|
|
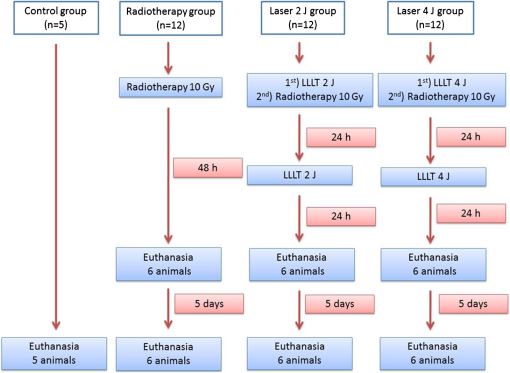
1.IntroductionHead and neck radiotherapy often involves the major salivary glands, which undergo morphological and functional changes, resulting in hyposalivation and xerostomia. Approximately 70% of patients irradiated in the head and neck have a progressive loss of salivary gland function.1 Quantitative and qualitative salivary changes cause a total or partial loss of taste, pain and burning mouth, dysphagia, dysphonia, increased susceptibility to oral infections and dental caries, among other complications.2 There are no studies that clearly show how radiotherapy affects the function of the salivary glands. Although acinar cells are functionally mature and do not have a high mitotic rate, they respond quickly to radiation.3–6 The parotid gland accounts for of the saliva and is considered the most radiosensitive of the major salivary glands.4,7–9 The acute and late changes observed in irradiated glands include loss and atrophy of acinar cells, decrease in glandular weight, and formation of fibrous tissue.4,5,10,11 Several studies have demonstrated the role of apoptosis in radiotherapy-induced glandular dysfunction.4,5 Dysfunction of the salivary glands is directly related to the decrease in acinar cells after radiation.4,5,12 To assess cell death after radiation therapy, studies have investigated the expression of caspase-3, a protein that plays an important role in cell apoptosis.3–5 Activation of caspase-3 protein is an acute event of apoptosis, which occurs because the ionizing radiation alters mitochondrial membrane permeability, causing the release of cytochrome C into the cytoplasm.13 As a result of radiation therapy, there is also loss of cell homeostasis, production of reactive oxygen species (ROS), and disruption of adenosine triphosphate synthesis. High levels of ROS further increase mitochondrial membrane permeability and activation of caspase-9 and -3.14 In an attempt to avoid the adverse effects of radiotherapy in the salivary glands, different methods of prevention and treatment of xerostomia have been studied. These include especially cytoprotective agents, growth factors, muscarinic cholinergic agonists, stem/progenitor cell-based therapy, and low-level laser radiation.4,5,15–20 Low-level laser therapy (LLLT) has been used due to its potential to induce several metabolic and biochemical processes, promoting tissue biomodulation, analgesia, and modulation of the inflammatory process.21,22 It can regulate the synthesis of nucleic acids and proteins and modulate the levels of cytokines, growth factors, and inflammatory mediators, as well as stimulate cell proliferation and differentiation.22 Simões et al.23 used LLLT on the major salivary glands of mice and observed an increase in salivary flow at the end of treatment. Simões et al.19 evaluated the response of the salivary glands to LLLT in patients undergoing radiotherapy. When applied concomitantly with head and neck radiotherapy, LLLT prevented the reduction in salivary flow. Loncar et al.24 applied LLLT to the parotid, submandibular, and sublingual glands of patients with xerostomia, for 10 consecutive days. The authors observed that the amount of saliva produced in the laser group increased gradually during the study. Onizawa et al.,25 in an in vitro study with mouse parotid acinar cells, observed that LLLT-induced cell proliferation and increased expression of the antiapoptotic proteins Bcl-2 and HSP25. Considering the aforementioned evidence, the aim of this study was to evaluate the effect of LLLT on radiotherapy-induced morphological changes and immunodetection of caspase-3 protein in parotids of mice. 2.Materials and MethodsThis study was approved by the Ethics Committee on Animal Use of the Pontifical Catholic University of Rio Grande do Sul (PUCRS), Brazil. The sample consisted of 41 male Swiss mice, weighing 25 to 30 g at the beginning of the experiment. The animals were kept in the Center for Experimental Biological Models of PUCRS in temperature-controlled () chambers equipped with input and output air filters and with a 12-h light-dark cycle. They were housed in cages appropriate for rodents with free access to water and food. The animals were randomly divided into four groups: control group (), radiotherapy group (), 2-J (J) laser group (), and 4-J laser group (). The radiotherapy group and 2- and 4-J laser groups were divided into two experimental times, i.e., six animals were euthanized 48 h after radiotherapy and the other animals, 7 days after receiving the ionizing radiation. The study flow diagram is shown in Fig. 1. 2.1.RadiotherapyRadiotherapy was performed in a single session in the Radiotherapy Department of the São Lucas Hospital. During radiotherapy, the animals were immobilized by means of a restraint for mice up to 50 g (Insight EB 286P, Brazil). The animals were placed in the prone position and irradiated with using a teletherapy unit (Philips, XK5101, The Netherlands). The radiation dose used was 10 grays (Gy), based on the study of Limesand et al.5 The yield of the radiation source was , the distance between the emission point of the radioactive beam and the animals was 54.5 cm and the area of the radiation field was . The animals of the radiotherapy group and 2- and 4-J laser groups were subjected to ionizing radiation. 2.2.Low-Level Laser TherapyA GaAlAs diode laser was used (Thera Lase, DMC Equipment Ltda, Brazil); the area of the spot tip of this tool was . Laser irradiation was performed in continuous wave mode. The following parameters were used: 830-nm (infrared) wavelength, 100-mW output power, and power density.
LLLT was performed on the laser groups immediately before and after 24 h the radiotherapy. The spot tip was placed in contact with the mouse skin in the region corresponding to the parotid glands. One point was applied in each parotid gland. The angle of incidence of the light beam on the tissue was as perpendicular as possible, minimizing refraction. The laser was calibrated before each LLLT session; the laser device had a calibration system coupled to the instrument. Furthermore, after calibration, we used the power meter to check the output power. 2.3.Euthanasia and Preparation of TissuesThe animals were euthanized using a chamber. In the radiotherapy and 2- and 4-J laser groups, six mice were euthanized 48 h after radiotherapy and the other animals after 7 days. At this time, the animals in the control group were also euthanized. The right and left parotid glands of each animal were dissected and immersed for 24 h in 10% buffered formalin. They were then dehydrated in increasing concentrations of alcohol, cleared, and embedded in paraffin. The left and right glands of each animal were included in the same paraffin block, from which two -thick sections were obtained. One section was stained with hematoxylin–eosin (HE) and the other processed to immunohistochemical with anticaspase-3 antibody. For immunohistochemistry, the section was mounted on a silanized slide (Flex DAKO, Santa Cruz, California). These slides were subjected to immunohistochemistry with anticleaved caspase-3 antibody () (Cell Signaling # 9661). Accordingly, histological sections were deparaffinized in xylene and rehydrated in a decreasing ethanol series. Antigen retrieval was performed using citric acid in a water bath at 95°C for 30 min. Endogenous peroxidase block was performed with hydrogen peroxide diluted 20 times with methanol. Incubation with primary antibody was for 60 min at room temperature. The detection system used was EasyLink One (EasyPath, Brazil) and the development of the reaction was with the DAB chromogen kit (EasyPath, Brazil). Slides were counter stained with Mayer’s hematoxylin and dehydrated in an increasing ethanol series. The sections were cleared with xylol, and glass coverslips were mounted with Permount (Fisher Scientific, Waltham, Massachusetts). A normal lymph node served as the positive control for the reactions. Negative controls were obtained by omitting the primary antibody. Histological evaluation of the glandular tissue was performed by a single blinded and calibrated examiner. Initially, we carried out a descriptive analysis of the HE-stained slides, observing the acinar structure (acinar disorganization), vascularization, presence of cytoplasmic vacuolation, inflammatory infiltrate, fibrosis, and edema. For each parameter, the following histological scores were established: absent (−), slight (+), moderate (++), or severe (+++). Those parameters not observed on the slides were considered absent. The score was considered slight when the histological parameter was observed in isolated areas on the slide. A severe score was given when the parameter was observed distributed in the entire slide. When an intermediate pattern was found, between slight and severe changes, the score was considered moderate. A slight score (+) was given for vascularization within the normal range as observed in nonirradiated glands, while a moderate score was considered when the amount of blood vessels was greater than normal. For immunodetection of caspase-3, we selected from each slide five equidistant fields, captured at magnification using an image capture system (Moticam 5—System ShiftCapture, China) connected to a light microscope (Olympus BX50, Japan). Captured images were saved in TIFF format and analyzed using the software ImageJ 1.48v. In each image, an automated analysis was performed to quantify the area staining positive for caspase-3 in acinar cells. Immunostaining percentage was determined for the five fields analyzed. Areas corresponding to ducts and blood vessels were omitted to avoid an error in detection. 2.4.Data AnalysisThe data were analyzed using descriptive statistics. We used the Kruskal–Wallis test to compare the percentage of caspase-3 immunostaining between groups. To compare the different time points within each group, the Mann–Whitney test was used at a significance level of 5%. Statistical analysis was performed with SPSS version 18.0. 3.Results3.1.Morphological AnalysisThe control group showed normal acinar structure and vascularization [Figs. 2(a) and 2(b)], but some vacuolated cells were seen. The glands of the animals in the radiotherapy group showed marked acinar disorganization, characterized by altered morphology and size of the acini, which was most significant 48 h after radiation. Also in this group, the presence of vacuolated cells was more evident than in the control, mainly 7 days after radiotherapy [Figs. 2(e) and 2(f)]. Fig. 2Histologic examination of parotid gland structure. (a) Control group, 200x and (b) 400x showing normal acinar structure. (c) 4-J laser group, 48 h after radiotherapy, showing greater vascularization, 200x. (d) 2-J laser group, 7 days after radiotherapy, displaying acinar disorganization, and vacuolated cells (arrow) 400x. (e) Radiotherapy group showing acinar disorganization and (f) vacuolated cells (arrow) 200x. 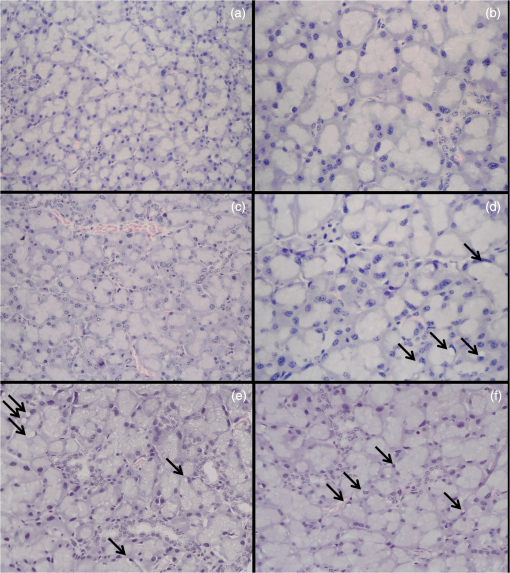 In the 2- and 4-J laser groups, 48 h after radiotherapy, cytoplasmic vacuolation was similar to that observed in the control group. Acinar disorganization areas were present but were less evident than in the radiotherapy group. Greater vascularization was observed in the glands of the 4-J laser group at this experimental time [Fig. 2(c)]. Seven days after radiotherapy, the 2- and 4-J laser groups had higher vascularization compared to the other groups. At this time, the presence of vacuolated cells was more pronounced compared to the control group but less evident than in the radiotherapy group. As for acinar structure, the pattern in the 4-J laser group at 7 days after radiation was the same as that observed at 48 h. In the 2-J laser group, we observed marked acinar disorganization, resembling that in the radiotherapy group (Table 1) [Fig. 2(d)]. There were no areas of fibrosis, inflammatory infiltrate, or edema in the study groups. Table 1Changes in glandular morphology based on descriptive analysis, in the control, 2-J laser, 4-J laser, and radiotherapy groups, at different time points (48 h and 7 days).
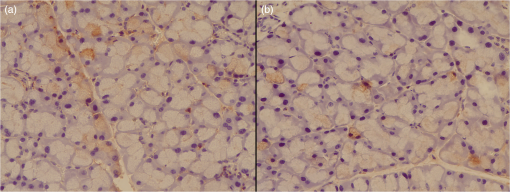
(−) absent; (+) slight; (++) moderate; (+++) severe. 3.2.Immunodetection of Caspase-3There was no significant difference in caspase-3 immunodetection between groups or between time points (Table 2). However, the data showed a lower percentage in the control group and greater percentage in the radiotherapy group. In the laser groups, percentage of caspase-3 immunodetection was intermediate between the values found in the radiotherapy and control groups [Figs. 3(a) and 3(b)]. Table 2Percentage (median and percentiles 25 to 75) of caspase-3 immunodetection in the radiotherapy, 2 J laser, 4 J laser, and control groups, at different time points (48 h and 7 days). 4.DiscussionThe dysfunction of the salivary glands is a frequent complication of head and neck radiotherapy and is directly related to structural damage.26 Changes in acinar structure are described within the first 48 and 72 h after radiotherapy.27 The acute effects of ionizing radiation on the salivary glands appear to be due to high levels of cell death, and many studies have suggested that chronic effects may be due to the damage initially produced.9,27 The present study investigated the effect of LLLT on acute morphological changes and on the detection of caspase-3 protein in parotid glands of irradiated mice. In this study, in both laser groups, 48 h after radiotherapy, cytoplasmic vacuolation as well as acinar structure appeared similar to that found in the control group. These results indicate that LLLT could have preserved glandular morphology during the first hours after exposure to ionizing radiation. Seven days after radiotherapy, however, there was an increase in cytoplasmic vacuolation and changes in acinar structure, which were more pronounced in the 2-J laser group. However, most of these changes were still less evident than that observed in the radiotherapy group. In our study, LLLT was applied in two sessions, i.e., immediately before and 24 h after radiation. If more sessions of LLLT had been used in this 7-day period, it is possible that the morphological changes found a week after radiotherapy could have been less evident. Several authors have described the occurrence of cytoplasmic vacuolation in salivary glands of irradiated animals,6,27–29 evident in the first hours after radiotherapy.6,27,28 The vacuoles in the cytoplasm represent an active process of autophagy,30 induced by nutritional shortage, infection, or oxidative stress.31 Vacuolated cells were more evident in the radiotherapy group than the other groups. However, even the salivary glands in the control group showed some vacuolated cells. The presence of cytoplasmic vacuolation was also recorded by Radfar and Sirois11 in salivary glands of nonirradiated minipigs. The salivary glands of the laser group had higher vascularization compared to control and radiotherapy groups. Laser stimulation of microcirculation has been described by other authors, when applying LLLT to different tissues.32,33 On the other hand, the literature demonstrates that salivary glands of irradiated animals display hypovascularization with changes in blood flow and distribution of vessels immediately after radiotherapy.29,34,35 These alterations were not observed in this study when comparing the radiotherapy and control groups, a result that can be explained by the difference in the methods for analyzing vascularization, since in the literature, other techniques such as immunohistochemistry and doppler have been used.29,34,35 In this study, there were no areas of fibrosis, inflammatory infiltrate, or edema. These results are consistent with most studies reporting the occurrence of fibrosis as a late effect in irradiated salivary glands, where it is found more often after the 30-day postirradiation.6,11,36 The presence of inflammatory infiltrate in salivary glands resulting from radiotherapy is rarely mentioned in the literature, and only observed in studies using minipigs and rhesus monkeys as experimental model.27,37 The occurrence of edema, although not manifesting late, was quoted in the literature only on the 10th day after exposure to ionizing radiation, which would explain why we did not observe this up to the seventh day.6 Since apoptosis of acinar cells has been identified as an acute event caused by radiotherapy,4,5,38,39 which influences the loss of function of the salivary glands, we chose to investigate the immunodetection of caspase-3 protein. Although not significantly different between the groups, the percentage of caspase-3 immunostaining was higher in the radiotherapy group and lower in the control group, while the laser groups showed intermediate values, indicating that LLLT might have influenced acinar cell apoptosis. In the literature, quantification of caspase-3 in the salivary glands of irradiated mice varied from 2% to 27%, depending on the radiation dose and observation time.4,5,30,39 These percentages are higher than those found in our study, probably because of the quantification methods used. In these studies, the percentage was obtained by the number of labeled cells. In our study, caspase-3 was quantified by the stained area in relation to the total area of each histological field, because cytoplasmic staining appeared diffuse in some slides and because cell counts alone could produce bias. Another issue to be considered is that studies differ on the peak occurrence of apoptosis. Avila et al.4 observed in transgenic animals an increased number of apoptotic cells 48 h after radiation. In the study by Limesand et al.,39 the expression of caspase-3 was higher 24 h after radiotherapy, decreasing drastically when assessed at 48 h. The authors explained this result as being due to the removal of these cells by phagocytosis,30 which could also explain the low percentage of detection of this protein in our study. The lack of a therapeutic protocol for LLLT made methodological definitions in this study difficult. In the literature, laser parameters differ considerably in studies using LLLT to treat xerostomia.19,24 There have been differences in wavelength, power, energy, and frequency of sessions.7,19,24 We opted for an infrared wavelength due to the depth of glandular parenchyma to be irradiated.24 The determination of the energy of 2 J supplied per point was based on the study of Saleh et al.,40 and for comparison, we decided to use also an LLLT protocol with energy of 4 J. Given the methodological limitations of this study, further research should be conducted in irradiated animals, using different LLLT protocols and observing glandular response, not only in the short term but also long term, when the occurrence of late changes in the salivary glands can be analyzed. 5.ConclusionsThe present study investigated the effect of LLLT on radiotherapy-induced morphological changes and immunodetection of caspase-3 protein in parotids of mice. The results suggest that when using the protocols defined in this study, LLLT had a tendency to reduce cell apoptosis by reducing active caspase-3. In addition, LLLT promoted the preservation of acinar structure, with regard to the organization of acini, reduced the occurrence of vacuolation, and even stimulated the vascularization of the parotid glands of irradiated mice. Of the two LLLT protocols studied (2 and 4 J), the one using 4 J of energy showed better results. ReferencesF. R. Burlage et al.,
“Parotid and submandibular/sublingual salivary flow during high dose radiotherapy,”
Radiother. Oncol., 61
(3), 271
–274
(2001). http://dx.doi.org/10.1016/S0167-8140(01)00427-3 Google Scholar
P. Dirix et al.,
“The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer,”
Support Care Cancer, 16
(2), 171
–179
(2008). http://dx.doi.org/10.1007/s00520-007-0300-5 Google Scholar
G. M. Paardekooper et al.,
“Radiation-induced apoptosis in relation to acute impairment of rat salivary gland function,”
Int. J. Radiat. Biol., 73
(6), 641
–648
(1998). http://dx.doi.org/10.1080/095530098141898 Google Scholar
J. L. Avila et al.,
“Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis,”
Int. J. Radiat. Oncol. Biol. Phys., 73
(2), 523
–529
(2009). http://dx.doi.org/10.1016/j.ijrobp.2008.09.036 Google Scholar
K. H. Limesand, K. L. Schwertfeger and S. M. Anderson,
“MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells,”
Mol. Cell. Biol., 26
(23), 8840
–8856
(2006). http://dx.doi.org/10.1128/MCB.01846-05 Google Scholar
R. P. Coppes et al.,
“Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists,”
Br. J. Cancer, 85
(7), 1055
–1063
(2001). http://dx.doi.org/10.1054/bjoc.2001.2038 Google Scholar
A. Simões et al.,
“Laser phototherapy effect on protein metabolism parameters of rat salivary glands,”
Lasers Med. Sci., 24
(2), 202
–208
(2009). http://dx.doi.org/10.1007/s10103-008-0548-0 Google Scholar
C. A. Murdoch-Kinch et al.,
“Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy,”
Int. J. Radiat. Oncol. Biol. Phys., 72
(2), 373
–382
(2008). http://dx.doi.org/10.1016/j.ijrobp.2007.12.033 Google Scholar
Y. Li et al.,
“The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy,”
Int. J. Radiat. Oncol. Biol. Phys., 67
(3), 660
–669
(2007). http://dx.doi.org/10.1016/j.ijrobp.2006.09.021 Google Scholar
R. M. Nagler et al.,
“Long-term salivary effects of single-dose head and neck irradiation in the rat,”
Arch. Oral Biol., 43
(4), 297
–303
(1998). http://dx.doi.org/10.1016/S0003-9969(97)00120-9 Google Scholar
L. Radfar and D. A. Sirois,
“Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 96
(3), 267
–274
(2003). http://dx.doi.org/10.1016/S1079-2104(03)00369-X Google Scholar
A. W. Konings, R. P. Coppes and A. Vissink,
“On the mechanism of salivary gland radiosensitivity,”
Int. J. Radiat. Oncol. Biol. Phys., 62
(4), 1187
–1194
(2005). http://dx.doi.org/10.1016/j.ijrobp.2004.12.051 Google Scholar
S. Desagher and J. C. Martinou,
“Mitochondrial as the central control point of apoptosis,”
Trends Cell Biol., 10
(9), 369
–376
(2000). http://dx.doi.org/10.1016/S0962-8924(00)01803-1 Google Scholar
G. Kroemer and J. C. Reed,
“Mitochondrial control of cell death,”
Nat. Med., 6
(5), 513
–516
(2000). http://dx.doi.org/10.1038/74994 Google Scholar
B. C. Jham et al.,
“A randomized phase III prospective trial of bethanechol to prevent radiotherapy-induced salivary gland damage in patients with head and neck cancer,”
Oral Oncol., 43
(2), 137
–142
(2007). http://dx.doi.org/10.1016/j.oraloncology.2006.01.013 EJCCER 1368-8375 Google Scholar
K. Ono et al.,
“Distinct effects of cevimeline and pilocarpine on salivary mechanisms, cardiovascular response and thirst sensation in rats,”
Arch. Oral Biol., 57
(4), 421
–428
(2012). http://dx.doi.org/10.1016/j.archoralbio.2011.09.013 Google Scholar
D. M. Brizel et al.,
“Phase III randomized trial of amifostine as a radioprotector in head and neck cancer,”
J. Clin. Oncol., 18
(19), 3339
–3345
(2000). Google Scholar
I. M. Lombaert et al.,
“Rescue of salivary gland function after stem cell transplantation in irradiated glands,”
PLoS One, 3
(4), e2063
(2008). http://dx.doi.org/10.1371/journal.pone.0002063 POLNCL 1932-6203 Google Scholar
A. Simões et al.,
“Laser phototherapy as topical prophylaxis against radiation-induced xerostomia,”
Photomed. Laser Surg., 28
(3), 357
–363
(2010). http://dx.doi.org/10.1089/pho.2009.2486 Google Scholar
O. Grundmann, G. C. Mitchell and K. H. Limesand,
“Sensitive of salivary glands to radiation: from animal models to therapies,”
J. Dent. Res., 88
(10), 894
–903
(2009). http://dx.doi.org/10.1177/0022034509343143 Google Scholar
T. Karu,
“Primary and secondary mechanisms of action of visible to near-IR radiation on cells,”
J. Photochem. Photobiol. B., 49
(1), 1
–17
(1999). http://dx.doi.org/10.1016/S1011-1344(98)00219-X Google Scholar
J. B. Dawson et al.,
“A theoretical and experimental study of light absorption and scattering by in vivo skin,”
Phys. Med. Biol., 25
(4), 695
–709
(1980). http://dx.doi.org/10.1088/0031-9155/25/4/008 Google Scholar
A. Simões et al.,
“Effect of defocused infrared diode laser on salivary flow rate and some salivary parameters of rats,”
Clin. Oral Invest., 12
(1), 25
–30
(2008). http://dx.doi.org/10.1007/s00784-007-0135-y Google Scholar
B. Loncar et al.,
“The effect of low-level laser therapy on salivary glands in patients with xerostomia,”
Photomed. Laser Surg., 29
(3), 171
–175
(2011). http://dx.doi.org/10.1089/pho.2010.2792 Google Scholar
K. Onizawa et al.,
“Low-level (gallium-aluminum-arsenide) laser irradiation of Par-C10 cells and acinar cells of rat parotid gland,”
Lasers Med. Sci., 24
(2), 155
–161
(2009). http://dx.doi.org/10.1007/s10103-008-0541-7 Google Scholar
K. Mossman, A. Shatzman and J. Chencharick,
“Long-term effects of radiotherapy on taste and salivary function in man,”
Int. J. Radiat. Oncol. Biol. Phys., 8
(6), 991
–997
(1982). http://dx.doi.org/10.1016/0360-3016(82)90166-3 Google Scholar
L. C. Stephens et al.,
“Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death,”
Am. J. Pathol., 124
(3), 469
–478
(1986). Google Scholar
S. G. Hakim et al.,
“A comparative study on the protection profile of lidocaine, amifostine, and pilocarpin on the parotid gland during radiotherapy,”
Cancer Res., 65
(22), 10486
–10493
(2005). http://dx.doi.org/10.1158/0008-5472.CAN-05-0023 Google Scholar
J. Xu et al.,
“Effect of irradiation on microvascular endothelial cells of parotid glands in the miniature pig,”
Int. J. Radiat. Oncol. Biol. Phys., 78
(3), 897
–903
(2010). http://dx.doi.org/10.1016/j.ijrobp.2010.05.048 Google Scholar
K. H. Limesand et al.,
“Insulin-like growth factor–1 preserves salivary gland function after fractionated radiation,”
Int. J. Radiat. Oncol. Biol. Phys., 78
(2), 579
–586
(2010). http://dx.doi.org/10.1016/j.ijrobp.2010.03.035 Google Scholar
B. Levine and G. Kroemer,
“Autophagy in the pathogenesis of disease,”
Cell, 132
(1), 27
–42
(2008). http://dx.doi.org/10.1016/j.cell.2007.12.018 CELLB5 0092-8674 Google Scholar
A. Domínguez et al.,
“Effect of low level laser therapy on dental pulp during orthodontic movement,”
World J. Methodol., 3
(2), 19
–26
(2013). http://dx.doi.org/10.5662/wjm.v3.i2.19 Google Scholar
Y. Maegawa et al.,
“Effects of near-infrared low-level laser irradiation on microcirculation,”
Lasers Surg. Med., 27
(5), 427
–437
(2000). http://dx.doi.org/10.1002/1096-9101(2000)27:5<427::AID-LSM1004>3.0.CO;2-A Google Scholar
B. H. Ahlner and M. G. Lind,
“The effect of irradiation on blood flow through rabbit submandibular glands,”
Eur. Arch. Otorhinolaryngol., 251
(2), 72
–75
(1994). http://dx.doi.org/10.1007/BF00179895 Google Scholar
N. W. Savage, B. J. Kruger and K. F. Adkins,
“Rat submandibular gland microvasculature following fractionated megavoltage irradiation,”
Aust. Dent. J., 30
(2), 99
–103
(1985). http://dx.doi.org/10.1111/j.1834-7819.1985.tb05351.x Google Scholar
R. Henriksson et al.,
“Increase in mast cells and hyaluronic acid correlates to radiation-induced damage and loss of serous acinar cells in salivary glands: the parotid and submandibular glands differ in radiation sensitivity,”
Br. J. Cancer, 69
(2), 320
–326
(1994). http://dx.doi.org/10.1038/bjc.1994.58 Google Scholar
J. Li et al.,
“Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig,”
Int. J. Radiat. Oncol. Biol. Phys., 62
(5), 1510
–1516
(2005). http://dx.doi.org/10.1016/j.ijrobp.2005.04.029 Google Scholar
K. L Martin et al.,
“Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model,”
PLoS One, 7
(12), e51363
(2012). http://dx.doi.org/10.1371/journal.pone.0051363 POLNCL 1932-6203 Google Scholar
K. H. Limesand, S. Said and S. M. Anderson,
“Suppression of radiation-induced salivary gland dysfunction by IGF-1,”
PLoS One, 4
(3), e4663
(2009). http://dx.doi.org/10.1371/journal.pone.0004663 POLNCL 1932-6203 Google Scholar
J. Saleh et al.,
“Effect of low level laser therapy on radiotherapy-induced hyposalivation and xerostomia: a pilot study,”
Photomed. Laser Surg., 32
(10), 546
–552
(2014). http://dx.doi.org/10.1089/pho.2014.3741 Google Scholar
BiographyMonique Dossena Acauan received her DDS and MSc degrees from the Pontifical Catholic University of Rio Grande do Sul—PUCRS, Brazil. She was an MSc student in the Department of Oral Medicine, School of Dentistry (PUCRS), São Lucas Hospital, Brazil. Her personal research interest is the clinical and basic aspects of laser phototherapy in oral lesions, tissue regeneration, and endodontics. Ana Paula Neutziling Gomes received her DDS from the Federal University of Pelotas-UFPEL, Brazil, MSc, and PhD degrees from the São Paulo University—USP, Brazil. She is a senior lecturer in the Department of Oral Pathology, School of Dentistry—UFPEL, Brazil. Her personal research interest is focused on the diagnosis and treatment of oral diseases. Aroldo Braga-Filho received his DDS from the Catholic University of Pelotas, Brazil. He was a fellow in the Radiation Oncology Division in the MD Anderson Cancer Center. He is the head of the Radiotherapy Division at São Lucas Hospital. Maria Antonia Zancanaro de Figueiredo received her DDS and PhD degrees from the Pontifical Catholic University of Rio Grande do Sul—PUCRS, Brazil. She is a professor in the Department of Oral Medicine, School of Dentistry (PUCRS), São Lucas Hospital, Brazil. Her personal research interest is focused on the diagnosis and treatment of oral diseases. Karen Cherubini received her DDS and PhD degrees from the Pontifical Catholic University of Rio Grande do Sul—PUCRS, Brazil. She is a professor in the Department of Oral Medicine, School of Dentistry (PUCRS), São Lucas Hospital, Brazil. Her personal research interest is focused on the diagnosis and treatment of oral diseases. Fernanda Gonçalves Salum received her DDS from the Federal University of Pelotas, Brazil, and PhD degrees from Pontifical Catholic University of Rio Grande do Sul—PUCRS, Brazil. She is a senior lecturer in the Department of Oral Medicine, School of Dentistry—PUCRS, São Lucas Hospital, Brazil. Her personal research interest is focused on the clinical and basic aspects of laser phototherapy in oral lesions and tissue regeneration, besides diagnosis, and treatment of oral diseases. |