|
|
1.IntroductionPhotobiomodulation (PBM) has recently been defined as a form of light therapy that utilizes nonionizing forms of light sources, including lasers, light-emitting diodes (LED), and broadband light, in the visible and infrared spectrum. It is a nonthermal process involving endogenous chromophores eliciting photophysical (i.e., linear and nonlinear) and photochemical events at various biological scales.1 PBM with low-level laser, which falls within this comprehensive definition, promotes various beneficial effects, such as reduction of inflammation, alleviation of pain, and acceleration of tissue repair.2 The mechanisms underlying these therapeutic outcomes are not fully understood, but it is thought that PBM modulates cellular metabolic processes via a nonthermal action, leading to an enhanced tissue regenerative potential due to stimulation by light alone.2 Wound healing is one of the areas of main interest for PBM because laser promotes healing and reduces pain at the same time.3 PBM in musculoskeletal tissues has therapeutic benefits for the treatment of pain, osteoarthritis, and tendinitis.4,5 It is also able to accelerate the process of bone formation as well as the healing of bone defects, fractures, and delayed consolidation; PBM can counter the process of bone resorption in osteoporosis acting as a rebalancing factor for proper bone remodeling.6–9 Despite considerable improvements in the development of surgical treatments, bone substitutes or adjuvant therapies, such as ultrasonic treatment and pulsed electromagnetic field,10–12 providing optimal tissue healing and improving the quality of life of patients, remain a challenge for orthopedic, maxillofacial and oral surgery. In this context, PBM is a highly promising strategy because it is accessible, easy to administrate, safe, painless, does not require the concomitant use of drugs, and may be also applied in the presence of metal devices.8 Many clinical and experimental studies have investigated the influence of PBM on bone fracture healing,8,13–15 tendinopathy,16,17 osteoarthritis,18 alveolar bone healing after tooth extraction,19 and bone regeneration in the midpalatal suture after maxillary expansion.20 Moreover, in vivo experimental studies have assessed the effects of laser irradiation on oral wound healing.21–26 However, the wide range of laser sources, optical parameters and application times used in the many different studies make comparisons between studies very difficult and definite treatment protocols for the clinical practice almost impossible to be extrapolated. In vitro assays are, thus, required in order to overcome these problems because PBM parameters may be evaluated more rigorously at a cellular level as a first step toward standardization of treatment protocols for following preclinical and clinical applications. So far, the effect of PBM in the process of in vitro wound healing has been evaluated on adenocarcinoma human alveolar epithelial cells, rat kangaroo renal epithelial cells,27 oral keratinocytes,28 human gingival fibroblasts,29 human gingival epithelial cells,30 diabetic wounded fibroblasts,31 human epidermal stem cells,32 and tenocytes.33 Only one study has investigated the effect of laser and LED sources on human cells derived from an osteosarcoma line using U2OS cells, finding that both sources enhance wound closure and concluding that biochemical and functional investigations on the mechanism of action and downstream pathways are needed.27 However, U2OS cells are negative for most osteoblast markers and are considered closer to fibroblasts than osteoblasts.34,35 The aims of the current study were (1) using an in vitro scratch-wound healing assay to analyze the influence of irradiation by a gallium-aluminum-arsenide (GaAlAs) diode laser with a wavelength of 915 nm on the migration and proliferation of Saos-2 osteoblast-like cells, which resemble human mature osteoblast phenotype, play a key role in bone remodeling, differentiate, and form calcified matrix, (2) to compare the effects of different doses of 5, 10, and of laser irradiation, and (3) to evaluate modulation by laser irradiation of the differential gene expression and release of bone metabolism related proteins. The relative contribution of cell proliferation and migration to the scratch-wound closure was also investigated using the cell proliferation inhibitor Mitomycin C (MMC). 2.Materials and Methods2.1.Cell CultureSaos-2 human osteoblast-like cells (ATCC® HTB-85™) were cultured in Dulbecco modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, Missouri) enriched with 10% fetal calf serum (Lonza Walkersville Inc., Walkersville), penicillin, and streptomycin solution (Gibco Invitrogen SRL, San Giuliano Milanese, Milan). When confluent, cells were detached with 0.05% (w/v) trypsin and 0.02% (w/v) ethylenediamine tetra-acetic acid (EDTA), counted and seeded into black 24-multiwell tissue culture plates with clear bottoms (STEPBIO S.r.l., Bologna, Italy) at a density of . Plates were returned to the controlled humidified incubator (37°C in temperature, 95% air/5% ). 2.2.In Vitro Micro Wound ModelAfter confluence had been reached, Saos-2 cultures were wounded with a sterile Eppendorf tip to create a cell free zone in the monolayer (at baseline, T0, wound area measured ). Cells were extensively washed with sterile phosphate buffered solution (PBS, Gibco Invitrogen SRL, San Giuliano Milanese, Milan), then laser irradiated. To discriminate the contribution of cell proliferation and migration to the process of wound closure, half of the wells were treated with MMC (Sigma-Aldrich) at a concentration of . Cell cultures were incubated and observed with an inverted microscope (Nikon Eclipse Ti-U, Nikon Italia, Italy) equipped with a digital camera (Sight DS-Fi2, Nikon Italia, Italy) after 4, 24, 48, 72, and 96 h from laser irradiation. Each well was photographed at magnification to cover the wounded area. The image acquisition software (NiS Elements Advanced Research, Nikon Italia, Italy) was used to measure the area of the cell free zone of the artificially created wounds. 2.3.Laser IrradiationCells were exposed to irradiation with a GaAlAs diode laser (Pocket Laser, Orotig s.r.l., Verona, Italy), which has a wavelength of and a maximum power output of . A 100 Hz pulse irradiation mode, a duty cycle of 50%, and a set power of 1 W (corresponding to an output power of 0.575 W, as measured at hand piece aperture) were used for 48, 96, and 144 s. The administered doses were, respectively, of 5, 10, and . The laser beam was delivered perpendicularly to each well by an optical fiber 0.6 mm in diameter that was defocused at the tip by a concave lens to cover the growth area of each well () at a distance of 19 mm. To avoid overlapping or scattered irradiation, black multiwell plates were used for all the assays. During the period of laser irradiation, the cover plate was removed and DMEM was replaced with PBS to avoid serum interference during irradiation.2 Control cells were not irradiated. Both control and laser-treated cells were cultivated under the same experimental conditions. 2.4.Viability Test and DNA QuantificationAlamar Blue assay (AbD Serotec, Oxford, United Kingdom) was used to evaluate cell viability after 24, 48, and 72 h from laser irradiation. Alamar was added to each culture well (1:10 v/v) for 4 h at 37°C. The colorimetric reaction was measured spectrophotometrically on supernatants at 570 and 625 nm wavelengths with a microplate absorbance reader (iMark, Biorad-Laboratoires Inc., Hercules, USA). DNA quantification (Quant-iT™ PicoGreen® dsDNA) was performed following manufacturer’s instructions. Briefly, cells were repeatedly washed with PBS, frozen at , and thawed at room temperature three times. Cell lysis was obtained by adding of Tris-EDTA buffer with sodium dodecyl sulphate 0.01% solution. A working solution of the PicoGreen® reagent was added and incubated with cell lysates in the dark for 3 min at room temperature. The fluorescence was read at 490ex-520em wavelengths, the readings were expressed as relative fluorescence units, and the DNA amount of each sample was calculated above a standard curve. 2.5.Quantification of mRNA Expression Levels by Quantitative Polymerase Chain ReactionAfter 24, 48, and 72 h from laser irradiation, Saos-2 cells grown in the presence of DMEM (10% fetal bovine serum, 1% penicillin-streptomycin and plasmocin) were homogenized and total RNA extraction was performed by the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. Total RNA was eluted with RNase-free water, quantified by NanoDrop 2000 (Thermo Scientific, Waltham, Massachusetts), and kept at until reverse transcription. Each RNA sample (2500 ng) was reverse transcribed to cDNA using the Super Script VILO cDNA Synthesis kit (Invitrogen) according to manufacturer’s instructions and diluted to the final concentration of . Quantification of gene expression for collagen type 1 alpha (COL1A1), transforming growth factor beta 1 (TGFbeta1), interleukin 1 beta (IL1beta), matrix metallopeptidase 1 (MMP1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (used as reference gene) was performed in a LightCycler Instrument (Roche Diagnostics GmbH, Mannheim, Germany) with the use of the Quanti Tect SYBR Green PCR Master Mix (Qiagen). Primer details are reported in Table 1. The protocol included
Table 1Primer specifications.
Each sample was tested in duplicate. Data were collected using the LightCycler Software 4.1. Relative quantification was performed using the comparative threshold (Ct) method (ΔCt), where relative gene expression levels equal . Gene expression levels of the target genes were calculated by normalization to the reference gene GAPDH, using the cells untreated as calibrators. 2.6.Supernatant Enzyme-Linked Immunosorbent Assay MeasurementsAfter 24, 48, and 72 h from laser irradiation, supernatants were collected for collagen type 1 (COLL1), TGFbeta1, and prostaglandin () determinations by Enzyme-linked Immunosorbent Assay (ELISA) kits following manufacturer’s instructions (R&D Systems, Inc., Minneapolis, Minnesota, for and Boster Biological Technology Co, Fremont, California, for other proteins). For TGFbeta1 assays, cell supernatants were chemically activated before testing by two serial steps: 1 N HCl for 10 min followed by 1.2 N NaOH with 0.5 M Hepes for 10 min. The measured protein concentrations were normalized by DNA content. 2.7.Statistical AnalysisThe Shapiro-Wilk Test was used to assess the normality of data. For scratched-wound area, cell viability, COL1A1 and TGFbeta1 gene expression, the differences between the laser irradiation protocols for each experimental time were analyzed using a one-way multivariate analysis of variance (MANOVA) and Tukey’s honest significant difference post hoc test, while the differences between the three experimental times were evaluated with ANOVA with repeated measures and post hoc tests using the Bonferroni correction. For DNA content, release of COLL1 and TGFbeta1, the differences between the laser irradiation protocols for each experimental time were analyzed using a Kruskall–Wallis H test and a Bonferroni-corrected Mann–Whitney U post hoc test, while the differences between the three experimental times were evaluated with a Friedman test followed by a Bonferroni-corrected Wilcoxon paired sign-rank test for each laser irradiation protocol. All comparisons were performed between laser-irradiated and nonirradiated groups; inter laser-irradiated group comparisons were performed if the former ones were significant. Statistical analyses were performed using the statistical software SPSS for Windows (version 18.0; 2009; SPSS Inc., Chicago, Illinois). The limit for statistical significance was set at . 3.Results3.1.In Vitro Micro Wound HealingCells progressively participated in the healing process enabling a gradual wound closure (Fig. 1). The 5 and laser-treated groups were the first to reach complete wound closure after 72 h, followed by the laser-treated group after 96 h from irradiation. Nonirradiated controls still showed partial wound healing after 96 h (Fig. 1). Fig. 1Representative micrographs of the in vitro scratch wound of Saos-2 cells treated with different laser doses (5, 10, and or untreated () at different times (4, 24, 48, 72, and 96 h). Black lines mark the residual wound area. . 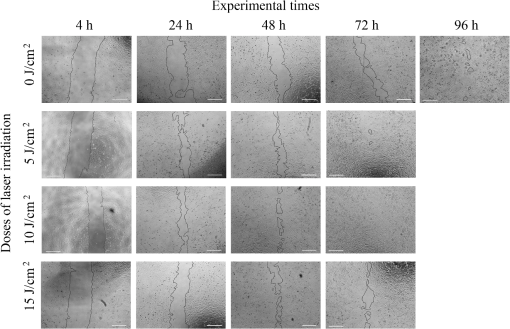 After 4 h, the 5 and laser-irradiated groups exhibited a significantly decreased wound area compared with the nonirradiated controls (, Fig. 2). After 48 and 72 h, all the laser-irradiated groups exhibited a significantly decreased wound area compared with nonirradiated controls ( for all, except for versus at 72 h with , Fig. 2) with a dose-dependent effect especially at 48 h ( versus 5 and with decreasing significance of and , respectively, Fig. 2). By analyzing data over the experimental times, the laser-irradiated group and nonirradiated controls significantly decreased the wound area between 4 and 72 h (, Fig. 2); in addition, control cell cultures significantly decreased the wound area between 4 and 24 h (, Fig. 2). The laser-irradiated group showed a significant decrease in wound area at almost all the experimental times (4 h versus 48 and 72 h, ; 48 h versus 72 h, , Fig. 2). Fig. 2Scratched wound area measured at different times (4, 24, 48, and 72 h) of Saos-2 cells treated with different laser doses (5, 10, and ) or untreated (). Data are means; bars are standard deviations. One-way multivariate analysis of variance (MANOVA) and Tukey’s honest significant difference (HSD) post hoc tests: 4 h: ***, versus 5 and , ; 48 h: ***, versus 5, 10, and , , ***, versus , , **, versus , ; 72 h: ***, versus 5 and , , **, versus , . ANOVA with repeated measures and post hoc tests using the Bonferroni correction: : a, 4 h versus 24 and 72 h, ; : b, 4 h versus 72 h, ; : c, 4 h versus 48 and 72 h, , d, 48 h versus 72 h, . 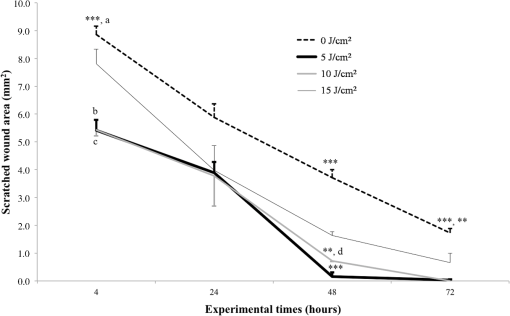 Similar to other cell cultures, in the presence of MMC, the laser-irradiated group reached complete wound closure after 72 h and the laser-irradiated group healed after 96 h, while nonirradiated controls still showed partial healing after 96 h from scratch (Fig. 3). The laser-irradiated group, however, reached a complete wound closure after 96 h (with MMC) rather than after 72 h (without MMC). Statistically significant differences between the groups were only slightly less pronounced and more time-delayed in the presence of the cell proliferation inhibitor. The 10 and laser-irradiated groups had decreased wound area compared with nonirradiated controls after 24 h ( and , respectively) and 72 h () (Fig. 3). Data analysis over the experimental times showed a statistically significant decrease in wound area only for the laser-treated group between 4 and 48 h (, Fig. 3). Fig. 3Scratched wound area measured at different times (4, 24, 48, and 72 h) of Saos-2 cells treated with different laser doses (5, 10, and ) or untreated () in the presence of Mitomycin C (MMC). Data are means; bars are standard deviations. One-way MANOVA and Tukey’s HSD post hoc tests: 24 h: **, versus 10 and , ; 72 h: **, versus 10 and , . ANOVA with repeated measures and post hoc tests using the Bonferroni correction: : a, 48 h versus 4 h, . 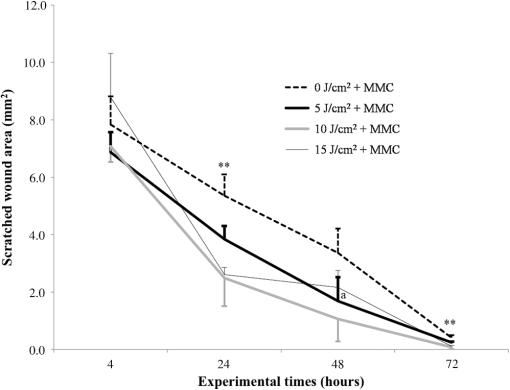 3.2.Viability Test and DNA QuantificationThere was no statistically significant difference in cell viability between laser-irradiated and nonirradiated groups for each experimental time (Fig. 4). Cell viability showed a statistically significant increase between 24 and 72 h for all laser-irradiated groups ( for 5 and , for ), between 24 and 48 h for the 5 and laser-irradiated groups (), and between 48 and 72 h for the laser-irradiated group (). Fig. 4Viability results of Saos-2 cells treated with different laser doses (5, 10, and ) or untreated () after 24, 48, and 72 h from irradiation. Data are means; bars are standard deviations. ANOVA with repeated measures and post hoc tests using the Bonferroni correction: 5 and : a, 24 h versus 48 and 72 h, ; : b, 48 h versus 72 h, ; : c, 24 h versus 72 h, . 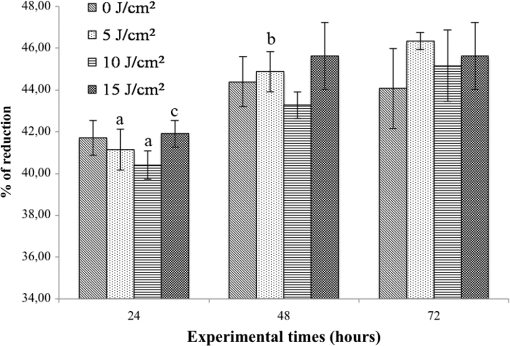 In agreement with the viability results, no statistically significant difference was found for DNA content between the different treatment protocols or experimental times. 3.3.Quantification of mRNA Expression Levels by Quantitative Polymerase Chain ReactionAfter 24 h, COL1A1 gene expression was increased in all laser-irradiated groups compared with nonirradiated controls (, Fig. 5). Moreover, the laser-irradiated group showed an increased COL1A1 gene expression compared with the 5 and laser-irradiated groups (, Fig. 5). The level of COL1A1 gene expression showed a significant increment at 48 h in the 10 and laser-irradiated groups compared with the nonirradiated group and reached the highest values in the laser-irradiated group (, Fig. 5). After 72 h, the laser-irradiated group showed an increased COL1A1 gene expression compared with the nonirradiated group (, Fig. 5). Over the experimental times, nonirradiated controls showed an increased COL1A1 gene expression at 72 h compared with 24 h (, Fig. 5). The laser-irradiated group showed an increased COL1A1 gene expression at 48 h compared with 24 h (, Fig. 5); the same trend was observed in the laser-irradiated group at 72 h compared with 48 h (, Fig. 5). Fig. 5Relative gene expression of COL1A1 of Saos-2 cells treated with different laser doses (5, 10, and ) or untreated () after 24, 48, and 72 h from irradiation. Data are means; bars are standard deviations. One-way MANOVA and Tukey’s HSD post hoc tests: 24 h: ***, versus 5, 10, and , ; **, versus 5 and , . 48 h: ***, 10 and versus , ; †, versus , . 72 h: **, versus , . ANOVA with repeated measures and post hoc tests using the Bonferroni correction: : a, 24 h versus 72 h, ; : b, 24 h versus 48 h, ; : b, 48 h versus 72 h, . 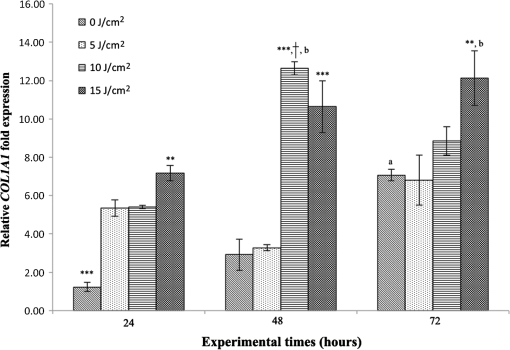 The gene expression of TGFbeta1 showed no statistically significant differences after 24 h between the experimental conditions; after 48 h, nonirradiated controls showed an increased expression compared with the () and the laser-irradiated groups () (Fig. 6). After 72 h, the laser-irradiated group exhibited a decreased TGFbeta1 gene expression compared with the nonirradiated group () (Fig. 6). Over the experimental times, nonirradiated controls showed an increased TGFbeta1 gene expression at 24 h compared with 48 h (, Fig. 6); at 72 h, the TGFbeta1 gene expression in the laser-irradiated group decreased compared with 24 h () (Fig. 6). Fig. 6Relative gene expression of TGFbeta1 of Saos-2 cells treated with different laser doses (5, 10, and ) or untreated () after 24, 48, and 72 h from irradiation. Data are means; bars are standard deviations. One-way MANOVA and Tukey’s HSD post hoc tests: 48 h: **, versus , ; ***, versus , . 72 h: ***, versus , . ANOVA with repeated measures and post hoc tests using the Bonferroni correction: : a, 24 h versus 48 h, ; : b, 24 h versus 72 h, . 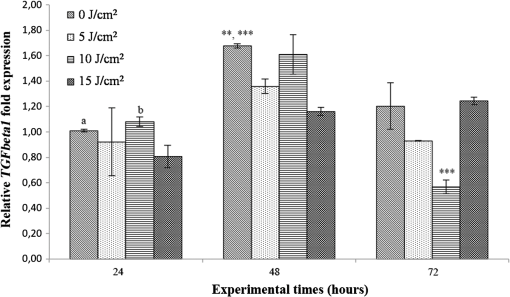 Gene expression for IL1beta and MMP1 was undetectable at each experimental time and condition. 4.Discussion and ConclusionsTo our knowledge, this is the first study that examines the effects of irradiation with a 915-nm GaAlAs diode laser on osteoblast migration and proliferation using an in vitro scratch-wound healing assay. This method has already been described as a convenient and inexpensive way to measure the cell healing capacity in vitro.38 Moreover, culture treatment with MMC allowed us to investigate the relative contribution of cell migration and proliferation to the process of scratch-wound closure. A Saos-2 human osteosarcoma-derived cell line was employed due to its resemblance to human mature osteoblast phenotype and its key role in bone healing, repair and remodeling.39,40 Unlike other human osteosarcoma-derived cell lines, such as U2OS cells used by Spitler and Berns,27 the cell line chosen for this study is able to differentiate and form calcified matrix and more closely resembles the osteoblast profile as far as the expression of bone remodeling proteins is concerned.34 A diode laser was employed in this study because it is one of the most popular in clinical practice and, at a wavelength of 915 nm, it is known to have a high penetration depth that is desirable for clinical applications on bone.2,41 Cells irradiated with a single laser application administered at doses of 5, 10, and showed an increased healing ability compared with nonirradiated controls. Nonirradiated controls still showed partial healing after 96 h, while the 5 and laser-irradiated groups were the first to reach complete wound closure after 72 h. The laser-irradiated group reached complete closure after 96 h and showed a tendency toward increased wound area compared with that of other laser-treated groups at each experimental time, suggesting a decreased healing ability for this dose of irradiation. When MMC was added to the culture medium, thus abolishing the contribution of cell proliferation, only the laser-irradiated group reached a complete wound closure in a time-delayed manner (after 96 h rather than after 72 h). Accordingly, with MMC, the laser-irradiated group failed to show a statistically significant decreased wound area, while the 10 and laser-irradiated groups maintained a decreased wound area compared with nonirradiated controls. These results suggest that laser irradiation with a wavelength of 915 nm promotes the closure of the scratched wound area mainly through stimulation of Saos-2 cell migration, in accordance with a previous report on human osteosarcoma cells using laser wavelengths of 652 and 806 nm.27 Previous studies have demonstrated the ability of PBM to stimulate proliferation and migration of many other cell phenotypes, but direct comparison with the current data is inappropriate due to different in vitro conditions.30,32,33,42 A significant increase in cell viability over the experimental times was detected for laser-treated groups and not for nonirradiated cells, but no statistically significant differences were found between laser-irradiated and nonirradiated groups. A previous report on healthy Saos-2 cells using the same laser equipment and parameters adopted in the present study concluded that, at 72 h from irradiation, the treated group showed a significantly higher viability compared with the nonirradiated controls.43 Probably, these contradictory results can be ascribed to the different in vitro models adopted. In accordance with the viability assays, laser irradiation had no statistically significant effect on DNA content. On the other hand, laser irradiation showed an influence on cellular anabolic properties through modulation of COL1A1 and TGFbeta1 gene expression. COL1A1 is a major protein in the bone extracellular matrix and it is intimately related to the achievement of bone tissue healing. Nonirradiated cells exhibited a constantly increasing COL1A1 gene expression over time; laser irradiation significantly increased its gene expression, reaching statistically significant differences for the 5, 10, and groups at 24 h and for the 10 and groups at 48 h, as well as for the group at 72 h compared with nonirradiated controls. These data suggested that a higher dose induced a longer-lasting effect on COL1A1 gene expression in the range between 5 and . This trend was confirmed by the protein analyses, although no statistically significant differences were detected. Despite the different laser equipment and parameters used, our results are consistent with those of other studies, which found an increased expression for this gene after PBM on mouse fibroblasts,44 human gingival fibroblasts,45 rat bone tissue,46 human keratinocytes,42 and porcine Achilles tendon fibroblasts.47 A slightly more delayed effect of laser irradiation was found on the gene expression of TGFbeta1, a potent cytokine that acts as a leading factor in the process of bone healing. Compared with nonirradiated cells, the 5 and laser-irradiated groups showed a decreased TGFbeta1 gene expression at 48 h, whereas only the laser-irradiated group retained comparable gene expression and then significantly decreased. Previous studies showed an enhanced TGFbeta1 production after low-level laser irradiation on osteoblast-like cells, but without performing the in vitro scratch-wound healing assay.48 Other reports supported the idea that its secretion after laser and LED irradiation decreases both in vivo on rats and in vitro on human umbilical vein endothelial cells.49,50 Moreover, TGFbeta1 levels have been shown to follow a phasic expression pattern over time postwounding in a clinical oral tooth extraction healing study.23 However, these biological effects seemed to be dependent on the individual cell phenotype as well as on the irradiation parameters, mainly wavelength, power density and irradiation time.2,49,50 Therefore, direct comparison with the current study is inappropriate because of different laser equipment, treatment protocol, in vitro model, and conditions used. In our experiment, at early experimental times, only the laser-irradiated group maintained a TGFbeta1 gene expression similar to untreated controls, while the 5 and laser-irradiated groups showed decreased gene expression, suggesting a biphasic dose response of osteoblasts-like cells treated with laser.33 The present study investigated the effects of a single session of laser irradiation on wound healing; the output power, the pulsing of the radiation, and the treated area were kept constant while varying the dose as the main study variable because it has been recognized as the most important laser parameter responsible for the biologic response.3 Further investigations with multiple sessions of laser irradiation should strengthen cell responses owing to a cumulative laser effect.51 Our results indicate that the in vitro scratch-wound healing assay induced a mechanical injury without an altered inflammatory status because of the lack of gene expression of catabolic and inflammatory proteins, such as IL1beta and MMP1 and because of the lack of production. For further investigations, it might be useful to employ primary human pathological cells and to add inflammatory cytokines to the culture medium in order to provide an in vitro environment that is closer to that of actual wound healing. Rigorous in vitro studies on the cellular and photobiological mechanisms of laser irradiation might be helpful to bridge the gap between in vitro research and biomedical applications. AcknowledgmentsThe study was supported by the Rizzoli Orthopaedic Institute (Ricerca Corrente) and partly by grants from Programma di Ricerca Regione Emilia Romagna-Università 2010–2012 to MT (Biological and Biophysical Stimulation on Implant Osteolysis and Aseptic Loosening Conditions: Effects of Pulsed Electromagnetic Fields and Platelet Derivatives Project) and by FIRB Project RPAB10MLK7 from Ministero dell’Istruzione, dell’Università e della Ricerca. The authors thank Orotig s.r.l., Verona, Italy, for providing the laser equipment. ReferencesJ. J. Anders, R. J. Lanzafame and P. R. Arany,
“Low-level light/laser therapy versus photobiomodulation therapy,”
Photomed. Laser Surg., 33
(4), 183
–184
(2015). http://dx.doi.org/10.1089/pho.2015.9848 Google Scholar
K. M. Alghamdi, A. Kumar and N. A. Moussa,
“Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells,”
Lasers Med. Sci., 27
(1), 237
–249
(2012). http://dx.doi.org/10.1007/s10103-011-0885-2 Google Scholar
J. Tuner and L. Hode, The Laser Therapy Handbook, 4th ed.Prima Books AB, Grangesberg, Sweden
(2004). Google Scholar
J. D. Carroll et al.,
“Developments in low level light therapy (LLLT) for dentistry,”
Dent. Mater., 30
(5), 465
–475
(2014). http://dx.doi.org/10.1016/j.dental.2014.02.006 Google Scholar
I. Marini, M. R. Gatto and G. A. Bonetti,
“Effects of superpulsed low-level laser therapy on temporomandibular joint pain,”
Clin. J. Pain, 26
(7), 611
–616
(2010). http://dx.doi.org/10.1097/AJP.0b013e3181e0190d 0749-8047 Google Scholar
S. Nesioonpour et al.,
“The effect of low-level laser on postoperative pain after tibial fracture surgery: a double-blind controlled randomized clinical trial,”
Anesth. Pain Med., 4
(3), e17350
(2014). http://dx.doi.org/10.5812/aapm.17350 Google Scholar
M. Briteño-Vázquez et al.,
“Low power laser stimulation of the bone consolidation in tibial fractures of rats: a radiologic and histopathological analysis,”
Lasers Med. Sci., 30
(1), 333
–338
(2015). http://dx.doi.org/10.1007/s10103-014-1673-6 Google Scholar
V. R. Sella et al.,
“Effect of low-level laser therapy on bone repair: a randomized controlled experimental study,”
Lasers Med. Sci., 30
(3), 1061
–1068
(2015). http://dx.doi.org/10.1007/s10103-015-1710-0 Google Scholar
M. H. Aras et al.,
“Effects of low-level laser therapy on changes in inflammation and in the activity of osteoblasts in the expanded premaxillary suture in an ovariectomized rat model,”
Photomed. Laser Surg., 33
(3), 136
–144
(2015). http://dx.doi.org/10.1089/pho.2014.3820 Google Scholar
M. Fini et al.,
“Current trends in the enhancement of biomaterial osteointegration: biophysical stimulation,”
Int. J. Artif. Organs, 27
(8), 681
–690
(2004). IJAODS 0391-3988 Google Scholar
S. Panseri et al.,
“Innovative magnetic scaffolds for orthopedic tissue engineering,”
J. Biomed. Mater. Res. A, 100
(9), 2278
–2286
(2012). Google Scholar
S. Incerti Parenti et al.,
“Effect of low-level laser irradiation on osteoblast-like cells cultured on porous hydroxyapatite scaffolds,”
Ann. Ist. Super. Sanita., 49
(3), 255
–60
(2013). Google Scholar
W. D. Chang et al.,
“Therapeutic outcomes of low-level laser therapy for closed bone fracture in the human wrist and hand,”
Photomed. Laser Surg., 32
(4), 212
–218
(2014). http://dx.doi.org/10.1089/pho.2012.3398 Google Scholar
B. Kan et al.,
“Histomorphometrical and radiological comparison of low-level laser therapy effects on distraction osteogenesis: experimental study,”
Lasers Med. Sci., 29
(1), 213
–220
(2014). http://dx.doi.org/10.1007/s10103-013-1308-3 Google Scholar
C. R. Tim et al.,
“Low-level laser therapy enhances the expression of osteogenic factors during bone repair in rats,”
Lasers Med. Sci., 29
(1), 147
–156
(2014). http://dx.doi.org/10.1007/s10103-013-1302-9 Google Scholar
S. Tumilty et al.,
“Clinical effectiveness of low-level laser therapy as an adjunct to eccentric exercise for the treatment of Achilles’ tendinopathy: a randomized controlled trial,”
Arch. Phys. Med. Rehabil., 93
(5), 733
–739
(2012). http://dx.doi.org/10.1016/j.apmr.2011.08.049 APMHAI 0003-9993 Google Scholar
A. Stergioulas et al.,
“Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic Achilles tendinopathy,”
Am. J. Sports Med., 36
(5), 881
–887
(2008). http://dx.doi.org/10.1177/0363546507312165 Google Scholar
K. Gworys et al.,
“Influence of various laser therapy methods on knee joint pain and function in patients with knee osteoarthritis,”
Ortop. Traumatol. Rehabil., 14
(3), 269
–277
(2012). http://dx.doi.org/10.5604/15093492.1002257 Google Scholar
M. Mozzati et al.,
“Influence of superpulsed laser therapy on healing processes following tooth extraction,”
Photomed. Laser Surg., 29
(8), 565
–571
(2011). http://dx.doi.org/10.1089/pho.2010.2921 Google Scholar
P. Angeletti et al.,
“Effect of low-level laser therapy (GaAlAs) on bone regeneration in midpalatal anterior suture after surgically assisted rapid maxillary expansion,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 109
(3), e38
–e46
(2010). http://dx.doi.org/10.1016/j.tripleo.2009.10.043 OSOMAE 0030-4220 Google Scholar
A. K. Devaiah et al.,
“Surgical utility of a new carbon dioxide laser fiber: functional and histological study,”
Laryngoscope, 115
(8), 1463
–1468
(2005). http://dx.doi.org/10.1097/01.mlg.0000171021.73635.3b Google Scholar
X. Wang, C. Zhang and K. Matsumoto,
“In vivo study of the healing processes that occur in the jaws of rabbits following perforation by an Er, Cr:YSGG laser,”
Lasers Med. Sci., 20
(1), 21
–27
(2005). http://dx.doi.org/10.1007/s10103-005-0329-y Google Scholar
P. R. Arany et al.,
“Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing,”
Wound Repair Regen., 15
(6), 866
–874
(2007). http://dx.doi.org/10.1111/j.1524-475X.2007.00306.x Google Scholar
T. Zeinoun et al.,
“Eosinophils and mastocytes in healing laser excision wounds,”
Lasers Med. Sci., 24
(3), 307
–312
(2009). http://dx.doi.org/10.1007/s10103-008-0554-2 Google Scholar
T. C. Silva et al.,
“In vivo effects on the expression of vascular endothelial growth factor-A165 messenger ribonucleic acid of an infrared diode laser associated or not with a visible red diode laser,”
Photomed. Laser Surg., 28
(1), 63
–68
(2010). http://dx.doi.org/10.1089/pho.2008.2403 Google Scholar
P. R. Arany et al.,
“Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration,”
Sci. Transl. Med., 6
(238), 238ra69
(2014). http://dx.doi.org/10.1126/scitranslmed.3008234 STMCBQ 1946-6234 Google Scholar
R. Spitler and M. W. Berns,
“Comparison of laser and diode sources for acceleration of in vitro wound healing by low-level light therapy,”
J. Biomed. Opt., 19
(3), 038001
(2014). http://dx.doi.org/10.1117/1.JBO.19.3.038001 JBOPFO 1083-3668 Google Scholar
J. Y. Lee et al.,
“Effect of low-level laser therapy on oral keratinocytes exposed to bisphosphonate,”
Lasers Med. Sci., 30
(2), 635
–643
(2015). http://dx.doi.org/10.1007/s10103-013-1382-6 Google Scholar
W. Lim et al.,
“Anti-inflammatory effect of 635 nm irradiations on in vitro direct/indirect irradiation model,”
J. Oral Pathol. Med., 44
(2), 94
–102
(2015). http://dx.doi.org/10.1111/jop.12204 JPMEEA 0904-2512 Google Scholar
K. Ejiri et al.,
“High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells,”
Lasers Med. Sci., 29
(4), 1339
–1347
(2014). http://dx.doi.org/10.1007/s10103-013-1292-7 Google Scholar
S. M. Ayuk, N. N. Houreld and H. Abrahamse,
“Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm,”
Diabetes Technol. Ther., 14
(12), 1110
–1117
(2012). http://dx.doi.org/10.1089/dia.2012.0125 Google Scholar
X. Liao et al.,
“Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization,”
Photomed. Laser Surg., 32
(4), 219
–225
(2014). http://dx.doi.org/10.1089/pho.2013.3667 Google Scholar
W. C. Tsai et al.,
“Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression,”
PLoS One, 7
(5), e38235
(2012). http://dx.doi.org/10.1371/journal.pone.0038235 POLNCL 1932-6203 Google Scholar
A. Burmester et al.,
“Comparison of the reaction of bone-derived cells to enhanced -salt concentrations,”
Biomatter, 4
(1), e967616
(2014). http://dx.doi.org/10.4161/21592527.2014.967616 Google Scholar
K. M. Niforou et al.,
“The proteome profile of the human osteosarcoma U2OS cell line,”
Cancer Genomics Proteomics, 5
(1), 63
–78
(2008). Google Scholar
J. Ye et al.,
“Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction,”
BMC Bioinf., 13 134
(2012). http://dx.doi.org/10.1186/1471-2105-13-134 Google Scholar
C. Cavallo et al.,
“Chondrocytes from patients with osteoarthritis express typical extracellular matrix molecules once grown onto a three-dimensional hyaluronan-based scaffold,”
J. Biomed. Mater. Res. A, 93
(1), 86
–95
(2010). http://dx.doi.org/10.1002/jbm.a.32547 Google Scholar
C. C. Liang, A. Y. Park and J. L. Guan,
“In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro,”
Nat. Protoc., 2
(2), 329
–333
(2007). http://dx.doi.org/10.1038/nprot.2007.30 1754-2189 Google Scholar
E. M. Czekanska et al.,
“A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing,”
J. Biomed. Mater. Res., 102
(8), 2636
–2643
(2014). http://dx.doi.org/10.1002/jbm.a.34937 Google Scholar
M. Griffin et al.,
“Degenerate wave and capacitive coupling increase human MSC invasion and proliferation while reducing cytotoxicity in an in vitro wound healing model,”
PLoS One, 6
(8), e23404
(2011). http://dx.doi.org/10.1371/journal.pone.0023404 POLNCL 1932-6203 Google Scholar
R. M. Huertas et al.,
“Effect and clinical implications of the low-energy diode laser on bone cell proliferation,”
Biol. Res. Nurs., 16
(2), 191
–196
(2014). http://dx.doi.org/10.1177/1099800413482695 Google Scholar
F. G. Basso et al.,
“Biostimulatory effect of low-level laser therapy on keratinocytes in vitro,”
Lasers Med. Sci., 28
(2), 367
–374
(2013). http://dx.doi.org/10.1007/s10103-012-1057-8 Google Scholar
S. Incerti Parenti et al.,
“Different doses of low-level laser irradiation modulate the in vitro response of osteoblast-like cells,”
J. Biomed. Opt., 19
(10), 108002
(2014). http://dx.doi.org/10.1117/1.JBO.19.10.108002 JBOPFO 1083-3668 Google Scholar
C. C. Martignago et al.,
“Effect of low-level laser therapy on the gene expression of collagen and vascular endothelial growth factor in a culture of fibroblast cells in mice,”
Lasers Med. Sci., 30
(1), 203
–208
(2015). http://dx.doi.org/10.1007/s10103-014-1644-y Google Scholar
A. Frozanfar et al.,
“The effects of low level laser therapy on the expression of collagen type I gene and proliferation of human gingival fibroblasts (Hgf3-Pi 53): in vitro study,”
Iran. J. Basic Med. Sci., 16
(10), 1071
–1074
(2013). Google Scholar
J. B. Park et al.,
“Effects of increased low-level diode laser irradiation time on extraction socket healing in rats,”
Lasers Med. Sci., 30
(2), 719
–726
(2015). http://dx.doi.org/10.1007/s10103-013-1402-6 Google Scholar
C. H. Chen et al.,
“Low-level laser irradiation promotes cell proliferation and mRNA expression of type I collagen and decorin in porcine Achilles tendon fibroblasts in vitro,”
J. Orthop. Res., 27
(5), 646
–650
(2009). http://dx.doi.org/10.1002/jor.20800 JOREDR 0736-0266 Google Scholar
M. Khadra et al.,
“Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material,”
Biomaterials, 26
(17), 3503
–3509
(2005). http://dx.doi.org/10.1016/j.biomaterials.2004.09.033 Google Scholar
A. P. de Sousa et al.,
“Effect of LED phototherapy () on expression during wound healing: an immunohistochemical study in a rodent model,”
Photomed. Laser Surg., 29
(9), 605
–611
(2011). http://dx.doi.org/10.1089/pho.2010.2833 Google Scholar
J. Szymanska et al.,
“Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion,”
J. Physiol. Pharmacol., 64
(3), 387
–391
(2013). Google Scholar
A. R. Coombe et al.,
“The effects of low level laser irradiation on osteoblastic cells,”
Clin. Orthod. Res., 4
(1), 3
–14
(2001). http://dx.doi.org/10.1034/j.1600-0544.2001.040102.x Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||

