|
|
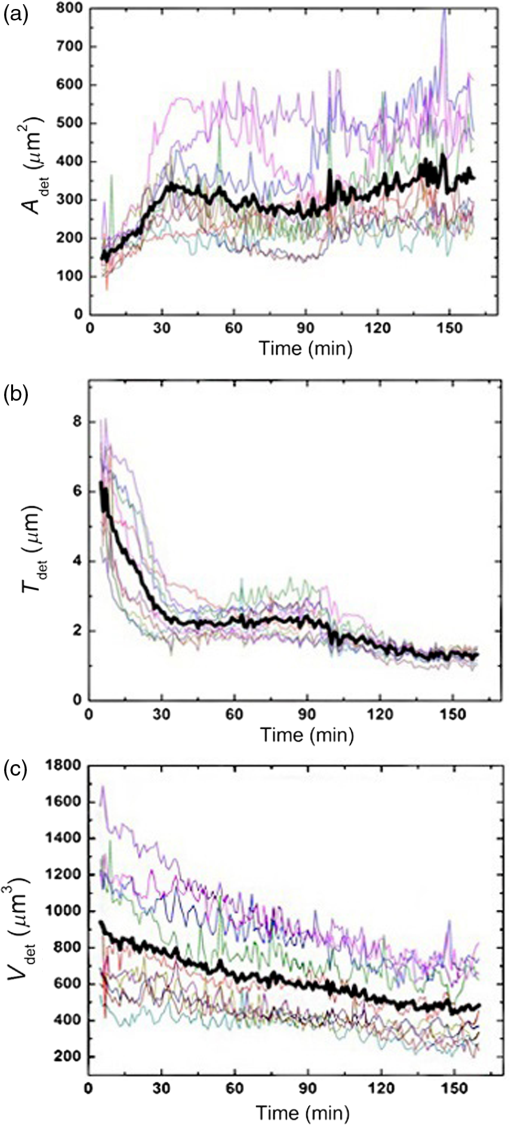
1.IntroductionDigital holographic microscopy is a high-resolution imaging technique that offers real-time imaging and quantitative measurements of cell morphological and physiological parameters without labeling the observed cells.1,2 This technique explores the phase shift of the probing laser light that has been reflected or transmitted through the monitored object. The amount of phase shift is determined by the optical thickness of the sample, which depends on the index of refraction inside the object and its physical thickness. The optical thickness can be measured with a precision down to the nanometer scale.1,2 In the present work, we demonstrate the capabilities of the recently introduced Holomonitor M4, which integrates a phase-contrast microscope with a digital holographic function, and it can create three-dimensional (3-D) images of the observed living cells. Previous versions of the Holomonitor (M2 and M3) are too large to be put into a cell culture incubator, which causes serious drawbacks in the observations of the living cells under relevant physiological conditions over long periods of time without any disturbance. For example, to maintain and keep the cells at a 37°C environment during the measurements, a heated plate was used, but clearly, this is not as efficient as an incubator (due to the differences in the temperature, humidity, and level).2 Persson et al. recommended the development of a Holomonitor system with controlled temperature and levels. During our experiments, the newly developed remarkably small-sized M4 Holomonitor was inside a cell culture incubator continuously. The parts of the appliance are specially selected to withstand the harsh climate of the cell incubator. Furthermore, a special mechanical stage was also developed in order to position the sample into that range of the optical arrangement where digital autofocusing works with high reproducibility and precision. In this range, there are no focusing issues, as there is a “built in” digital autofocus in the software. This is possible since the optical data are collected from a relatively wide span rather than from a focal plane. Image quality ameliorates due to our mechanical stage compared to the original arrangement, where the vertical position of the sample was set by adapter sheets with various thicknesses. Thus, with this stage, we can set sharp images faster and easier. The in-situ monitoring of living cell movements are more and more important today.2 Several other techniques exist to study cellular movements, but they have been mainly directed at migration studies and they have their drawbacks. For example, filter assays measure the cell migration over a membrane in response to chemoattractants (Boyden chamber, Zigmond and Dunn chambers). The disadvantage is that they are very specialized, requiring cells to migrate through both a matrix and the pores of the filter.2 Very few cell lines can migrate through both of them. Single cell movements can be studied by using time-lapse imaging, often with fluorescent markers.2 Fluorescent imaging also has disadvantages. It may disturb the cells and the imaging time is limited by the bleaching of the fluorescent marker.2 In contrast with fluorescent imaging, holographic microscopy is a label-free technique.1,2 To understand the behavior of the cells in such environments and to draw conclusions to figure out further therapeutic possibilities, it is crucial to observe and quantitatively record live cell behavior, the random movement (motility), and directional movement (migration).2 For example, observation of the movement of the tumor cells is a very important topic: the formation of tumors and metastasis arise when cells migrate and move.1 Highly tumorigenic cells have been shown earlier to move faster than nontumorigenic cells; thus, it is essential to follow even the single cells, not just the populations.2 Developing drugs that inhibit migration, therefore, has been important and these are in the focus of many research projects.1 It has to be also emphasized that traditional herbal extracts became more and more popular in the cure of these illnesses, but the literature of systematic quantitative studies is quite limited. Some studies showed that the main extract of the green tea [epigallocatechin gallate (EGCg)] is effective against many cancer cell types.3–5 The action of EGCg on cell motility and migration is an intensively researched topic today.6–9 Cell motility and cell stiffness are closely related to metastatic activity in cancer cells. Earlier experiments also showed that EGCg treatment dose dependently inhibited cell motility and increased cell stiffness in human lung cancer cells and inhibited the expression of vimentin and Slug.9 Obviously, hypoxia has stimulatory effects on cancer cell invasion and migration. EGCg has inhibitory effects on chemoattractant- and hypoxia-stimulated migration of HeLa cells.6 Another study also showed that EGCg inhibits even the motility of the Plasmodium sporozoite, the causative agent of malaria. It might occur due to the binding of EGCg to the adhesion molecules on the parasite surface, for example circumsporozoite protein (CSP) or thrombospondin-related anonymous protein (TRAP) that are important proteins for its motility.7 Here, we observed the dynamic properties of tumor cells under exposure of a natural chemical compound: EGCg, a green tea polyphenol. We demonstrate that the M4 system is a highly efficient research tool in this field. The effect of substrate nanostructuring on live cell behavior has recently come into prominence and is relevant for various fields, such as biomedical applications, separation science, and stem cell research.10,11 The observation of bone cells on these types of coatings is critical for further biomedical applications. For example, it is anticipated that these unique nanostructures have the potential to be considered for the development of next generation orthopedic and dental implants.12 Holographic microscopy can also be successfully applied in cell morphology studies. However, analyzing cell behavior and morphology on nanostructured surfaces using these novel techniques is not a straightforward and trivial task. Here, preosteoblast cell adhesion and spreading were monitored on a novel transparent titanate nanotube (TNT) coating recently introduced by us.13 The developed coating enhances the adsorption of proteins and the adhesion of cells by increasing the roughness of the surface. The homogeneity and the transparency of the coating, and the easy, fast, and cheap preparation procedure make it a promising candidate for biomedical and biosensing applications.13 Stable TNT sols and high quality transparent thin films made from them were prepared without using any additives.14 The novel coating is fabricated by spin coating, and this technique cannot be used on standard Holomonitor cellular chambers or Ibidi slides. We demonstrate that after some methodological developments, the present optical system is also compatible with the observation of live cell behavior on nanostructured coatings. It has to be also emphasized that for many cell imaging studies, image contrast is simply too low unless dyes are added. Routine dyes are used extensively in fluorescence microscopy for crude analysis of live cells.15 Visualization of the morphological changes that occurs also typically rely on the use of fluorochromes to enhance contrast.15 The high contrast images obtained by these labeling techniques are suitable for downstream analysis, but these methods require the interruption and manipulation of normal cell culture conditions, potentially perturbing optimal cell function and it is likely that the cell division process is also affected.15 New imaging techniques, for example, digital holographic microscopy and ptychography, do not use any labels or dyes.15 However, according to several authors, it would be useful to develop cell labels that can be detected in a nondestructive, noninvasive way to increase the scope of digital holography.16 Using the present optical arrangement, one can create sharp 3-D images of the investigated cells, but due to the limited vertical resolution of the Holomonitor, very thin parts of the cell lamellipodium are not detected at all. As a result, the cell contact area and volume values determined by the system are underestimated, while the averaged cell thickness is overestimated. In the case of morphological parameter wanderings, we propose a simple method to correct the recorded data to obtain more reliable single-cell morphological values. Moreover, we compare the corrected data with kinetic parameters obtained earlier by a surface-sensitive label-free optical biosensor.13 2.Materials and Methods2.1.Cell Cultures, Solutions and Related ProtocolsCell lines and chemicals of reagent grade were obtained from Sigma-Aldrich (Hungary), unless stated otherwise. The cervical cancer cell line, HeLa, was used in our first experiment. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (Gibco, Invitrogen Inc., Paisley, United Kingdom), 4 mM l-glutamine, penicillin, streptomycin solution, and amphotericin B. In the second experiment, we used preosteoblast cells. The osteoblastic cell line MC3T3-E1 was maintained in -modified minimal essential medium, containing ribonucleosides, deoxyribonucleosides, and sodium bicarbonate, supplemented with 10% fetal bovine serum (Gibco, Invitrogen Inc.), 2 mM l-glutamine, penicillin, streptomycin solution, and amphotericin B. During the Holomonitor experiment, the MC3T3 cells were seeded at an appropriate density ( cells in ) onto the titanate-coated cover glasses. Cells were cultured in a humidified atmosphere containing 5% at 37°C. On reaching 80% confluence, cells were detached every three to five days using 0.25% trypsin/ethylenediaminetetraacetic acid solution and were not used beyond passage 20. EGCg solution was prepared in DMEM medium at a concentration . The 1 ml HeLa cell suspension was applied into the chamber of the PLL-g-PEG/PLL-g-PEG-RGD coated Ibidi -Slide. The Ibidi slide was placed onto the sample stage. The cells were incubated and monitored for 20 h and images were taken every 5 min. During this time, the cells could spread, proliferate, and move. After 20 h, an additional 1 ml EGCg solution (freshly solved in DMEM media) was added to the cells. They were incubated and monitored for 20 h and images were again taken every 5 min. 2.2.Standard Ibidi Slide and Coating Procedure with Synthetic PolymersWe used a special flow chamber (Ibidi, -Slide I, hydrophobic, uncoated, sterile) in our experiments with the HeLa cells. The PLL-g-PEG [SuSoS, PLL(20)-g(3.5)-PEG(2)] and PLL-PEG-RGD solution [SuSoS, PLL(20)-g(3.5)-PEG(2.3)/PEG(3.4)-RGD (8%)] were mixed in 1:1 ratio and pipetted into the chamber for 30 min. All polymers were dissolved in 10 mM HEPES, . Irreversibly adsorbed polymers were removed by an intense washing step with solvent. After that, we filled the channel of the -Slide with cell suspension in DMEM media (). 2.3.Developed Chamber and Coating Procedure to Create the Nanostructured Titanate FilmsBorosilicate cover glasses (, 0.16-mm thick) from VWR International and Kalrez O-ring (Compound 4079 AS no. 012 inner ) from Angst+Pfister were used. The spin coating of the TNT sol was carried out at room temperature at a speed of 3000 RPM for 20 s.13 MC3T3 cells were seeded at an appropriate density ( cells in ) onto the cover glasses. 3.Results and Discussions3.1.Instrumentational Development—Sample Stage to Improve Image Quality and the Employed Cell ChambersA special mechanical stage was developed in order to position the sample into that range of the optical arrangement where digital autofocusing works with high reproducibility and precision. The geometrical constraints of this special aluminum stage guarantee that when the micrometer screw is rotated, the surface of the table remains horizontal, which does not tilt the sample but moves up or down in a highly precise manner. Due to its formation, we can set the height of the sample and keep it horizontal without bias [see Figs. 1(a) and 1(b)]. The necessity and importance of this stage is evident. If the sample is positioned too low, only the edge of the field of vision can be seen [see Fig. 1(f)], with a similar problem if the sample is positioned too high [see Fig. 1(d)]. In focus, we can create clear images [see Fig. 1(e)]. This is important, because obtaining sharp images is the main requirement for the further evaluations. Fig. 1Incubator proof Holomonitor M4 and the developed sample stage. (a) Representation of the basics of the working principle of the aluminum sample stage. (b) Appliance in the incubator. The sample stage is moveable upward and downward using the screw. (c) Ibidi -Slide filled with cell culture media with adhered cells in the channel of the slide. (d) Image recorded by the Holomonitor above focus (e) in focus and (f) below focus. Note the image above and below focus means that the sample stage is positioned above or below that range where the image focus can be found by the Holomonitor digitally. 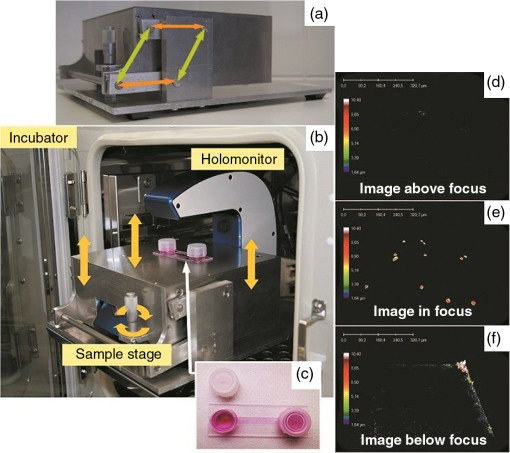 In the first experiments with EGCg, we used an Ibidi flow chamber made of the highest optical quality plastic [see Fig. 1(c)]. Ibidi -Slides and tissue culture flasks were also used for observing the effects of the anticancer drug on tumor cells by Holomonitor in earlier measurements.15,17–19 The coating solution, the cell suspension, and later the EGCg solution were pipetted into one of the chambers of the slide. From that chamber, the fluids flow into the other chamber through a channel between them. The images of the cells were taken at the channel area [see Fig. 1(c)]. In the second experiment with the nanostructured coatings, we could not use Ibidi -Slides. The employed TNT coating is fabricated by spin coating, but with standard Holomonitor cellular chambers or Ibidi slides direct spin-coating is not feasible; because these chambers are enclosed and can be filled up with a solution using tiny inlets and outlets, a large open surface is not available for spin coating. We solved this problem by developing a novel methodology for assembling the Holomonitor chamber. Here, the transparent nanostructured coating (Fig. 2) was prepared on a cover glass by the standard room temperature spin-coating process, employing exactly the same parameters as reported earlier.13 This resulted in TNT coatings of thick with a mean squared roughness of around 5 nm.13 After an O-ring was placed onto the coated cover glass, the cell suspension was pipetted into it until bulging, and another glass slide was put on the top, carefully preventing the ingress of air bubbles [Fig. 2(a)] so we could avoid evaporation and disturbing meniscus effects. The O-ring was previously lubricated with silicone grease. Fig. 2The measurement setup used in the second experiment where the cells are adhered onto a nanostructured titanate layer. (a) Cell suspension was pipetted into the O-ring placed on the nanostructured surface and then it was covered by another cover glass. (b) Atomic force microscopy image of the transparent nanostructured titanate coating. 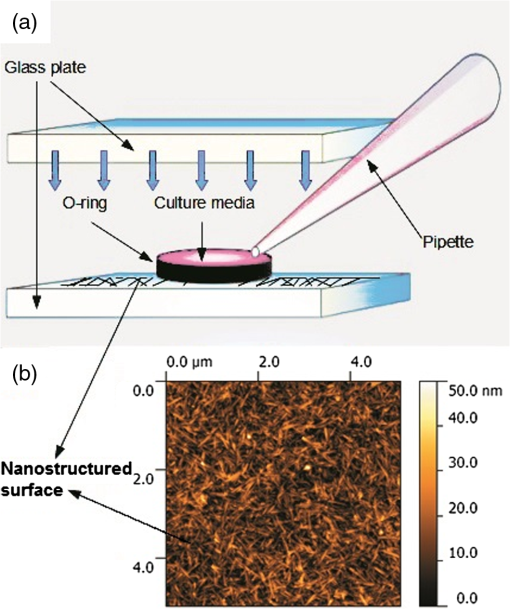 3.2.Effect of EGCg on Cellular Motility and MigrationIn the first experiment, we investigated the effect of EGCg on cellular motility and migration. From the captured images, a time-lapse video can be created by combining the images into a continuous film. Single cells were tracked in each image sequence to get information about cell movement: migration, motility, and motility speed. Note that the Holomonitor software uses the centroid for the cell tracking. A more detailed profile analysis can be made even of a single cell by the Holomonitor program and by cross-sectional images of the spread cells [see Fig. 3(d)]. Fig. 3Cell movement analysis by Holomonitor M4 when epigallocatechin gallate (EGCg) is added to the HeLa cancer cells. The black lines represent the mean of the values, averaged for five typical cells. (a) Motility, (b) migration, (c) average motility speed, the effect of EGCg is clearly seen, and (d) profile analysis of the spread HeLa cell by Holomonitor M4. 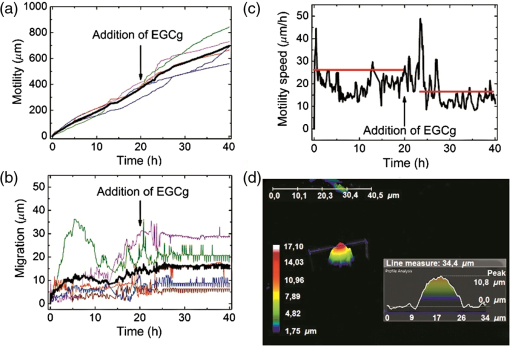 In the Holomonitor software, motility is calculated as the actual distance the cell has moved from the start to the end of the analysis and migration as the shortest distance from the starting point to the end point. Note that the motility can be very high even if the migration is close to zero. Figures 3(a)–3(c) show average motility, motility speed, and migration for typical five cells selected from the captured images. We found that after the addition of EGCg to the cells, mainly the migration, but also the motility and motility speed of the cells started to reduce or stagnate. With this experiment, we proved that our newly developed sample stage is excellent for monitoring the cells and to observe the effects of active substances. We first demonstrated that the movement of HeLa cells temperately reduced after the addition of the polyphenol EGCg and this effect was monitored in a completely noninvasive and label-free manner under physiologically relevant conditions. 3.3.Cell Morphology on TNT Coatings During Cellular Spreading and AdhesionIn the second experiment, the O-ring (lubricated with silicone grease) was placed on the TNT-coated cover glass and the MC3T3 cell suspension was pipetted into it. Then another cover glass was put on the O-ring. Thereafter, it was placed in the incubator on the sample stage of the Holomonitor. The cells were incubated and monitored for 24 h, and images were taken every minute for the first 3 h, and every 5 min for the remainder of the time (Fig. 4). Fig. 43-D images of the preosteoblast cells on the nanostructured titanate nanotube coating and cross-section images of typical cells in the beginning, after 3 h and after 24 h. 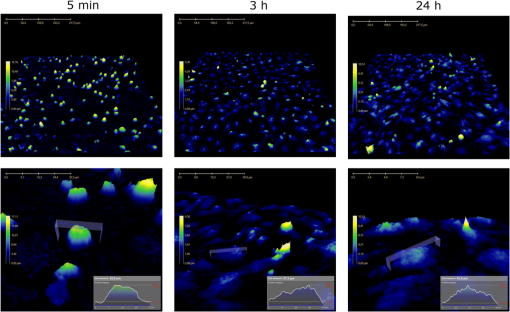 The area, the average thickness, and the volume of 10 typical cells on TNT are plotted as a function of time, and the mean of the values is represented by the black curve (Fig. 5). Since the cellular behavior did not change considerably after 3 h, the area and thickness values are plotted only for the first 160 min. It is clearly seen that during the first 30 min, the area increased considerably, while later the changes were negligible. The initial small variance of the area values was increasing in the first hour. By contrast, the measured cell thickness values decreased dramatically in the beginning of the adhesion, and the relatively large variation became much smaller after 2 h. 3.4.Limitations of the Measured Single-Cell Morphological ParametersThe lateral resolution of the Holomonitor M4 is , and the vertical resolution () is also , depending on the background noise. The noise levels are determined by various factors, for example, the cell culture vessel, the density of the cells, etc. We found that due to these factors, the vertical resolution of the system is limited, and sometimes the lamellipodia, the very thin parts of the cells, cannot be sensed perfectly by the instrument. This phenomenon is illustrated in Fig. 6(c). We suppose that under certain thicknesses, parts of the cell slick into the background surface; therefore, these thin parts of the cell cannot be distinguished from the background. The averaged thickness of the lamellipodia () is supposed to be in the present work. An anisotonic state under pathophysiological conditions generally develops slowly and gradually with time, which gives the volume-restoring mechanisms a chance to continuously preserve cell volume. The resulting shift in cell volume is usually negligible, a phenomenon designated isovolumetric volume regulation.20 The reduction of the detected cell volumes during our experiment is, therefore, unrealistic [see Fig. 5(c)] and we can also safely state that the determined spread cell contact areas are, therefore, strongly underestimated. We propose a simple method to correct the recorded Holomonitor data. Fig. 6The detected and corrected data of the preosteoblast cells: (a) the area and (b) the thickness curves. (c) Sometimes the lamellipodia, the very thin parts of the cells, cannot be sensed perfectly by the instrument. 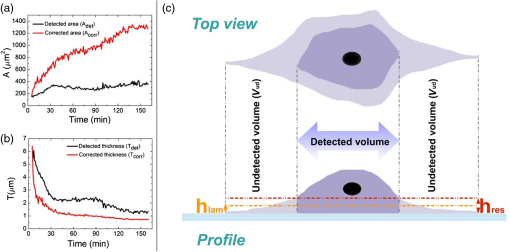 Our starting point is the assumption that the volume of the cells is constant during the cell adhesion experiment, and we can easily calculate the volume of the cell by multiplying its area and thickness. Our starting point was the preosteoblast average volume at the zeroth minute at the beginning of the measurement () and the other volume is the value that belongs to the 160th min () [see Fig. 5(c)]. The difference between the first and the last timepoint is where is the volume at the first timepoint and is the volume at the last timepoint of the measurement.We can get the undetected volume () at the ’th timepoint from this simple calculation: where is the volume at the ’th timepoint of the measurement and is the undetected volume.The undetected area can be calculated by the following equations: where is the undetected area and is the average thickness of the lamellipodia (). where is the corrected area and is the detected area. The difference between the detected and corrected area is shown in Fig. 6(a).The averaged cell thickness also needs to be corrected by using the following equation: where is the corrected thickness. The difference between the detected and corrected thickness is shown in Fig. 6(b). Figures. 6(a) and 6(b) clearly show that the corrected morphological values predict a much smoother spreading behavior, saturating after 2 h. Note that this is in fine agreement with our previous study using optical biosensors to monitor preosteoblast cell adhesion and spreading on these nanostructured films.134.ConclusionsIn the present work, we report on the successful application of the M4 Holomonitor to monitor cancer cell motility, migration, motility speed, and the spreading of preosteoblast cells on a nanostructured titanate coating. The M4 Holomonitor, applied in the present study, has a small size, and it is making it feasible to be directly put into a humidified cell culture incubator. This technique is completely noninvasive and label-free, therefore, nothing disturbs the cells during their movements. A special mechanical stage was also developed in order to position the sample into that range of the optical arrangement where digital autofocusing works with high reproducibility and precision. This new development significantly improved image quality. With the help of this novel development, we could perform two successful measurements demonstrating the capabilities of our novel arrangement. The Holomonitor was used to analyze the morphology and movements of living cells in a way that is automatic, cost efficient, and causes the cells no harm.16 In our first study, it is demonstrated that the movement of HeLa cells was temperately reduced after the addition of polyphenol EGCg. This phenomenon could be monitored in a completely noninvasive and label-free manner. With this experiment, we proved that our recently developed sample stage is an excellent tool for observing cell dynamical changes and monitoring the effects of chemical substances on living cells. In another experiment, the Holomonitor M4 was used to study cellular adhesion and spreading on nanostructured titanate coatings. A novel arrangement was developed to measure live cell behavior on spin-coated surfaces. We recorded the adhesion and spreading processes of the cells in real time. From the recorded data, we concluded that the averaged cell thickness and area detected by the instrument saturate after 30 min. These results are clearly contradictive to previous investigations where the preosteoblast adhesion on these nanostructured surfaces was monitored by an optical biosensor.13 Based on these findings, we concluded that under certain thicknesses, relatively large parts of the cells (parts of the thin lamellipodium) slick into the background surface due to the limited vertical resolution of the optical arrangement. We determined the time-dependent corrected single-cell contact area and averaged thickness by the assumption that the cell volume is constant during the adhesion process. As the thin and transparent coating did not decrease the image quality, the Holomonitor could be used to demonstrate the effects of these types of coatings on the adhesion kinetics, migration, and morphology of living cells. Our results clearly demonstrate that the recently introduced Holomonitor M4 has every potential to become an important tool in investigating cellular behavior on nanostructured coatings over long periods and under exposure to drugs or various natural compounds. Our new correction method for estimation of the undetected parts of lamellipodia resulted in more precise evaluations supported by earlier biosensor kinetic data.13 Measurements with a Holomonitor can be especially useful in combination with other novel label-free biosensing methods21,22 to obtain a high content analysis of live cell morphology and behavior. AcknowledgmentsThis work was supported by the Momentum Program (Lendület) of the Hungarian Academy of Sciences. This research was also realized in the frames of TÁMOP 4.2.4. A/2-11-1-2012-0001 “National Excellence Program – Elaborating and operating an inland student and researcher personal support system convergence program.” The project was subsidized by the European Union and co-financed by the European Social Fund. We would like to thank Kersti Alm for her suggestions and comments and we would like to thank Plósz Engineering Office (Hungary) as well for creating the sample stage. ReferencesZ. El-Schish et al., Digital Holographic MICROSCOPY–innovative and Non-destructive Analysis of Living Cells, 1055
–1062
(2010). Google Scholar
J. Persson et al., Cell motility studies using digital holographic microscopy, 1063
–1072
(2010). EDUCD6 Google Scholar
H. Mukhtar and N. Ahmad,
“Tea polyphenols: prevention of cancer and optimizing health,”
Am. J. Clin. Nutr., 71
(6 Suppl), 1698
–1702
(2000). AJCNAC 0002-9165 Google Scholar
C. J. Dufresne and E. R. Farnworth,
“A review of latest research findings on the health promotion properties of tea,”
J. Nutr. Biochem., 12
(7), 404
–421
(2001). http://dx.doi.org/10.1016/S0955-2863(01)00155-3 JNBIEL 0955-2863 Google Scholar
B. N. Singh, S. Shankar and R. K. Srivastava,
“Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications,”
Biochem. Pharmacol., 82
(12), 1807
–1821
(2011). http://dx.doi.org/10.1016/j.bcp.2011.07.093 BCPCA6 0006-2952 Google Scholar
Q. Zhang et al.,
“Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells,”
Mol. Cancer Ther., 5
(5), 1227
–1238
(2006). http://dx.doi.org/10.1158/1535-7163.MCT-05-0490 MCTOCF 1535-7163 Google Scholar
J. K. Hellmann et al.,
“Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility,”
PLoS One, 5
(1), 1
–7
(2010). http://dx.doi.org/10.1371/journal.pone.0008682 1932-6203 Google Scholar
O. Tudoran et al.,
“Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells,”
J. Cell. Mol. Med., 16
(3), 520
–530
(2012). http://dx.doi.org/10.1111/j.1582-4934.2011.01346.x JCMMC9 1582-1838 Google Scholar
A. Takahashi et al.,
“Mechanism-based inhibition of cancer metastasis with (-)-epigallocatechin gallate,”
Biochem. Biophys. Res. Commun., 443
(1), 1
–6
(2014). http://dx.doi.org/10.1016/j.bbrc.2013.10.094 BBRCA9 0006-291X Google Scholar
C. S. Chen et al.,
“Geometric control of cell life and death,”
Science, 276
(5317), 1425
–1428
(1997). http://dx.doi.org/10.1126/science.276.5317.1425 SCIEAS 0036-8075 Google Scholar
L. Bacakova et al.,
“Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants,”
Biotechnol. Adv., 29
(6), 739
–767
(2011). http://dx.doi.org/10.1016/j.biotechadv.2011.06.004 BIADDD 0734-9750 Google Scholar
N. Swami, Z. Cui and L. S. Nair,
“Titania nanotubes: novel nanostructures for improved osseointegration,”
J. Heat Transfer, 133
(3), 034002
(2011). http://dx.doi.org/10.1115/1.4002465 JHTRAO 0022-1481 Google Scholar
J. Nador et al.,
“Enhanced protein adsorption and cellular adhesion using transparent titanate nanotube thin films made by a simple and inexpensive room temperature process: application to optical biochips,”
Colloids Surf. B Biointerfaces, 122 491
–497
(2014). http://dx.doi.org/10.1016/j.colsurfb.2014.07.015 CSBBEQ 0927-7765 Google Scholar
L. Kőrösi et al.,
“Titanate nanotube thin films with enhanced thermal stability and high-transparency prepared from additive-free sols,”
J. Solid State Chem., 192 342
–350
(2012). http://dx.doi.org/10.1016/j.jssc.2012.04.038 JSSCBI 0022-4596 Google Scholar
J. Marrison et al.,
“Ptychography--a label free, high-contrast imaging technique for live cells using quantitative phase information,”
Sci. Rep., 3 2369
(2013). http://dx.doi.org/10.1038/srep02369 SRCEC3 2045-2322 Google Scholar
K. Alm et al., Cells and Holograms-Holograms and Digital Holographic Microscopy as a Tool to Study the Morphology of Living Cells, 335
–351
(2013). Google Scholar
M. Trulsson et al.,
“HAMLET binding to??-actinin facilitates tumor cell detachment,”
PLoS One, 6
(3),
(2011). http://dx.doi.org/10.1371/journal.pone.0017179 1932-6203 Google Scholar
M. Puthia et al.,
“Prevention and treatment of colon cancer by peroral administration of HAMLET (human α-lactalbumin made lethal to tumour cells),”
Gut, 63
(1), 131
–142
(2013). http://dx.doi.org/10.1136/gutjnl-2012-303715 GUTTAK 0017-5749 Google Scholar
J. H. CS et al.,
“Low resolution solution structure of HAMLET and the importance of its alpha-domains in tumoricidal activity,”
PLoS One, 7
(12),
(2012). http://dx.doi.org/10.1371/journal.pone.0053051 1932-6203 Google Scholar
E. K. Hoffmann, I. H. Lambert and S. F. Pedersen,
“Physiology of cell volume regulation in vertebrates,”
Physiol. Rev., 89
(1), 193
–277
(2009). http://dx.doi.org/10.1152/physrev.00037.2007 PHREA7 0031-9333 Google Scholar
N. Orgovan et al.,
“Dependence of cancer cell adhesion kinetics on integrin ligand surface density measured by a high-throughput label-free resonant waveguide grating biosensor,”
Sci. Rep., 4 4034
(2014). http://dx.doi.org/10.1038/srep04034 SRCEC3 2045-2322 Google Scholar
N. Orgovan et al.,
“In-situ and label-free optical monitoring of the adhesion andspreading of primary monocytes isolated from human blood: Dependence on serum concentration levels,”
Biosens. Bioelectron., 54 339
–344
(2014). http://dx.doi.org/10.1016/j.bios.2013.10.076 BBIOE4 0956-5663 Google Scholar
BiographyBeatrix Peter received her master’s degree from Budapest University of Engineering and Economics in 2012. Currently, she is a PhD student at the Doctoral School of Molecular- and Nanotechnology at the University of Pannonia. She conducts research at the Nanobiosensorics Group at MTA EK MFA. Her research topics include label-free biosensors, nanobiotechnology, physiological effects of green tea polyphenols, and cell adhesion. Judit Nador achieved her master’s degree at the Eotvos Lorand Science University. Currently she is a PhD student at the Doctoral School of Molecular- and Nanotechnology, University of Pannonia. She works in the Nanobiosensorics Group at MTA EK MFA. She prepares and characterizes coatings of titanate and titania nanoparticles, and studies protein adsorption and living cell adhesion on them using label-free biosensing methods. Krisztina Juhasz received her master’s degree in biophysics from the Eötvös Loránd University, Budapest. After graduation, she became a PhD student at University of Pannonia. Her main research interest was experimental investigation and theoretical modeling of live cell behavior. This research was conducted at the Nanobiosensorics Group at MTA EK MFA. At present, she is an ESR Marie Curie Fellow at Nanion Technologies. The main object of her work is to develop cardiomyocyte assays on biomedical devices. Agnes Dobos completed her bachelor’s degree at Budapest University of Engineering and Economics as a biochemical engineer. She was a research intern during her contribution to the present work. Currently, she is a master’s degree student in the field of tissue engineering and regenerative medicine at the Vienna University of Applied Sciences. Her current research interests are in biofabrication and material sciences. Laszlo Korosi obtained his PhD in chemistry at the University of Szeged in Hungary in 2008. He was a senior researcher at Enviroinvest Corp. during his contribution to this work. He is currently a research associate of the Research Institute for Viticulture and Oenology at University of Pécs. His research interests include nanostructured metal oxide photocatalysts, sol-gel chemistry, thin films, and antimicrobial activity of titania-based nanomaterials. Inna Székács has a PhD in biotechnology from Palladin Institute of Biochemistry of the National Academy of Sciences of Ukraine. She is currently a research fellow in the Nanobiosensorics Group at MTA EK MFA. Her research topics include biosensing, nanobiotechnology, cellular toxicology, and cell adhesion studies. Daniel Patko obtained his diploma in biophysics (MSc) from the University of Szeged in 2010. At present, he is conducting his research project at the Nanobiosensorics Group at MTA EK MFA. The research activities are supervised by Robert Horvath and include the development of label-free optical biosensors and their exploitation in novel applications areas, such as monitoring microvesicles, bacteria, and living cells. Robert Horvath is a physicist with specialization in biophysics. At present, he is the head of the Nanobiosensorics Group at MTA EK MFA. Their research activities cover various aspects of optical biosensing from theory to device fabrication and future applications, such as the detection and characterization of proteins, bacteria, and living cells ( www.nanobiosensorics.com). |