|
|
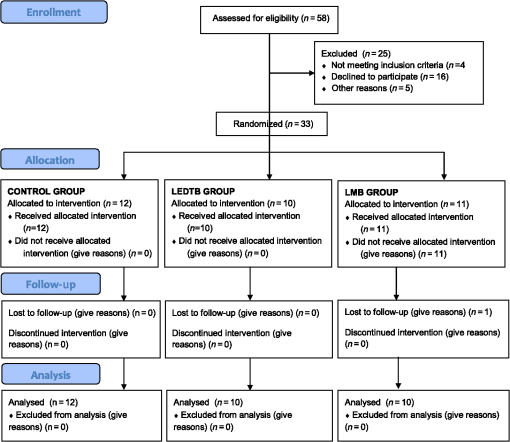
1.IntroductionThe widespread use of fluoride has led to the decline of dental caries worldwide.1 However, it is still a very common disease in preschool children, with high risk of developing caries in both primary and permanent teeth.2 This high-risk caries group therefore requires special dental care and innovative treatment techniques that prioritize minimal intervention. In the dynamic process of dental caries, after enamel demineralization, the lesion may progress slowly toward the dentin, initially characterized by an area of widely demineralized dentin located under a partially demineralized zone and infected with bacteria.3 The inner layer, known as the affected dentin, is usually contaminated with fewer bacteria, and although these microorganisms are capable of dissolving this mineralized tissue, the ultrastructure of the crossed bands from remaining collagen matrix makes it capable of being remineralized. The microbial composition of deep caries in dentin is diverse and the most prevalent species are Fusobacterium nucleatum, Atopobium genomospecies, Lactobacillus casei, Lactobacillus fermentum, Bifidobacterium species, and the Streptococcus species, mainly Streptococcus mutans.4 Traditionally, total removal of carious tissue can cause damage to the pulp, pain, and even weakening of the tooth structure. Nowadays, more conservative approaches, known as minimal intervention techniques for the management of caries, have proven to reduce adverse consequences of restorative treatment such as postoperative pain and pulp exposure5. Minimal intervention can be described as “removal of the superficial necrotic and demineralized dentin with complete excavation of the peripheral demineralized dentin, avoiding excavation close to the pulp”6. Therefore, this indirect pulp treatment is recommended for primary teeth with deep caries or reversible pulp inflammation. A proper diagnosis and treatment selection are based on history, clinical, and radiograph examinations, in addition to a good marginal seal of the restoration, in order to create an environment that would be inhospitable to the remaining bacteria.7 The use of hand or rotary instruments does not guarantee the cleanliness of the infected dentin and elimination of residual bacteria usually present in the cavity before restorative procedures.7 Thus, photodynamic antimicrobial chemotherapy (PACT) may be a promising alternative for elimination of the microorganisms still present in the remaining affected dentin and increasing the chances of successful treatment. PACT associates a photosensitive agent, activated by irradiation, with a light source at a specific wavelength to generate cytotoxic species, including singlet oxygen and free radicals.8 These products are capable of damaging the essential components of cells, or modifying the metabolic activity irreversibly, and may result in bacterial death.9 This mechanism of action avoids the development of bacterial resistance, since this is a limitation of conventional antimicrobial therapies.10 Studies have demonstrated that cariogenic bacteria are susceptible to death as a result of the photosensitization of microbial components, with the use of photosensitive agents such as ortho-toluidine blue11–13 and methylene blue14 in association with light sources. Furthermore, S. mutans, the most cariogenic microorganism, may be killed under conditions similar to those found in a caries lesion.11 This has also been observed in different studies using dentin collagen matrix, in vitro bovine carious dentin, in vitro human carious dentin, ex vivo carious dentin, and in vivo carious dentin.13–20 If the microorganisms associated with dental caries could be reduced, in vivo, by PACT, the results could be very positive for conservative dentistry, since it does not imply removal of the affected dentin, thereby reducing the risk of pulp exposure, and can thus be considered less traumatic treatment for children.10 Therefore, the aim of this study was to test, in vivo, two PACT protocols in the treatment of deep caries lesions in primary teeth by means of microbiological and clinical evaluations. 2.Material and Methods2.1.Ethical ConsiderationsThis parallel-designed clinical trial was conducted in accordance with the Helsinki declaration and was approved by the ethics committee of the School of Dentistry, University of São Paulo (#150/10). Each child’s parent signed a written term of free and informed consent allowing the children to participate in the study, and the children themselves also granted permission for the treatment. This study was conducted in accordance with the standards of reporting trials—Consort Statement21 and was registered with ClinicalTrials.gov (NCT# 02479958). 2.2.Sample CollectionParticipants, recruited from the School of Dentistry of the University of São Paulo between May and October 2011, were children in the age range from 5 to 7 years. The sample size was estimated based on the means and standard deviations of a previous study using BioEstat 5.3 software (Mamiraua, Tefé, AM, Brazil).14 Calculation of statistical significance was estimated using the paired -test for the paired samples (; ). The following inclusion criteria were applied: children with good general health, without syndromes or chronic systemic diseases. They should also have at least one primary molar with deep carious lesion, which should extend two-thirds of the inner half of dentin (Fig. 1) without pain symptoms and be compatible with reversible pulpits. Children whose parents refused to sign the informed consent document, who did not cooperate with the clinical exams, whose teeth had the treatment choice changed (pain or evolution to irreversible pulpits), who did not keep the appointments for the scheduled treatments, or who needed antibiotic treatment for other medical reasons were excluded from the study without prejudice. For all of these reasons, out of the 58 children enrolled, 32 (18 girls and 14 boys) were included in the final sample, according to the CONSORT flow diagram (Fig. 2).21 Digital photographs and initial radiographs were taken of the selected teeth; demographic features of the children were evaluated by DMFT (decayed, missing, and filled teeth) and DMFS (decayed, missing, and filled surfaces) indexes of primary dentition, as well as the biofilm and gingival indexes.22,23 Fig. 1Radiograph showing the extent of the carious lesions included in the study. EDJ indicates the enamel–dentin junction and PC, the pulp chamber limit. Lesions should extend two-thirds of the inner half of dentin (interrupted line). 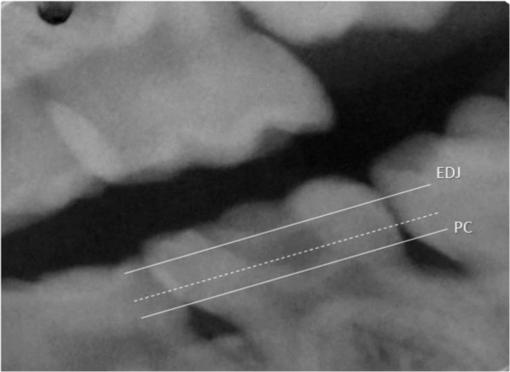 The participants were randomly assigned to their groups by a simple randomization method, generated by a software spreadsheet (Excel 2007; Microsoft, Redmond, Washington). Clinical procedures were all performed by one experienced operator, and both the operator and patient were able to see which treatment was being carried out (although they were children), because the light source could not be disguised. However, dentin samples collected from each patient were coded, so the researcher was blind to the laboratory phase of the microbiological analysis. Encoding was performed by applying previously created labels to the microcentrifuge tubes into which the carious dentin was introduced. Labels were only decoded at the end of the experiment, after the microbiological analysis. 2.3.Treatment GroupsAll treatments were conducted after isolation with rubber dam (Table 1). The carious tissue was partially removed from the lateral walls, and only the superficial demineralized necrotic tissue was removed from the pulp wall with the help of a conventional dentin curette. The dentin was initially collected from the pulp wall with the active part of a sterile 1-mm diameter micropunch (Ritcher, São Paulo, Brazil) with a penetration depth of 0.5 mm.14 After this, the teeth were treated according to the designated groups as follows:
Table 1Characteristics of the experimental groups.
Table 2 shows the demographic features of the participants, such as age and gender distribution, and clinical variables such as DMFT and DMFS values, % of visible biofilm, gingival and biofilm index values. Table 2Means (±SD) of clinical variables such as age and gender distribution, DMFT and DMFS values, % of visible biofilm, gingival, and biofilm index values evaluated.
All patients were clinically and radiographically followed up 6 and 12 months after the end of the treatments. They were also submitted to full dental care, when a complete treatment was performed. They were instructed to make contact if they experienced any signs of pain in any of their teeth. 2.4.Microbiological AnalysisThe carious dentin collected before and after each treatment was placed in separate microtubes containing of TE buffer (10 mM Tris–HCl and 0.1 mM EDTA, pH 7.6) and stored at until use. Microbiological analyses of the samples were performed by quantitative analysis of total bacteria and five specific species, by real-time polymerase chain reaction (PCR). 2.4.1.Bacterial strains and growth conditionsS. mutans (Sm, ATCC 25175), Streptococcus sobrinus (Ss, ATCC 44378), L. casei (Lc, L324m), F. nucleatum (Fn, ATCC 25586), and Atopobium rimae (Ar, CCUG 31168) were used as a source of chromosomal deoxyribose nucleic acid (DNA) for amplification and later as standard in PCR quantitative curves. Sm strains were cultured in mitis salivarius agar (Difco, Sparks, Maryland) with bacitracin at 37°C under microaerophilic conditions (10% in air; Shel Lab, Cornelius, Oregon) for 48 h; Ss strains were cultured in mitis salivarius agar (Difco, Sparks, Maryland) at 37°C under microaerophilic conditions (10% in air; Shel Lab, Cornelius, Oregon) for 48 h; Lc strains were cultured in Rogosa agar (Difco, Sparks, Maryland) at 37°C under microaerophilic conditions (10% in air; Shel Lab, Cornelius, Oregon) for 48 h; Fn strains were grown at 37°C in BHI agar under anaerobic conditions (85% , 5% , and 10% ) generated in an anaerobic chamber (Plaslabs, Lansing, Michigan) for 96 h; Ar strains were grown at 37°C in BHI agar under anaerobic conditions (85% , 5% , and 10% ) generated in an anaerobic chamber (Plaslabs, Lansing, Michigan) for 96 h. 2.4.2.Deoxyribose nucleic acid extractionThe carious dentin collected was thoroughly vortexed, and total DNA from bacterial standard strains and carious dentin collected was extracted using a MasterPure™ Complete DNA and RNA EPICENTRE kit (Epicentre Biotechnologies, Madison, Wisconsin), according to the manufacturer’s instructions. DNA was eluted in TE buffer; the quantity and quality were estimated by spectrometry (Nanodrop ND1000, Thermo Fisher Scientific Inc., Wilmington, Delaware), and then were stored at until use. In order to check the specificity and to produce amplicons to PCR standard curves of each standard DNA, conventional PCR was performed using the primers described in Table 3. Table 3Type of primers, sequence (5′–3′), amplicon size (bp), and annealing temperatures (°C) used for each group/species.
2.4.3.Quantitative polymerase chain reactionQuantitative analysis of the different bacteria was performed by real-time PCR with the thermal cycler Step One Plus Real-Time PCR System (Applied Biosystems, Foster City, California), and the product was detected by fluorescence using SYBR® Green system (Applied Biosystems, Foster City, California) in accordance with the manufacturer’s recommended protocol. PCR reactions were set up in 96-well plates in a total volume of 20 ml containing 10-ml Sybr Green mix, 100 ng of sample DNA, and 200 mM of each primer (Table 3). Internal region of the 16SrRNA gene specific to identify Universal, Lc, Fn, and Ar, while an excerpt inside gtfB and gtfU was used to identify Sm and Ss, respectively. Temperature cycling profiles included a preincubation at 94°C for 5 min followed by 40 cycles of 94°C for 15 s, annealing temperature according to Table 3, for 30 s, 72°C for 1 min, and final extension at 72°C for 10 min. As negative control for each reaction, MilliQ sterile water was added instead of a DNA template. After the amplification reactions, the melt curve analysis was performed using the following temperatures: 95°C for 15 s, 65°C for 1 min, and 0.5°C to 95°C for 15 s.28 Fluorescence was detected after each cycle and represented on a graph using the Step One Plus Real-Time PCR System software (Applied Biosystems, Foster City, California). Quantification of Universal, Sm, Ss, Lc, Fn, and Ar gene copies of purified amplification DNA products was obtained by comparison with standard curves ( to 10 copies of each specific microorganism gene). Curve standards and clinical samples were run in duplicate, and mean values were used to calculate the bacterial load. Data were analyzed using iQ5 Optical System Bio-Rad software (Hercules, California). 2.5.Statistical AnalysisThe number of DNA gene copies of each species/bacterial group was evaluated by two-way analysis of variance, treatment, and time, as the repeated measures factor. The homogeneity of variances was checked and the data were transformed to . The Spearman correlation test was used to check if there was any correlation between the bacterial gene copies and the clinical variables. The level of significance for all statistical tests was . 3.ResultsAfter the follow-up periods of both 6 and 12 months, no signs of pain or restoration failure were observed. The radiographs also showed no abnormal images. Figure 3 shows the number of gene copies of each microorganism () before and after each treatment group. Table 4 shows the statistical results with values. Independent of the treatment, a significant reduction in microorganisms was found for universal bacteria, Sm, Lc, Fn, and Ar before and after treatments when values were compared. No differences were found between treatments. No strong correlations were found among any of the clinical variables tested. Fig. 3Quantification of microorganisms () and standard deviation (SD) before and after each treatment. 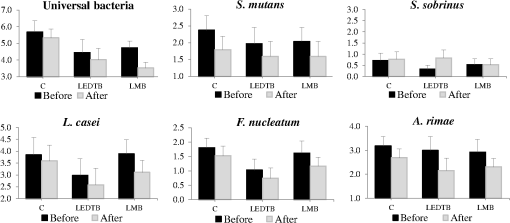 Table 4Average and standard error of quantification of microorganisms (log10) before and after each treatment.
4.DiscussionThe aim of the present study was to evaluate the in vivo effects of photodynamic antimicrobial therapy on the carious dentin of primary teeth. In both in vitro and in situ studies, the literature has shown this therapy to be effective in reducing the number of microorganisms in planktonic cultures and in carious dentin.11–20 The results of the present study demonstrated that PACT reduced the number of all the microorganisms tested after each treatment, except for S. sobrinus; however, for the LED and low-power laser parameters used, no differences could be found among the groups. The classic treatment for deep caries lesions, as was performed in our control group, is to remove the infected dentin, clean the remaining affected tissue, and seal the cavity with a restorative material. These procedures alone guarantee a reduction in bacteria,7 as shown in our results. However, the main goal of the present study was to perform a conservative technique for treating deep caries lesions, to reduce the number of microorganisms in the remaining affected dentin even further, by means of PACT, and in many cases, to avoid the need for endodontic treatment. A tendency for this to be achieved in the PACT groups was observed; however, the reduction did not differ statistically from that of the control group. This is in agreement with studies conducted by Teixeira et al.,29 who were unable to demonstrate the antimicrobial effect of PACT in bacteria present in oral biofilm, and by Tonon et al.,20 who found only a discrete bacterial reduction in a clinical isolate strain of S. mutans. Bacteria have different behaviors when treated in planktonic cultures, in biofilms, and in carious dentin.15,17,20 PACT is less prone to work in biofilms or in the carious dentin matrix due to the limited penetration of the photosensitizing agent into this surface and the deficient light propagation through the demineralized tissues.18,19,30 A relevant in vivo study conducted with 26 young adult patients with deep caries in permanent molars, by using methylene blue as the photosensitive agent and low-power red laser (InGaAIP , 100 mW, , 90 s, 9 J), was able to show a modest reduction in microorganisms (),14 however, differing from the high values achieved in in vitro or in situ studies. In the LMB group in the present study, we used the same PACT parameters as those used in the above-mentioned study, and we were unable to find this statistical microbial reduction in vivo, although there was a numerical trend toward this (Fig. 3). Several photosensitive agents have been proposed for PACT in carious dentin, and they have shown good results in vitro and in situ. Among them, toluidine blue,11–13,15–18 methylene blue,14,17 and, more recently, curcumin.19,20 The authors did not expect to find differences between the two PACT techniques proposed, because provided the PS agent selected is activated by a proper light source wavelength, the reactive products, such as singlet oxygen, would be generated and make direct or indirect contact with bacteria and damage their vital structures.31 Although there was a lack of difference between the PACT groups and the control group, the trend toward bacteria reductions highlights the need for future clinical studies testing new PACT protocols for carious dentin, since there have been new findings on the choice of light source and type of photosensitive agents. A recent study evaluated ortho-toluidine blue dye penetration into carious dentin and showed it could be detected up to deep in situ, as measured by confocal Raman microspectroscopy. This reveals the capacity of the photosensitizer to really penetrate into a carious lesion, resulting in the death of microorganisms deep within dentin.18 A limitation of this clinical trial was the final number of patients enrolled to participate. It is especially critical to maintain children within the inclusion criteria, because of their higher rates of sickness and parents’ cooperation. During the follow-up recalls at 6 and 12 months, none of the treated teeth showed any pain symptoms or radiographic abnormalities. The demographic features of the children confirmed the high caries risk group, as shown by the DMFT of 5.3 and DMFS of 8.3. The acceptable value proposed by the WHO for the year of 2000 was of 50% of caries-free children at 5 years of age and DMFT lower than 3.0 at 12 years.32 The values of the present investigation are way above these predictions and emphasize the urgent need for primary dental care for this population. This study was unable to show that PACT protocols were better than the control protocol used, nevertheless, all therapies reduced the number of microorganisms in dentin, except for S. sobrinus. Therefore, all therapies can be considered effective modern approaches to minimal intervention for the management of deep primary caries. However, it is important to emphasize that these results could be a starting point for new clinical searches for parameters for the use of low-power lasers and LED in PACT for microbial reduction. AcknowledgmentsThis study was supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP” (Grants #2010/07212-5 and #2011/08392-0). The authors thank the Special Laboratory of Lasers in Dentistry LELO-FOUSP and the Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, and also the assistance given by Dr. Jun Kim and Leo Batista Cruz of the University of São Paulo during the clinical and laboratory phases of the study. ReferencesT. M. Marthaler,
“Changes in dental caries 1953–2003,”
Caries Res., 38
(3), 173
–181
(2004). http://dx.doi.org/10.1159/000077752 CAREBK 0008-6568 Google Scholar
R. Leroy et al.,
“Multivariate survival analysis for the identification of factors associated with cavity formation in permanent first molars,”
Eur. J. Oral Sci., 113
(2), 145
–152
(2005). http://dx.doi.org/10.1111/j.1600-0722.2005.00199.x Google Scholar
E. A. M. Kidd and S. Joyston-Bechal, Essentials of Dental Caries. The Disease and Its Management, Oxford University Press, Oxford
(2000). Google Scholar
K. C. Lima et al.,
“Microbiota of dentinal caries as assessed by reverse-capture checkerboard analysis,”
Caries Res., 45
(1), 21
–30
(2011). http://dx.doi.org/10.1159/000322299 CAREBK 0008-6568 Google Scholar
D. Ricketts et al.,
“Operative caries management in adults and children,”
Cochrane Database Syst. Rev., 28
(3), CD003808
(2013). http://dx.doi.org/10.1002/14651858.CD003808.pub3 Google Scholar
L. Bjørndal et al.,
“Stepwise excavation may enhance pulp preservation in permanent teeth affected by dental caries,”
J Evid Based Dent Pract., 11
(4), 175
–177
(2011). http://dx.doi.org/10.1016/j.jebdp.2011.09.005 Google Scholar
E. J. Mertz-Fairhurst et al.,
“Ultraconservative and cariostatic sealed restorations: results at year 10,”
J. Am. Dent. Assoc., 129
(1), 55
–66
(1998). http://dx.doi.org/10.14219/jada.archive.1998.0022 Google Scholar
T. J. Dougherty et al.,
“Photodynamic therapy,”
J. Natl. Cancer Inst., 90
(12), 889
–905
(1998). http://dx.doi.org/10.1093/jnci/90.12.889 Google Scholar
A. P. Castano, T. N. Demidova and M. R. Hamblin,
“Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization,”
Photodiagn. Photodyn. Ther., 1
(4), 279
–293
(2004). http://dx.doi.org/10.1016/S1572-1000(05)00007-4 Google Scholar
M. Wilson,
“Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections,”
Photochem. Photobiol. Sci., 3
(5), 412
–418
(2004). http://dx.doi.org/10.1039/b211266c Google Scholar
T. Burns, M. Wilson and G. J. Pearson,
“Effect of dentine and collagen on the lethal photosensitization of Streptococcus mutans,”
Caries Res., 29
(3), 192
–197
(1995). http://dx.doi.org/10.1159/000262068 CAREBK 0008-6568 Google Scholar
I. C. Zanin et al.,
“Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode,”
Eur. J. Oral Sci., 114
(1), 64
–69
(2006). http://dx.doi.org/10.1111/j.1600-0722.2006.00263.x Google Scholar
J. P. Lima et al.,
“Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries,”
Eur. J. Oral Sci., 117
(5), 568
–574
(2009). http://dx.doi.org/10.1111/j.1600-0722.2009.00662.x Google Scholar
A. Guglielmi et al.,
“Clinical use of photodynamic antimicrobial chemotherapy for the treatment of deep carious lesions,”
J. Biomed. Opt., 16
(8), 088003
(2011). http://dx.doi.org/10.1117/1.3611009 Google Scholar
J. A. Williams et al.,
“The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentine,”
Caries Res., 38
(6), 530
–536
(2004). http://dx.doi.org/10.1159/000080582 CAREBK 0008-6568 Google Scholar
J. S. Giusti et al.,
“Antimicrobial photodynamic action on dentin using a light-emitting diode light source,”
Photomed. Laser Surg., 26
(4), 281
–287
(2008). http://dx.doi.org/10.1089/pho.2007.2149 Google Scholar
J. P. Rolim et al.,
“The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers,”
J. Photochem. Photobiol. B, 106 40
–46
(2012). http://dx.doi.org/10.1016/j.jphotobiol.2011.10.001 Google Scholar
M. A. Melo et al.,
“A comparative study of the photosensitizer penetration into artificial caries lesions in dentin measured by the confocal Raman microscopy,”
Photochem. Photobiol., 15
(10), 12186
(2013). http://dx.doi.org/10.1111/php.12186 Google Scholar
N. C. Araujo et al.,
“Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine,”
Lasers Med. Sci., 29
(2), 629
–635
(2014). http://dx.doi.org/10.1007/s10103-013-1369-3 Google Scholar
C. C. Tonon et al.,
“Comparative effects of photodynamic therapy mediated by curcumin on standard and clinical isolate of Streptococcus mutans,”
J. Contemp. Dent. Pract., 16
(1), 1
–6
(2015). http://dx.doi.org/10.5005/jp-journals-10024-1626 Google Scholar
K. F. Schulz, D. G. Altman and D. Moher,
“CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials,”
Trials, 11 32
(2010). http://dx.doi.org/10.1186/1745-6215-1111-1132 Google Scholar
J. C. Greene and J. R. Vermillion,
“The simplified oral hygiene index,”
J. Am. Dent. Assoc., 68 7
–13
(1964). http://dx.doi.org/10.14219/jada.archive.1964.0034 Google Scholar
K. R. Ekstrand et al.,
“Detection, diagnosing, monitoring and logical treatment of occlusal caries in relation to lesion activity and severity: an in vivo examination with histological validation,”
Caries Res., 32
(4), 247
–254
(1998). http://dx.doi.org/10.1159/000016460 CAREBK 0008-6568 Google Scholar
A. Yoshida et al.,
“Development of a 5’ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus,”
J. Clin. Microbiol., 41
(9), 4438
–4441
(2003). http://dx.doi.org/10.1128/JCM.41.9.4438-4441.2003 Google Scholar
K. Matsuda et al.,
“Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules,”
Appl. Environ. Microbiol., 75
(7), 1961
–1969
(2009). http://dx.doi.org/10.1128/AEM.01843-08 Google Scholar
R. R. Price et al.,
“Targeted profiling of oral bacteria in human saliva and in vitro biofilms with quantitative real-time PCR,”
Biofouling, 23
(3–4), 203
–213
(2007). http://dx.doi.org/10.1080/08927010701251169 BFOUEC 0892-7014 Google Scholar
B. J. Paster et al.,
“Bacterial diversity in human subgingival plaque,”
J. Bacteriol., 183
(12), 3770
–3783
(2001). http://dx.doi.org/10.1128/JB.183.12.3770-3783.2001 Google Scholar
S. R. Teixeira et al.,
“Quantification of Porphyromonas gingivalis and fimA genotypes in smoker chronic periodontitis,”
J. Clin. Periodontol., 36
(6), 482
–487
(2009). http://dx.doi.org/10.1111/j.1600-051X.2009.01411.x Google Scholar
A. H. Teixeira et al.,
“Effect of photodynamic antimicrobial chemotherapy on in vitro and in situ biofilms,”
Caries Res., 46
(6), 549
–554
(2012). http://dx.doi.org/10.1159/000341190 CAREBK 0008-6568 Google Scholar
A. C. Nogueira et al.,
“Photosensitizer and light diffusion through dentin in photodynamic therapy,”
J. Biomed. Opt., 18
(5), 055004
(2013). http://dx.doi.org/10.1117/1.JBO.18.5.055004 Google Scholar
J. Y. Nagata et al.,
“Antibacterial photodynamic therapy for dental caries: evaluation of the photosensitizers used and light source properties,”
Photodiagn. Photodyn. Ther., 9
(2), 122
–131
(2012). http://dx.doi.org/10.1016/j.pdpdt.2011.11.006 Google Scholar
World Health Organization, “Oral health information systems, oral health surveillance,”
(2003) http://www.who.int/oral_health/action/information/surveillance/en/ Google Scholar
|