Physiol., 288 G1036
–G1047
(2005). http://dx.doi.org/10.1152/ajpgi.00418.2004 0193-1857 Google Scholar
B. Aukst-Margetic et al.,
“Levomepromazine helps to reduce sleep problems in patients with PTSD,”
Eur. Psychiatry, 19 235
–236
(2004). http://dx.doi.org/10.1016/j.eurpsy.2003.12.007 0924-9338 Google Scholar
C. Popa et al.,
“Spectroscopic studies of ethylene and ammonia as biomarkers at patients with different medical disorders,”
U. P. B. Sci. Bull. Ser. A, 73
(2), 167
–174
(2011). Google Scholar
C. Popa et al.,
“Qualitative and quantitative determination of human biomarkers by laser photoacoustic spectroscopy methods,”
Laser Phys., 21
(7), 1336
–1342
(2011). http://dx.doi.org/10.1134/S1054660X11130238 LAPHEJ 1054-660X Google Scholar
C. Popa et al.,
“Exertion in Kangoo Jumps aerobic: evaluation and interpretation using spectroscopic technique determinations,”
J. Spectrosc., 2013 602434
(2013) http://dx.doi.org/10.1155/2013/602434 Google Scholar
C. Popa et al.,
“The level of ethylene biomarker in renal failure of elderly patients analyzed by photoacoustic spectroscopy,”
Laser Phys., 23
(12), 125701
(2013). http://dx.doi.org/10.1088/1054-660X/23/12/125701 LAPHEJ 1054-660X Google Scholar
C. Popa et al.,
“Testing fruits quality by photoacoustic spectroscopy assay,”
Laser Phys., 24
(10), 105702
(2014). http://dx.doi.org/10.1088/1054-660X/24/10/105702 LAPHEJ 1054-660X Google Scholar
F. Harren et al.,
“Sensitive intracavity photoacoustic measurements with a CO2 waveguide laser,”
Appl. Phys. B, 50 137
(1990). http://dx.doi.org/10.1007/BF00331909 APBOEM 0946-2171 Google Scholar
M. Bratu et al.,
“Removal of interfering gases in breath biomarker measurements,”
J. Optoelectron. Adv. Mater., 13
(8), 1045
–1050
(2011). 1454-4164 Google Scholar
D. C. Dumitras et al.,
“Evaluation of ammonia absorption coefficients by photoacoustic spectroscopy for detection of ammonia levels in human breath,”
Laser Phys., 21
(4), 796
–800
(2011). http://dx.doi.org/10.1134/S1054660X11070061 LAPHEJ 1054-660X Google Scholar
C. Popa et al.,
“Ethylene and ammonia traces measurements from the patients’ breath with renal failure via LPAS method,”
Appl. Phys. B, 105
(3), 669
–674
(2011). http://dx.doi.org/10.1007/s00340-011-4716-8 APBOEM 0946-2171 Google Scholar
B. M. Ross, S. Shah and M. Peet,
“Increased breath ethane and pentane concentrations in currently unmedicated patients with schizophrenia,”
Open J. Psychiatry, 1 1
–7
(2011). http://dx.doi.org/10.4236/ojpsych.2011.11001 2161-7325 Google Scholar
M. Yanik et al.,
“Is the arginine-nitric oxide pathway involved in the pathogenesis of schizophrenia?,”
Neuropsychobiology, 47
(2), 61
–65
(2003). http://dx.doi.org/10.1159/000070010 NPBYAL 0302-282X Google Scholar
K. Q. Do et al.,
“Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo,”
Eur. J. Neurosci., 12
(10), 3721
–3728
(2000). http://dx.doi.org/10.1046/j.1460-9568.2000.00229.x EJONEI 0953-816X Google Scholar
B. Uttara et al.,
“Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options,”
Curr. Neuropharmacol., 7
(1), 65
–74
(2009). http://dx.doi.org/10.2174/157015909787602823 CNUEAN 1570-159X Google Scholar
B. Halliwell,
“Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment,”
Drugs Aging, 18
(9), 685
–716
(2001). http://dx.doi.org/10.2165/00002512-200118090-00004 DRAGE6 1170-229X Google Scholar
S. P. Mahadik, D. Evans and H. Lal,
“Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia,”
Prog. Neuropsychopharmacol. Biol. Psychiatry, 25
(3), 463
–493
(2001). http://dx.doi.org/10.1016/S0278-5846(00)00181-0 PNPPD7 0278-5846 Google Scholar
C. Fendri et al.,
“Oxidative stress involvement in schizophrenia pathophysiology: a review,”
Encephale, 32
(2 Pt 1), 244
–252
(2006). http://dx.doi.org/10.1016/S0013-7006(06)76151-6 ENCEAN 0013-7006 Google Scholar
T. M. Michel et al.,
“Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis,”
J. Neural Transm., 111
(9), 1191
–1201
(2004). http://dx.doi.org/10.1007/s00702-004-0160-9 JNTMAH 0300-9564 Google Scholar
D. Pavlovic, V. Tamburic and I. Stojanovic,
“Oxidative stress as marker of positive symptoms in schizophrenia,”
Facta Univ., 9
(2), 157
–161
(2002). Google Scholar
M. M. Khan et al.,
“Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics,”
Schizophr. Res., 58
(1), 1
–10
(2002). http://dx.doi.org/10.1016/S0920-9964(01)00334-6 SCRSEH 0920-9964 Google Scholar
M. Phillips et al.,
“Volatile organic compounds in the breath of patients with schizophrenia,”
J. Clin. Pathol., 48 466
–469
(1995). http://dx.doi.org/10.1136/jcp.48.5.466 AJCPAI 0002-9173 Google Scholar
P. K. Ranjekar et al.,
“Decreased antioxidant enzymes and membrane essential poly-unsaturated fatty acids in schizophrenic and bipolar mood disorder patients,”
Psychiatry Res., 121
(2), 109
–122
(2003). PSRSDR Google Scholar
M. Boskovic et al.,
“Oxidative stress in schizophrenia,”
Curr. Neuropharmacol., 9 301
–312
(2011). http://dx.doi.org/10.2174/157015911795596595 CNUEAN 1570-159X Google Scholar
A. Ciobica et al.,
“Oxidative stress in schizophrenia—focusing on the main markers,”
Psychiatr. Danub., 23
(3), 237
–245
(2011). PSYDEI 0353-5053 Google Scholar
O. Akyol et al.,
“The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance,”
Prog. Neuropsychopharmacol. Biol. Psychiatry, 26
(5), 995
–1005
(2002). http://dx.doi.org/10.1016/S0278-5846(02)00220-8 PNPPD7 0278-5846 Google Scholar
A. Dietrich-Muszalska, B. Olas and J. Rabe-Jablonska,
“Oxidative stress in blood platelets from schizophrenic patients,”
Platelets, 16
(7), 386
–391
(2005). http://dx.doi.org/10.1080/09537100500128872 PLTEEF 1369-1635 Google Scholar
L. B. Othmen et al.,
“Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings,”
Prog. Neuropsychopharmacol. Biol. Psychiatry, 32
(1), 155
–159
(2008). http://dx.doi.org/10.1016/j.pnpbp.2007.08.003 PNPPD7 0278-5846 Google Scholar
I. Altuntas et al.,
“Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients,”
Clin. Chem. Lab. Med., 38
(12), 1277
–1281
(2000). http://dx.doi.org/10.1515/CCLM.2000.201 CCLMFW 1434-6621 Google Scholar
G. Dakhale et al.,
“Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics,”
Neuropsychobiology, 49
(4), 205
–209
(2004). http://dx.doi.org/10.1159/000077368 NPBYAL 0302-282X Google Scholar
B. D’souza and V. D’souza,
“Oxidative injury and antioxidant vitamins E and C in schizophrenia,”
Indian J. Clin. Biochem., 18
(1), 87
–90
(2003). IJCBEY Google Scholar
B. Halliwell and M. Whiteman,
“Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean?,”
Br. J. Pharmacol., 142
(2), 231
–255
(2004). http://dx.doi.org/10.1038/sj.bjp.0705776 BJPCBM 0007-1188 Google Scholar
M. Kuloglu et al.,
“Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder,”
Cell Biochem. Funct., 20
(2), 171
–175
(2002). http://dx.doi.org/10.1002/(ISSN)1099-0844 CBFUDH 1099-0844 Google Scholar
M. Rukmini, B. D’souza and V. D’souza,
“Superoxide dismutase and catalase activities and their correlation with malondialdehyde in schizophrenic patients,”
Indian J. Clin. Biochem., 19
(2), 114
–118
(2004). IJCBEY Google Scholar
K. Surapaneni,
“Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in schizophrenic patients,”
J. Clin. Diagn. Res., 1
(2), 39
–44
(2007). JCDRAB 0973-709X Google Scholar
X. Zhang et al.,
“The effects of Ginkgo biloba extract added to haloperidol on peripheral T cell subsets in drug-free schizophrenia: a double-blind, placebo-controlled trial,”
Psychopharmacology, 188
(1), 12
–17
(2006). http://dx.doi.org/10.1007/s00213-006-0476-2 0033-3158 Google Scholar
P. Sivaraman, R. D. Rattehalli and M. B. Jayaram,
“Levomepromazine for schizophrenia (review),”
(2015) http://www.update-software.com/BCP/WileyPDF/EN/CD007779.pdf May ). 2015). Google Scholar
T. Hibbard, K. Crowley and A. J. Killard,
“Direct measurement of ammonia in simulated human breath using an inkjet-printed polyaniline nanoparticle sensor,”
Anal. Chim. Acta, 779 56
–63
(2013). http://dx.doi.org/10.1016/j.aca.2013.03.051 ACACAM 0003-2670 Google Scholar
R. Lynne and M. Andrew,
“Palliative and end-of-life care in advanced renal failure,”
Clin. Med., 10
(3), 279
–281
(2010). http://dx.doi.org/10.7861/clinmedicine.10-3-279 CLMEA3 1470-2118 Google Scholar
BiographyCristina Popa has a PhD in physics and since 2006 has worked as a scientist researcher at the National Institute for Laser, Plasma and Radiation Physics, Optics and Lasers in Life Sciences, Environment and Manufacturing Group. She has considerable results in laser applications in medicine and biology, numerous studies on laser photoacoustic spectroscopy, and she has published more than 30 articles in ISI journals, 1 book, and 2 book chapters. |