|
|
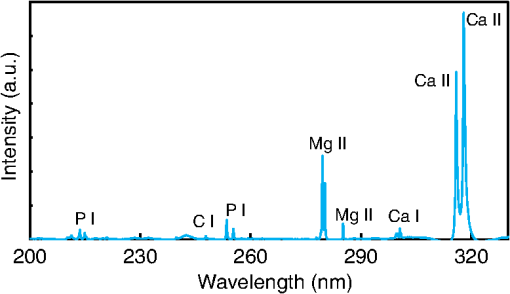
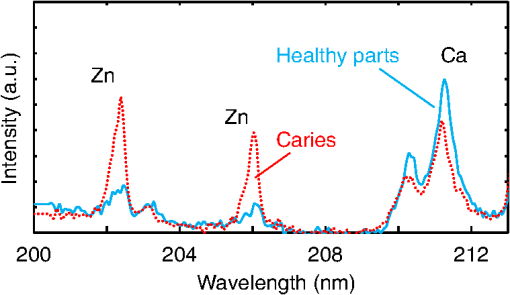
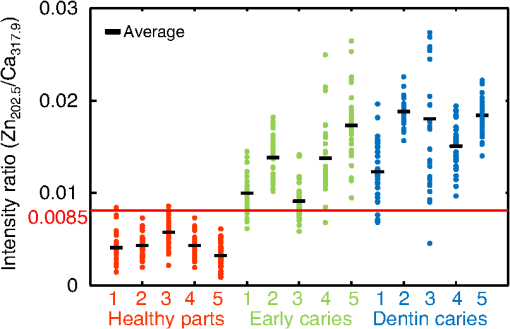
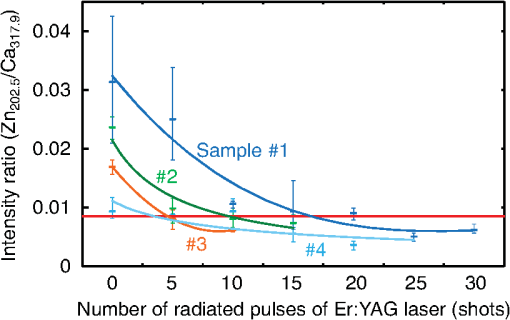
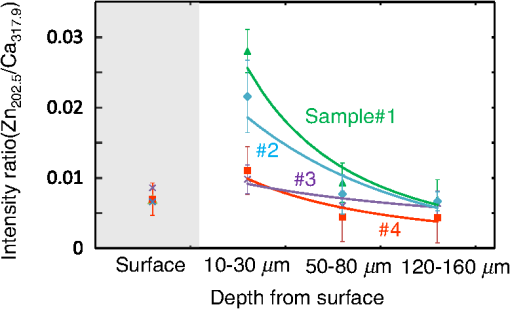
1.IntroductionVisual observation and contact methods using a dental probe have been applied for diagnosing dental caries, although they sometimes lead to misdiagnosis and pain in patients. To improve diagnostic accuracy and to avoid causing pain, many noncontact methods based on the radiation of electromagnetic waves have been developed. Transversal microradiography1 uses a microfocus x-ray source and thus is difficult to apply to in vivo diagnosis. Digital imaging fiber-optic transillumination2 and other methods based on fluorescence observation, such as quantitative light-induced fluorescence2,3 and infrared-laser or visible light-induced fluorescence,4,5 have been well established. Moreover, some diagnostic systems based on them are commercially available. Some groups have proposed to use diagnosis methods based on fluorescence observation,6 photoacoustic signals,7,8 and infrared spectra8–10 for feedback controlling of laser ablation of dental tissues. Although a definitive method has not appeared yet, Chan et al.11 and Alexander and Fried12 succeeded in selectively removing dental composite from teeth surfaces by using spectral feedback.11,12 They focused on the difference in concentration of calcium between dental composites and dental hard tissues. However, these methods usually differentiate dental caries or dental composites from healthy dental tissues by comparing the data obtained from those two different parts. In contrast, caries detection methods based on laser-induced breakdown spectroscopy (LIBS) enable absolute and quantitative analysis of elements contained in teeth. This method for elemental analysis is based on the spectral analysis of plasma emissions generated by the irradiation of high-powered laser pulses.13 LIBS is different from other element analysis methods such as inductively coupled argon plasma-atomic emission spectroscopy (ICP-AES),14 and LIBS needs no pretreatment of samples. Thus, it can analyze very small amounts in real time. Many groups have applied LIBS methods to biomedical applications because they can be used in vivo and are less invasive for diagnosis in a variety of soft and hard tissues.15–18 In dental applications, many groups have proposed LIBS methods for caries detection that analyze element contents in teeth,19,20 and some groups have shown results from in vivo studies.21 Enamel, which is the outermost layer of the tooth, is a biochemical composite whose components are made up of 96% inorganic materials [including minerals, hydroxyapatites (), and a small amount of various metals], 3% water, and 1% organics such as protein and fat. In the decayed parts of teeth, the amounts of these components vary in accordance with the degree of caries progress. Therefore, by analyzing these elements, one can detect caries and execute an accurate diagnosis of the decaying stages. These methods usually detect relatively strong emission lines of elements such as Ca and P in hydroxyapatite and C, Mg, Cu, and Sr in visible wavelengths. However, the sensitivity and accuracy were insufficient for detecting early caries. We built an optical-fiber–based LIBS system for in vivo and real-time analysis of tooth enamel during dental treatment using a dental Er:YAG laser system. Niemz22 showed that by using an Nd:YLF picosecond-pulse laser, ablation and analysis of dental hydroxyapatite could be simultaneously performed. In this paper, a more conventional Er:YAG laser is used for dental tissue ablation and a hollow optical fiber that transmits both a Q-switched Nd:YAG laser for LIBS and an infrared Er:YAG laser for tooth ablation is used for building the system that could be used for in vivo applications. We describe our expansion of the spectral region under analysis to ultraviolet (UV) light to improve the sensitivity of caries detection, and we show that early caries can be detected at high accuracy by analyzing the emission peaks of zinc (Zn) using UV light. 2.Experimental SetupFigure 1 shows the schematic of the experimental setup. A Q-switched Nd:YAG laser with an operating wavelength of 1064 nm, a pulse width of 7 to 8 ns, and a repetition rate of 10 pps was used as the light source for plasma generation. The laser light was coupled to a hollow optical fiber with an inner diameter of by a convex lens with a focal length of 250 mm. By using a hollow optical fiber for delivery of the laser light to the sample surface, the system is capable of both diagnosis based on LIBS and caries removal because the hollow optical fibers deliver high-powered infrared laser light for tooth ablation as well.23,24 Fig. 1Schematic of experimental setup of fiber-based laser-induced breakdown spectroscopy (LIBS) system. 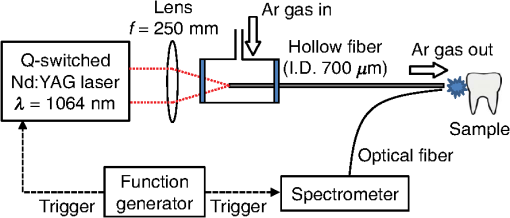 Figure 2 shows a schematic of the distal end of a fiber optic probe. Plasma emissions induced by laser radiation were detected using a step-index, pure-silica-glass optical fiber with a core diameter of and a numerical aperture of 0.22. The detected emissions were delivered to a fiber-coupled spectrometer (Ocean Optics HR2000+, slit width 10 mm, ) to measure the power spectra of emitted light from 200 to 340 nm wavelengths with a resolution of 0.14 nm. Experiments were performed at atmospheric pressure, and argon gas was injected onto the sample via the bore of a hollow optical fiber to enhance emitted plasma intensities of low concentration elements.25 Extracted human teeth in different levels of decay were prepared as measurement samples. Visual inspection by a dentist and palpation by using a dental probe was performed and the samples were stored in saline. The samples were divided into three stages: early stages of decay called “E1”; stages called “E2,” where the decay and cavities stayed only in the tooth enamel; advanced stages called “D1” and “D2,” where the cavities reached into the dentin; and healthy teeth without any decay or cavities. More than 10 samples from each stage, including both incisors and molars, were prepared and the samples were washed with brushes and pure water before being tested. 3.Results and DiscussionHealthy teeth are known to usually contain Ca and P, which are the main components of hydroxyapatite at high concentrations. As tooth decay progresses, other inorganic elements such as Mg and Cu precipitate when crystals of hydroxyapatite are demineralized. Therefore, one can detect the development of dental caries as increases in the density of minor inorganic materials in LIBS spectra. However, in our preliminary tests, we found that the differences in the intensities of these elements showing strong emission peaks in visible and near-infrared wavelengths are too small between the decayed and healthy parts to detect early caries. Therefore, in this study, we focused on the UV region, where characteristic emission peaks of various minor components appear. A measured LIBS spectrum of a healthy tooth in a wavelength region of 200 to 340 nm is shown in Fig. 3. This is an averaged spectrum of emissions taken using 50 laser shots, and the integration time of each of the emissions was 100 ms. The radiated pulse energy was 21 to 22 mJ. In the LIBS spectrum in Fig. 3, we found that—in addition to components of hydroxyapatite—minor inorganic components such as Mg were detected. Figure 4 shows the LIBS spectra of a healthy tooth and a decayed tooth. The radiated pulse energy in the measurement of caries was set to 15 to 16 mJ. We found from the figure that, for a decayed tooth, the density of C was higher and that the density of Ca was lower than the respective density for each in a healthy tooth. Based on this result, we first tried to set an evaluation standard for the diagnosis of caries progress by using the peak intensities of C and Ca, especially showing relatively high peak intensities and large differences between caries and healthy teeth. We prepared five samples from each of the healthy teeth, early caries (E1 and E2), and dentin caries (D1 and D2). We performed 30 measurements for each sample and calculated the intensity ratios of C at 247.7 nm and Ca at 317.9 nm for quantitative evaluation as these were not affected by fluctuation in the emission intensities.26,27 Figure 5 shows a scatter diagram of the measured results. From these results, we found that the dentin caries samples significantly differed from the other samples. However, the averaged value of the healthy teeth and the early caries was 0.0050 and 0.0059, respectively, and the difference was too small to distinguish. Fig. 4LIBS spectra of healthy tooth and decayed tooth in wavelength regions of (a) 200–340 nm and (b) 200–250 nm. 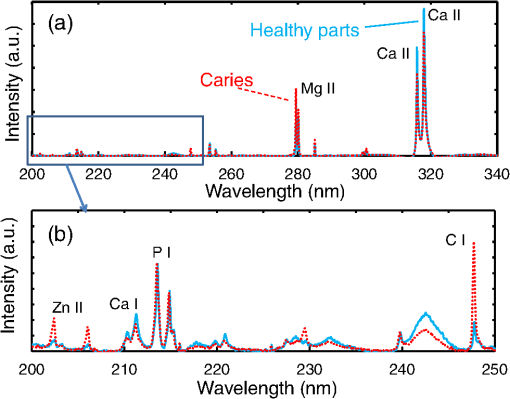 Fig. 5Scattered diagram of calculated intensity ratios of C at 247.7 nm and Ca at 317.9 nm for three different stages of caries development. 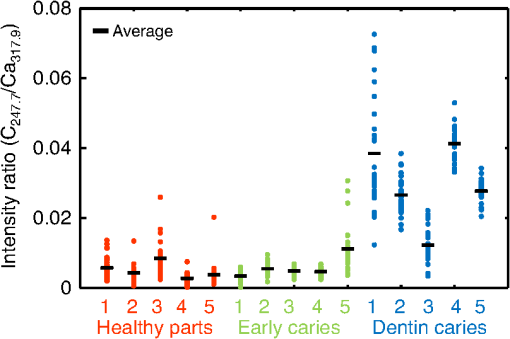 On the basis of these results, we repeatedly performed similar experiments while changing the combination of focusing elements to choose the optimum target elements for detection of early caries. In the spectra shown in Fig. 6, we found that the peak height of Zn also increases in caries teeth. Compared with other inorganic materials, zinc is known to exist at high concentrations in the outer layer of enamel.28,29 Although various inorganic elements precipitate when hydroxyapatite is demineralized, a change in the concentration due to tooth decay is seen more clearly in zinc because early caries exist in enamel that is close to the surface. Therefore, we assumed that zinc was strongly detected in this early stage of dental decay. Figure 7 shows a scatter diagram of measured intensity ratios between Zn at 202.5 nm and Ca at 317.9 nm. In this figure, the average value of the intensity ratios was 0.0044 for the healthy teeth and 0.013 for the early caries. By setting a boundary at around 0.008, one can clearly distinguish between healthy teeth and early caries. The accuracy of the diagnosis based on this result was as high as 98.2% for the healthy teeth, 85.2% for the early caries, and 96.6% for the dentin caries. In additional experiments using more than five samples, the healthy parts and early caries were repeatedly tested. The results were that the diagnostic accuracy for early caries was higher than 80%. Next, we evaluated the feasibility of real-time analysis of our method during laser treatment using an Er:YAG dental laser system. We utilized a dental laser system (J. Morita, Erwin AdvErl), and laser pulses with a wavelength of and pulse energy of 100 mJ were radiated onto early caries by using a hollow optical fiber. Simultaneously, the aforementioned LIBS analysis was performed. The results are shown in Figure 8 as the measured intensity ratio Zn/Ca as a function of the number of radiated pulses of the Er:YAG laser. For all five samples that we tested, decreases in the intensity ratio were observed. Therefore, we demonstrated that our system can be used to inform a practitioner that the caries parts have been removed. 4.DiscussionThe tooth samples used in the above experiments were visually inspected and palpated by dental probes. Since histological tests were not performed, there was a suspicion that the detected Zn component might not be from carious lesions but from stains or other substances such as tooth pastes and mouthwashes. In these experiments, the spot size of the laser beam radiated on the teeth surface was around 0.5 mm, which was usually larger than the stained part of caries lesions. To check that Zn emissions in the measured spectra did not originate only from stain, we took LIBS spectra of healthy teeth while ablating the surface by Er:YAG laser light. In this experiment, three healthy tooth samples were prepared and LIBS analyses were performed by radiating pulse energies of 20 to 21 mJ of Nd:YAG laser light. A totally of four LIBS analyses were performed for each samples while ablating the samples’ surfaces with Er:YAG laser light with a pulse energy of 100 mJ. Figure 9 shows the change of the measured intensity ratio Zn/Ca in the depth direction. Since there were variations in the ablated depth per radiated pulse, the horizontal values range in depth. From this result, it is found that Zn exists at relatively high concentrations in the enamel layer that is close to the surface and that the Zn concentration is less at the very surface of the enamel where stain and other substances are present in higher concentration. This result shows that the Zn component detected in the experiments is originated not only from stains, although those stains also contain Zn in some concentrations. Although we showed that Zn emissions in the LIBS spectra of teeth are sensitively effected by early caries, there may be some questions on safety aspects when utilizing this method for a feedback system controlling a therapeutic laser. First, it is still not clear why Zn preferentially locates in caries lesions. To make this clear, a more thorough investigation including histological inspection should be performed and, by combining Zn emissions with those of other components, such as Mg, that have been well established in LIBS-based diagnosis of caries, the diagnosis certainty should be largely improved. Second, it is known that the Zn correlates well with remineralization of enamel30,31 and that Zn in saliva can reduce enamel demineralization and modify remineralization,32 therefore, Zn is sometimes added to fluoride toothpastes. This is supported by the fact that elevated Zn levels are present at the neonatal line and the postnatally formed dentin.33,34 Therefore, a diagnosis based on only Zn content may induce removal of newly formed dental hard tissues that are important for defense against caries formation. To avoid this trouble, LIBS analyses of multielements should be used for more precise analysis and considering that Zn content enhances the accuracy of diagnosis, especially for early caries. The proposed method based on LIBS may not be applied for detection of caries lesion that spreads in dentin down through the sound enamel. However, these lesions underneath enamel usually show the appearance of early caries. Therefore, this method can be a cue for follow-up detailed examinations based on other methods such as optical coherence tomography35,36 and x-ray inspection. 5.ConclusionWe designed an LIBS system for in vivo analysis of tooth enamel. The system utilizes a hollow optical fiber that transmits both a Q-switched Nd:YAG laser light for LIBS and an infrared Er:YAG laser light for tooth ablation. Thus, the system enables real-time analysis of teeth during laser dental treatment. By expanding the spectral region under analysis to UV light and by focusing on emission peaks of Zn in the UV region, we substantially improved the sensitivity of caries detection. We showed, by using the ratios of peak intensities of Zn and Ca, that early caries were distinguished from healthy teeth with accuracy rates higher than 80%. We then applied this LIBS analysis to decayed teeth while ablating the caries part with Er:YAG laser light. We also showed that the intensity ratio Zn/Ca decreases as the amount of radiation from Er:YAG laser pulses increases. Therefore, our system would be useful for informing a practitioner that the caries parts have been removed. The certainty of the diagnoses is expected to be improved by combining this method based on emission of Zn with that of the other elements such as Mg and Sr. This may also help avoid misdiagnoses that induce the removal of newly formed dental hard tissues. ReferencesE. de Josselin de Jong, J. J. ten Bosch and J. Noordmans,
“Optimized microcomputer-guided quantitative microradiography on dental mineralized tissue slices,”
Phys. Med. Biol., 32
(7), 887
–899
(1987). http://dx.doi.org/10.1088/0031-9155/32/7/008 PHMBA7 0031-9155 Google Scholar
G. K. Stookey and C. Gonzales-Cabezas,
“Emerging methods of caries diagnosis,”
J. Dent. Educ., 65
(10), 1001
–1006
(2001). 0022-0337 Google Scholar
E. de Josselin de Jong et al.,
“A new method for in vivo quantification of changes in initial enamel caries with laser fluorescence,”
Caries Res, 29
(1), 2
–7
(1995). http://dx.doi.org/10.1159/000262032 CAREBK 0008-6568 Google Scholar
K. Takamori et al.,
“Detection of occlusal caries under sealants by use of a laser fluorescence system,”
J. Clin. Laser Med. Surg., 19
(5), 267
–271
(2001). http://dx.doi.org/10.1089/10445470152612008 JCLSEO Google Scholar
N. Subhash et al.,
“Tooth caries detection by curve fitting of laser-induced fluorescence emission: a comparative evaluation with reflectance spectroscopy,”
Laser Surg. Med., 37
(4), 320
–328
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Eberhard et al.,
“Evaluation of selective caries removal by a fluorescence feedback-controlled Er:YAG laser in vitro,”
Caries Res, 39
(6), 496
–504
(2005). http://dx.doi.org/10.1159/000088186 CAREBK 0008-6568 Google Scholar
K. Fan and D. Fried,
“Scanning ablation of root caries with acoustic feedback control,”
Proc. SPIE, 6425 64250J
(2007). http://dx.doi.org/10.1117/12.714789 PSISDG 0277-786X Google Scholar
D. Harris and D. Fried,
“Pulsed Nd:YAG laser selective ablation of surface enamel caries: I. Photoacoustic response and FTIR spectroscopy,”
Proc. SPIE, 3910 164
–170
(2000). http://dx.doi.org/10.1117/12.380823 PSISDG 0277-786X Google Scholar
T. Henry et al.,
“Selective removal of demineralized enamel using a laser coupled with near-IR reflectance imaging,”
Proc. SPIE, 9306 93060M
(2015). http://dx.doi.org/10.1117/12.2083647 PSISDG 0277-786X Google Scholar
J. Y. Cheng, K. Fan and D. Fried,
“Use of a compact fiber optic spectrometer for spectral feedback during the laser ablation of dental hard tissues and restorative materials,”
Proc. SPIE, 6137 61370F
(2006). http://dx.doi.org/10.1117/12.661789 PSISDG 0277-786X Google Scholar
K. H. Chan, K. Hirasuna and D. Fried,
“Rapid and selective removal of composite from tooth surfaces with a laser using spectral feedback,”
Lasers Surg. Med., 43
(8), 824
–832
(2011). http://dx.doi.org/10.1002/lsm.21111 LSMEDI 0196-8092 Google Scholar
R. Alexander and D. Fried,
“Selective removal of dental composite using 355-nm nanosecond laser pulses,”
Lasers Surg. Med., 30
(3), 240
–245
(2002). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
D. A. Cremers and L. J. Radziemski, Handbook of Laser-Induced Breakdown Spectroscopy, 2
–6 John Wiley & Sons, Wes Sussex
(2006). Google Scholar
L. T. Chew et al.,
“Zinc, lead and copper in human teeth measured by induced coupled argon plasma atomic emission spectroscopy (ICP-AES),”
Appl. Radiat. Isot., 53
(4–5), 633
–638
(2000). http://dx.doi.org/10.1016/S0969-8043(00)00243-8 ARISEF 0969-8043 Google Scholar
D. B. Sherman, M. P. Ruben and H. M. Goldman,
“The application of laser for the spectrochemical analysis of calcified tissues,”
Ann. N. Y. Acad. Sci., 122 767
–772
(1965). http://dx.doi.org/10.1111/j.1749-6632.1965.tb20258.x ANYAA9 0077-8923 Google Scholar
Q. Sun et al.,
“Zinc analysis in human skin by laser induced-breakdown spectroscopy,”
Talanta, 52
(2), 293
–300
(2000). http://dx.doi.org/10.1016/S0039-9140(00)00340-4 TLNTA2 0039-9140 Google Scholar
Z. Hosseinimakarem and S. H. Tavassoli,
“Analysis of human nails by laser-induced breakdown spectroscopy,”
J. Biomed. Opt., 16
(5), 057002
(2011). http://dx.doi.org/10.1117/1.3574757 JBOPFO 1083-3668 Google Scholar
V. K. Singh, V. Kumar and J. Sharma,
“Importance of laser-induced breakdown spectroscopy for hard tissues (bone, teeth) and other calcified tissue materials,”
Lasers Med. Sci.,
(2014). http://dx.doi.org/10.1007/s10103-014-1549-9 LMSCEZ 1435-604X Google Scholar
O. Samek et al.,
“Quantitative analysis of trace metal accumulation in teeth using laser-induced breakdown spectroscopy,”
Appl. Phys. A, 69
(S1), 179
–182
(1999). http://dx.doi.org/10.1007/s003399900277 APAMFC 0947-8396 Google Scholar
V. K. Unnikrishnan et al.,
“Biomedical and environmental applications of laser-induced breakdown spectroscopy,”
Pramana J. Phys., 82
(2), 397
–401
(2014). http://dx.doi.org/10.1007/s12043-014-0698-5 PRAMCI 0304-4289 Google Scholar
O. Samek, H. H. Telle and D. C. S. Beddows,
“Laser-induced breakdown spectroscopy: a tool for real-time, in vitro and in vivo identification of carious teeth,”
BMC Oral Health, 1
(2001). http://dx.doi.org/10.1186/1472-6831-1-1 BOHMBD 1472-6831 Google Scholar
M. H. Niemz,
“Investigation and spectral analysis of the plasma-induced ablation mechanism of dental hydroxyapatite,”
Appl. Phys. B, 58
(4), 273
–281
(1994). http://dx.doi.org/10.1007/BF01082621 APBOEM 0946-2171 Google Scholar
Y. Matsuura et al.,
“Infrared-laser delivery system based on polymer-coated hollow fibers,”
Opt. Laser Technol., 33
(5), 279
–283
(2001). http://dx.doi.org/10.1016/S0030-3992(01)00018-4 OLTCAS 0030-3992 Google Scholar
Y. Matsuura et al.,
“Hollow-fiber delivery of high-power pulsed Nd:YAG laser light,”
Opt. Lett., 23
(23), 1858
–1860
(1998). http://dx.doi.org/10.1364/OL.23.001858 OPLEDP 0146-9592 Google Scholar
N. Farid, S. Bashir and K. Mahmood,
“Effect of ambient gas conditions on laser-induced copper plasma and surface morphology,”
Phys. Scr., 85
(1), 1
–7
(2012). http://dx.doi.org/10.1088/0031-8949/85/01/015702 PHSTBO 0031-8949 Google Scholar
O. Samek et al.,
“Quantitative laser-induced breakdown spectroscopy analysis of calcified tissue samples,”
Appl. Phys. B, 56 865
–875
(2001). http://dx.doi.org/10.1016/S0584-8547(01)00198-7 APBOEM 0946-2171 Google Scholar
K. V. Singh and A. K. Rai,
“Potential of laser-induced breakdown spectroscopy for the rapid identification of carious teeth,”
Lasers Med. Sci., 26
(3), 307
–315
(2011). http://dx.doi.org/10.1007/s10103-010-0786-9 LMSCEZ 1435-604X Google Scholar
I. Zipkin, Biological Mineralization, 58
–61 John Wiley & Sons, New York
(1973). Google Scholar
E. Reitznerova et al.,
“Determination of some trace elements in human tooth enamel,”
Fresenius J. Anal. Chem., 367 748
–754
(2000). http://dx.doi.org/10.1007/s002160000461 FJACES 0937-0633 Google Scholar
R. J. Lynch,
“Zinc in the mouth, its interactions with dental enamel and possible effects on caries: a review of the literature,”
Int. Dent. J., 61
(s3), 46
–54
(2011). http://dx.doi.org/10.1111/j.1875-595X.2011.00049.x IDJOAS 0020-6539 Google Scholar
R. J. Lynch et al.,
“Effects of zinc and fluoride on the remineralisation of artificial carious lesions under simulated plaque fluid conditions,”
Caries Res, 45
(3), 313
–322
(2011). http://dx.doi.org/10.1159/000324804 CAREBK 0008-6568 Google Scholar
R. Tan-Walker and R. J. Gilbert,
“Oral delivery of zinc from slurries and separated supernatant fractions of dentifrices,”
J. Dent. Res., 68 1708
–1709
(1989). JDREAF 0022-0345 Google Scholar
D. Kang, D. Amarasiriwardena and A. H. Goodman,
“Application of laser ablation–inductively coupled plasma–mass spectrometry (LA–ICP–MS) to investigate trace metal spatial distributions in human tooth enamel and dentine growth layers and pulp,”
Anal. Bioanal. Chem., 378
(6), 1608
–1615
(2004). http://dx.doi.org/10.1007/s00216-004-2504-6 ABCNBP 1618-2642 Google Scholar
T. Tange,
“A study of trace elements in deciduous teeth: the differences of Cd, Zn, Pb, and levels in prenatally and postnatally formed deciduous teeth [in Japanese],”
Kanagawa Shigaku, 24
(4), 653
–670
(1990). KSHGDM Google Scholar
D. Fried et al.,
“Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography,”
J. Biomed. Opt., 7
(4), 618
–627
(2002). http://dx.doi.org/10.1117/1.1509752 JBOPFO 1083-3668 Google Scholar
K. H. Chan et al.,
“A method for monitoring enamel erosion using laser irradiated surfaces and optical coherence tomography,”
Lasers Surg. Med., 46
(9), 672
–678
(2014). http://dx.doi.org/10.1002/lsm.v46.9 LSMEDI 0196-8092 Google Scholar
BiographyShuhei Sasazawa received his BS degree in electrical communications engineering from Tohoku University, Japan, in 2013, and then received his MS degree from Graduate School of Biomedical Engineering, Tohoku University, Japan, in 2015. He had been working on the development of fiber-based dental diagnosis systems. Satoko Kakino received his DDS degree from Tokyo Medical and Dental University (TMDU), Japan, in 2004 and her PhD in pediatric dentistry from TMDU in 2008. Currently, she is an assistant professor of the Pediatric Dentistry Graduate School of TMDU. Her research interests include noninvasive diagnosis of the tooth viability in relation to circulation of the dental pulp by optical methods. Yuji Matsuura has been a professor of the Graduate School of Biomedical Engineering, Tohoku University, Japan, since 2008. His research interests include application of specialty fiber optics in medical fields, hollow waveguides for ultraviolet and infrared light, and optics for soft and hard x-rays. He is a member of the SPIE and the Optical Society of America. |