|
|
1.IntroductionThe 2014 Nobel Prize in physics was awarded to Isamu Akasaki and Hiroshi Amano (Japan) and Shuji Nakamura (United States) “for the invention of efficient blue light-emitting diodes which has enabled bright and energy-saving white light sources.”1 The development of light-emitting diodes (LEDs) has advanced them to a stage where their use in phototherapy is possible. LEDs offer several advantages for clinical and laboratory use. The most important one is a wide choice of emission wavelengths from ultraviolet A to near infrared with a narrow bandwidth of 5 to 10 nm. In addition, LEDs are long-life inexpensive light sources with uniquely high efficiency.2 Moreover, they can be arranged in different geometric combinations to compensate for difficult anatomic areas3–5 that can be useful and effective to use in dentistry for the treatment and prophylaxis of gingivitis. Gingivitis is an inflammation of the gums caused by plaque and bacteria accumulation. According to the data of WHO, the majority of children have signs of gingivitis and a severe periodontal disease, which may result in tooth loss. This is also found in 15% to 20% of middle-aged adults.6 Dental plaque consists of a mixed bacterial flora, sometimes with desquamated epithelial cells and migrated polymorphonuclear leukocytes.7,8 Bacteria in plaque around the teeth release enzymes (collagenases) that can damage and erode the gum tissues. The infected gums swell, bleed easily, recede, and result in loosening of the teeth. A treatment for dental active therapy usually includes debridement of tooth surfaces to remove supra- and subgingival plaque and dental calculus, and application of antimicrobial and antiplaque agents or devices.9 For instance, a key procedure for treatment of periodontal disease is plaque reduction or elimination by mechanical/chemical means.8–12 For many cases, mechanical removal of plaque and plaque-derived products10,11 leads to disease resolution. However, many clinical trials indicate that self-administered plaque control alone, without periodic professional reinforcement, is inconsistent in providing a long-term inhibition of gingivitis.8,12,13 Antibacterial therapy is also considered as a very important component of complex treatment.8,9 Medicamental antibacterial therapy currently available for periodontic disease is sufficiently effective and adequate. However, there are many patients who cannot take the treatment. This is caused by the following factors: high frequency of allergic reactions, contra-indications and side effects of the prescription of drugs, adverse effect on oral microbiocenosis, and others. In addition, bacteria growing in biofilms exhibits resistance mechanisms.14 Therefore, recently, nonmedicamental methods of the treatment are being intensively developed.13,15–18 Some periodontal pathogens are chromogenic bacteria, which are accumulating porphyrins.8 The amounts of endogenous porphyrin in oral black-pigmented bacteria from dental plaque samples have been evaluated in Ref. 19 as (Prevotella intermedia), (Prevotella nigrescens), (Prevotella melaninogenica), and (Porphyromonas gingivalis). Lipovsky et al.20,21 and Mohl et al.22 have reported the presence of endogenous porphyrins in Staphylococcus aureus. The UV–visible absorption spectrum of porphyrins exhibits an intense peak at around 405 to 415 nm.23 As a result, excitation of porphyrin molecules by blue light causes energy transfer from its triplet state to molecular oxygen to produce the excited-state singlet oxygen, which can then oxidize and destroy various biological molecules such as lipids, proteins, and nucleic acids.24 Inactivation of oral bacteria by visible light has been reported elsewhere.13,15,19,25–29 The killing efficiency of 405-nm LED light for Propionibacterium acnes and Staphylococcus epidermidis at constant doses of 35, 70, and with five different irradiation times from 30 to 240 min has been studied in Ref. 30. In addition, a significant increase in the mitotic rate of normal cells has been determined when illuminated with with a maximum at .31 Designing of the LED light source in a form of toothbrush allows a combination of mechanical/chemical means of plaque elimination with photodynamic antibacterial therapy. We have hypothesized that it can exert a synergic effect on the treatment impact. Thus, the goal of our pilot clinical study is an evaluation of the efficacy of the treatment of gingivitis with low intensive blue light-emitting toothbrushes (B-LETBs) based on photodynamic therapy (PDT) and biostimulation principles of recovery of inflammatory diseases. 2.Materials and Methods2.1.Light SourcesB-LETBs have been designed and manufactured by the Laser Center of St. Petersburg State University of Information Technologies, Mechanics, and Optics (St. Petersburg, Russia) in cooperation with Palomar Medical Technologies Inc. The tested B-LETBs are prototypes of a standard mechanical toothbrush with a blue LED.32,33 The central wavelength of the B-LETB is 412 nm with a spectral width of about 25 nm. The photographs of the B-LETB and toothbrush head are presented in Figs. 1(a)–1(c). The scheme of the toothbrush head is shown in Fig. 1. An LED-matrix is placed on a Cu-plate which is cooled by water from a heat exchanger in the brush handle. The B-LETB contains 10 light transparent bristle bundles. LED emits light through and between the bristles. The Cu-plate surface surrounding the LED-matrix is coated with a reflecting thin layer of silver with a reflectance of 85% to 86% at the emission wavelength range that returns light from the tooth surface (a so-called photon-recycling mirror). Fig. 1Photographs of a blue light-emitting toothbrush: (a) general view, toothbrush head in (b) OFF and (c) ON, and (d) regimes and scheme of toothbrush head. 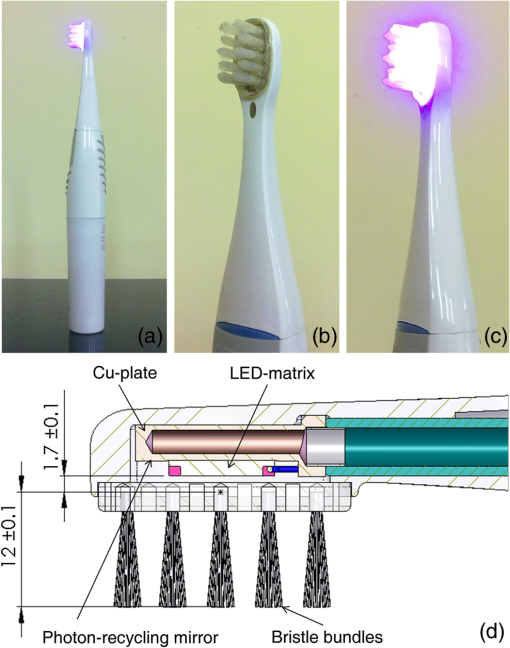 The B-LETB initial mean power density measured with a standard power meter (IMO-2N, Etalon, Russia) for all brushes (60 pcs.) used in the clinical study was . With increasing time of the LETB use, we found some degradation of the bristle hardness as well as decay of the light power density that was, however, not less than at the end of study for each device. 2.2.Microbiological StudyThere is a wide spectrum of bacteria found in the pockets between the teeth and gums.34 The prevalence rate of Staphylococcus species is found to be 73% in dental plaque and 84% in saliva.35 In this work, a microbiological test was done with the aim of comparison of mechanical and photochemical cleaning facilities of the low-intensity B-LETB. Samples of subgingival plaques were obtained clinically by a standard dental applicator. Bacterial suspension was prepared as was described in our earlier paper.13 The surfaces of three cover glasses were covered by the suspension (0.05 ml of the suspension on each glass). The glasses with suspension were put into a thermostat with a temperature of 37°C for 30 min. The first sample served as a control, the second was treated mechanically with six circular motions and chemically with commercial toothbrush and toothpaste, and the third one was treated by the B-LETB (six circular motions) (PDT and mechanically) and a toothpaste (chemically). After the treatment, the glasses were put into a bath with 2 ml of isotonic buffer solution. Then 10-fold consecutive dilutions (from to ) of all samples were prepared. Test tubes with these solutions were put into the thermostat with a temperature of 37°C for 24 h. Bacterial growth suppression efficiency was estimated by counting the cell colony-forming units (CFU). 2.3.Subject SelectionSubject selection for the clinical study was done according to the American Dental Association Acceptance Program Guidelines.34 The clinical trial was carried out in the Dental Clinic of Saratov State Medical University (Russia). Experiments were performed in accordance with the ethical standards of the Declaration of Helsinki.35 Clinical protocols have been approved by the Ethic Committee of Saratov State Medical University. The volunteers gave their written informed consent prior to participation in the study. Before the clinical study, an investigator examined the subject’s oral cavity to confirm the eligibility for the study. Selection criteria were the following: the subject had gingivitis, the subject had read and signed a written informed consent form, and the subject was a healthy volunteer and was free of any systemic diseases other than gingivitis that would interfere with the light exposure results or increase the risk of adverse reactions. Exclusion criteria were the following: the subject was on systemic antibiotics within the treatment period, the subject had severe concurrent diseases, tobacco smoking, and the subject was not able to comply with the study requirements. A total of 60 subjects from 17- to 39-years old of both genders with mild (73% of volunteers) to moderate (27% of volunteers) gingivitis were enrolled for testing of the B-LETBs. Assessment of disease severity was carried out with a Shiller–Pisarev probe: gingival mucosa was anointed with Shiller solution (1 g of crystalline iodide, 2 g of potassium iodide, and 40 ml of distilled water). Coloration varied, depending on the intensity of the inflammation. Gingivitis index PMA (P, papilla interdentalis; M, gingiva marginalis; and A, gingiva alveolaris)36 characterizing inflammatory state of gingiva by color was used: for a healthy gum, mucosa was straw-yellow colored, at chronic inflammation due to glycogen store it was brown colored. Inflammation of papilla, marginal gingival, and alveolar gingiva was evaluated as 1, 2, and 3, respectively. All highest grades for each tooth were summarized. PMA was evaluated with the following equation:13,36 The value of the index up to 30% corresponded to gingivitis of a mild degree, 30% to 60% to gingivitis of a moderate degree, and more than 60% to gingivitis of a severe degree.The condition of gingival mucosa was evaluated from the following parameters (as “Yes” or “No”): anemic, atrophic, hyperemic, hydropic, bleed at probing, cyanotic, ulcerous, hypertrophied, changed fibrously, and exfoliated from cervix of the tooth The conditions of teeth and tooth plaques were also evaluated. Based on the investigation, both the diagnosis and severity of the disease were determined by a professional dentist. 2.4.Study DesignSelected subjects were randomly divided into two groups of 30 persons each. Group I (B-LETB treated) included the volunteers (17 females and 13 males), who were treated by the B-LETB, and group II (control) included the volunteers (16 females and 14 males), who used a standard Braun oral-B manual toothbrush (Procter & Gamble). In both groups, the same toothpaste “Blend-a-Med cavity protection mineral action” (Procter & Gamble) was used. The duration of the treatment study was 4 weeks. The subjects from the first group were instructed how to use the B-LETBs, and all subjects were instructed regarding the right procedure of the tooth brushing. For both groups, the method of the brushing was similar. The time of the full-mouth brushing was 2 min. It had to be carried out two times per day: in the morning and in the evening following meals. The study of the toothbrushes was designed as a single-blind [the examiner did not know which group (experimental or control) the patient belonged to], randomized, prospective clinical study. 2.5.Method of EvaluationThree visits were made by each volunteer to score the state of their oral cavity: baseline (before B-LETB use), 2 week period, and after a month of use. Effects of the treatment were evaluated using the comparison of the patient’s scores from each follow-up visit to the baseline scores, and the average scores of the treatment and the control groups.34 Clinical evaluation of gingivitis severity was visually assessed using an original augmented approximate hygiene index (AHI)13 and standard indices complying with the American Dental Association Acceptance Program Guidelines:34,37 gingival index of Löe-SilnessADA (LSI);38,39 gingival bleeding indexADA (GBI);40 gingivitis index PMA;36 and Turesky modification of the Quigley–Hein plaque indexADA (TI).41 All indices were measured by a single examiner to exclude examiner bias. To evaluate TI, a score of 0 (no plaque) to 5 (plaque covering two-thirds or more of the crown of the tooth) was assigned to each facial and lingual nonrestored surface of all the teeth except the third molars.41 Augmented AHI13 was based on the method of evaluation of TI, but instead of using the investigation of the facial and lingual surfaces, medial and distal tooth surfaces were taken into account. TI and AHI for the entire mouth were determined by dividing the total cumulative score by the number of surfaces examined.13,41 The measurement of the state of oral hygiene LSI was based on recording both soft debris and mineralized deposits on a tooth within a gingival sulcus.38,39 Each of the four areas of a tooth (buccal, lingual, medial, and distal) was given a score from 0 (no plaque) to 3 (abundance of soft matter within the gingival pocket and/or on the tooth and gingival margin). The scores from the four areas of the tooth were added and divided by four in order to give the plaque index for the tooth. Evaluation of GBI was carried out on both vestibular and oral surfaces of a tooth by a special dulled probe.13,40 To evaluate the gingival bleeding degree, a score from 0 (gingival bleeding was absent) to 3 (gingival bleeding appeared during food intake or tooth brushing) was assigned. Both indices LSI and GBI for the patient were obtained by summing the indices for six teeth and dividing by six. Each index was determined for each individual, and the average index was determined for the group. The improvement of tooth status in the both groups was calculated by dividing the difference between the baseline index value and the current index value by the baseline index value where the current index values were scored on the 15th and the 30th days. The percentage improvement of the tooth state of the patients from the first group (treatment) in comparison with that of the patients from the second group (control) was calculated by dividing the difference between the control and B-LETB treatment scores by the control scores on the 15th and the 30th daysBrushes were compared using the independent -test (the statistical method for comparing two unrelated groups on the same conditions). At the level of significance , differences between average values of the baseline and current indices and between average values of indices for the treatment and control groups were accepted as statistically significant. 3.Results and DiscussionFigure 2 shows the result of different treatments of bacteria from the dental plaque. The first column corresponds to the control sample without any treatment. The second column presents the result of mechanical/chemical treatment. The third column shows a combined effect of mechanical/chemical treatment and blue irradiation with the low-intensity B-LETB on bacteria from the dental plaque. The average number of CFU without treatment has been evaluated as . A significant reduction in the average number of CFU down to (95%) in comparison with the control sample has been observed for the mechanical/chemical treatment. The mechanical/chemical + PDT treatment using the B-LETB has decreased the average number of the colonies down to (97.5%). It can be supposed that with application of the B-LETB by the volunteers, a suppression of the pathological flora that are sensitive to blue light action has been observed. Fig. 2Average value of in vitro measured number of bacterial colonies (CFU) taken from tooth plaque: without treatment (control), after a standard toothbrush and a toothpaste, and the B-LETB with the standard toothpaste.  Thus, the use of the B-LETB has resulted in multifactor therapeutic action on oral pathological microflora: in addition to mechanical removal of the bacteria as in ordinary tooth-brushing procedure, it has shown additional suppression action on microorganisms due to photodynamic action. Before the experiment, we have evaluated average values of the indices and standard deviations in both groups. The results are presented in Table 1. At the level of significance, equal or less than 0.05, the difference of initial values of the indices between the treatment and control groups can be considered to be statistically significant. However, as follows from Table 1, the value for the studied groups is greater than 0.05, therefore, the differences can be accepted as statistically insignificant, i.e., values of the indices before experiment are homogeneous enough. Table 1Initial average values of clinical indices and standard deviations.
Figures 3(a)–3(e) present a temporal evolution of the studied indices normalized to their initial values for the both treated and control volunteer groups used the B-LETBs. Three of them (TI, AHI, and LSI) characterize the degree of the covering the tooth by plaque. Fig. 3Dynamics of some dental indices for evaluation of gingivitis severity: (a) Turesky modification of the Quigley–Hein plaque indexADA, (b) approximate hygiene index, (c) gingival index of Löe-SilnessADA, (d) gingival bleeding indexADA, (e) and gingival index PMA. Shaded and solid columns correspond to the results of tooth brushing by the B-LETB and the standard toothbrush, respectively. Bars show standard deviation. 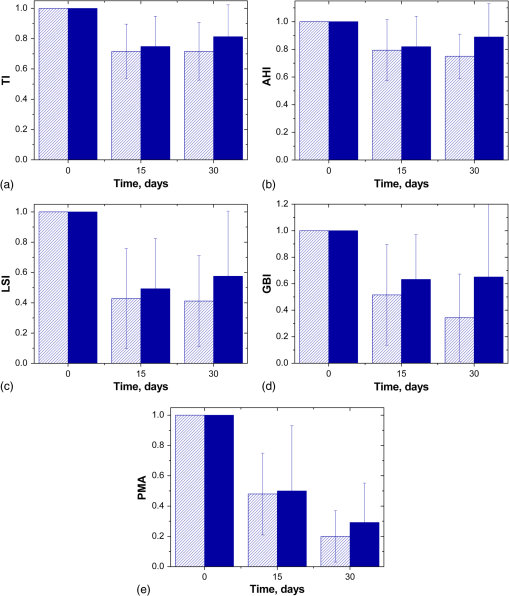 In Figs. 3(a)–3(c), it is well seen that the indices have decreased for both groups of the subjects; a significant reduction is observed on the 15th day of the treatment. The average improvement of the studied indices relative to the baseline for all subjects calculated by Eq. (2) is shown in Table 2 in the second and the third columns; the second column relates to the improvement of the indices in the treatment group, and the third one in the control group. It is seen that the corresponding values in these columns are close to each other. The fourth column shows the improvement of the indices from the treatment group in relation to the control group calculated by Eq. (3). Statistical analysis has showed that the differences in the indices after the treatment by the B-LETBs and standard toothbrushes are not significant by the 15th day. The second column of Table 3 shows the levels of significance obtained by comparison of these groups. All of them are greater than 0.05, thus differences between the treatment and control groups are statistically insignificant. Table 2Results of average improvement of the studied indices relative to the baseline for the subjects from different groups and the B-LETB treated group relative to the control group.
Table 3Level of significance of differences between average values of the clinical indices in the B-LETB-treated group and the control group (level of significance p<0.05 corresponds to a significant difference between the groups).
For the group treated by the B-LETBs, reduction of plaque size has continued during the month of examination; at the same time, the control group has demonstrated partial recovery of plaque [see Figs. 3(a)–3(c) and Table 2, columns 5 and 6]. Results show that on the 30th day of the treatment, differences between the indices in the treatment and control groups have increased (Table 2, columns 4 and 7). In Table 3, the third column corresponds to the level of significance of differences between the studied groups by the 30th day. All values are less than 0.05, which confirms the good cleaning properties of the B-LETBs. Bacterial endotoxins, cytotoxins, and other pathogenic substances are released from plaques and diffuse into the adjacent soft tissues where they elicit an inflammatory response that results in tissue disruption and degradation.42 Therefore, removal of plaque and plaque-derived products, according to many authors, has been a key procedure for treatment of periodontal disease.8–12 Our results have confirmed that improvement of gums is observed in both studied groups during the first half of the observation period. It can be explained by the “learning effect.” The dentist’s instructions relating to the guidelines of tooth brushing provide better results in plaque removal. The suggestion was made that in the beginning of the trials, the volunteers were brushing their teeth carefully and fulfilled all requirements of the investigator, but by the end of the trials, they reverted to their usual manner of brushing. Therefore, during the subsequent 2 weeks, the increase of the plaque indices in the control groups was observed. At the same time, the plaque indices (TI, AHI, and LSI) continued to fall down gradually in the groups with B-LETB testing and have shown a statistically significant difference between plaque removal actions of the B-LETBs and standard brushes. The difference between the two groups in all indices has increased in favor of the B-LETB group between 2 and 4 weeks. We can expect this trend to be more pronounced with an increase of the use of the B-LETB up to several months. Figure 3(d) shows the kinetics of GBI for the two studied groups; and Table 1 shows the percentage of improvement of the gingival bleeding. Figure 3(e) and Table 2 demonstrate the temporal evolution of gingival inflammation. For these indices, statistically significant differences between the groups have also been observed only after a month (see Table 3). From the PMA kinetics, it follows that proper tooth brushing decreases the inflammation in both groups of the volunteers. Kinetics of the GBI and LSI correlates with that of the gingival inflammation development; since the bleeding is caused by gingival inflammation, a decrease of the inflammation process leads to a reduction in the GBI and LSI. Our results have shown that the action of blue light in the same time interval gives an additional reduction in these indices, possibly due to photodynamic suppression of bacteria growth and tissue biostimulation effects.31 Blue light can be an alternative to a conventional antibiotic treatment due to the absence of drug side effects and bacterial resistance. Blue light is effective for phototherapy since exposure to blue light induces photoexcitation of bacterial porphyrins, singlet oxygen production, and subsequent bacterial destruction.43 The potential use of blue light sources (405, 415, 407 to 420 nm) for phototherapy of lesions caused by growth of pathological bacteria is discussed in the literature.2,16–22,25–30,44 4.ConclusionThe present pilot clinical study has been aimed at the evaluation of the effectiveness of the B-LETBs by the use of standard dental indices characterizing the status of teeth and gingiva. A microbiological study has demonstrated the suppression of pathological microorganisms by B-LETB irradiation (up to 97.5%). In both control and treated groups of volunteers, improvement of all indices compared to the baseline has been found. It can be explained by an improvement in oral hygiene for both groups due to careful and correct tooth brushing. However, for the B-LETB group, the efficiency of tooth brushing has been higher (25% to 82% in treatment group versus 11% to 70% in control group); differences between the control and treated groups are statistically significant. The study has also shown that the B-LETB-treatment has a great potential to be much more effective for use at a longer time interval. The use of the LETB can significantly simplify the procedure of the treatment of gingivitis and allows carrying out phototherapy by patients themselves at home. Thus, replacing a standard toothbrush with the B-LETB can be a very promising solution for treatment of gingivitis and prevention of periodontitis. Due to low cost of LED technology, we expect such a product to be affordable for most patients from children to the elder population. AcknowledgmentsWe are thankful to G.B. Altshuler for useful discussions, A.V. Lepilin for the help in microbiological experiments, and S.V. Eremina for the help in paper translation to English. The research was supported by the Government of the Russian Federation (Grant 14.Z50.31.0004 to support scientific research projects implemented under the supervision of leading scientists). References“The official web site of the Nobel prize,”
(2015) http://www.nobelprize.org/nobel_prizes/physics/laureates/2014/press.html September ). 2015). Google Scholar
E. F. Schubert, Light-Emitting Diodes, 327 Cambridge University Press, Cambridge, United Kingdom
(2003). Google Scholar
C. S. Enwemeka et al.,
“Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro,”
Lasers Surg. Med., 40
(10), 734
–737
(2008). http://dx.doi.org/10.1002/lsm.20724 LSMEDI 0196-8092 Google Scholar
M. Maclean et al.,
“Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array,”
Appl. Environ. Microbiol., 75
(7), 1932
–1937
(2009). http://dx.doi.org/10.1128/AEM.01892-08 AEMIDF 0099-2240 Google Scholar
R. Cicchi et al.,
“Improvement of the healing process in superficial skin wounds after treatment with EMOLED,”
Proc. SPIE, 9303 93030E
(2015). http://dx.doi.org/10.1117/12.2052188 PSISDG 0277-786X Google Scholar
“WHO media centre, oral health,”
(2015) http://www.who.int/mediacentre/factsheets/fs318/en/ September ). 2015). Google Scholar
J. J. Zambon,
“Periodontal diseases: microbial factors,”
Ann. Periodontol., 1
(1), 879
–925
(1996). http://dx.doi.org/10.1902/annals.1996.1.1.879 Google Scholar
“Treatment of plaque-induces gingivitis, chronic periodontitis, and other clinical conditions,”
J. Periodontol., 72
(12), 1790
–1800
(2001). http://dx.doi.org/10.1902/jop.2001.72.12.1790 Google Scholar
“Parameter on plaque-induced gingivitis,”
J. Periodontol., 71
(Suppl. 5), 851
–852
(2000). Google Scholar
J. N. Amato et al.,
“Changes in the oral-health-related quality of life of Brazilian children after an educational preventive programme: an 1-month longitudinal evaluation,”
Int. J. Dent. Hygiene, 12
(3), 226
–233
(2014). http://dx.doi.org/10.1111/idh.12075 Google Scholar
P. A. Versteeg et al.,
“Brushing with and without dentifrice on gingival abrasion,”
J. Clin. Periodontol., 32
(2), 158
–162
(2005). http://dx.doi.org/10.1111/j.1600-051X.2005.00652.x JCPEDZ 0303-6979 Google Scholar
R. P. C. Raman et al.,
“Effect of nonsurgical periodontal therapy verses oral hygiene instructions on type 2 diabetes subjects with chronic periodontitis: a randomised clinical trial,”
BMC Oral Health, 14 79
(2014). http://dx.doi.org/10.1186/1472-6831-14-79 Google Scholar
E. A. Genina et al.,
“Phototherapy of gingivitis: pilot clinical study,”
J. Innovative Opt. Health Sci., 4
(4), 437
–446
(2011). http://dx.doi.org/10.1142/S1793545811001745 Google Scholar
G. G. Anderson, G. A. O’Toole,
“Bacterial biofilms,”
Ser. Curr. Top. Microbiol. Immunol., 85
–105 Springer-Verlag, Berlin, Heidelberg
(2008). Google Scholar
N. S. Soukos et al.,
“Photodestruction of human dental plaque bacteria: enhancement of the photodynamic effect by photomechanical waves in an oral biofilm model,”
Lasers Surg. Med., 33
(3), 161
–168
(2003). http://dx.doi.org/10.1002/lsm.10208 LSMEDI 0196-8092 Google Scholar
R. G. E. C. Cauwels and L. C. Martens,
“Low level laser therapy in oral mucositis: a pilot study,”
Eur. Arch. Paediatr. Dent., 12
(2), 118
–123
(2011). http://dx.doi.org/10.1007/BF03262791 Google Scholar
G. B. Silva et al.,
“Photomedicine and laser surgery,”
Photomed. Laser Surg., 29
(1), 27
–31
(2011). http://dx.doi.org/10.1089/pho.2009.2699 Google Scholar
J. D. Carroll et al.,
“Developments in low level light therapy (LLLT) for dentistry,”
Dent. Mater., 30
(5), 465
–475
(2014). http://dx.doi.org/10.1016/j.dental.2014.02.006 Google Scholar
N. S. Soukos et al.,
“Phototargeting oral black-pigmented bacteria,”
Antimicrob. Agents Chemother., 49
(4), 1391
–1396
(2005). http://dx.doi.org/10.1128/AAC.49.4.1391-1396.2005 Google Scholar
A. Lipovsky et al.,
“Sensitivity of Staphylococcus aureus strains to broadband visible light,”
Photochem. Photobiol., 85
(1), 255
–260
(2009). http://dx.doi.org/10.1111/j.1751-1097.2008.00429.x Google Scholar
A. Lipovsky et al.,
“Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing,”
Lasers Surg. Med., 42
(6), 467
–472
(2010). http://dx.doi.org/10.1002/lsm.20948 LSMEDI 0196-8092 Google Scholar
M. Mohl et al.,
“Titania nanofibres in gypsum composites: an antibacterial and cytotoxicology study,”
J. Mater. Chem. B, 2
(10), 1307
–1316
(2014). http://dx.doi.org/10.1039/c3tb21644f Google Scholar
Porphyrins and Metalloporphyrins, 910 Elsevier, Amsterdam
(1975). Google Scholar
R. W. Redmond and J. N. Gamlin,
“A compilation of singlet oxygen yields from biologically relevant molecules,”
Photochem. Photobiol., 70
(4), 391
–475
(1999). http://dx.doi.org/10.1562/0031-8655(1999)070<0391:ACOSOY>2.3.CO;2 PHCBAP 0031-8655 Google Scholar
C. A. Henry et al.,
“Phototoxicity of argon laser irradiation on biofilms of Porphyromonas and Prevotella species,”
J. Photochem. Photobiol. B, 34
(2–3), 123
–128
(1996). http://dx.doi.org/10.1016/1011-1344(95)07239-X JPPBEG 1011-1344 Google Scholar
P. Meisel and T. Kocher,
“Photodynamic therapy for periodontal diseases: state of the art,”
J. Photochem. Photobiol. B, 79
(2), 159
–170
(2005). http://dx.doi.org/10.1016/j.jphotobiol.2004.11.023 JPPBEG 1011-1344 Google Scholar
N. Komerik and A. MacRobert,
“Photodynamic therapy as an alternative antimicrobial modality for oral infections,”
J. Environ. Pathol. Toxicol. Oncol., 25
(1–2), 487
–504
(2006). http://dx.doi.org/10.1615/JEnvironPatholToxicolOncol.v25.i1-2.310 JEPOEC 0731-8898 Google Scholar
A. Braun et al.,
“Short term clinical effects of adjunctive antimicrobial photodynamic therapy (aPDT) in periodontal treatment—a randomized clinical trial,”
J. Clin. Periodontol., 35
(10), 877
–884
(2008). http://dx.doi.org/10.1111/j.1600-051X.2008.01303.x JCPEDZ 0303-6979 Google Scholar
X. Jiang et al.,
“Toluidine blue O and porphyrin-mediated photodynamic therapy on three main pathogenic bacteria of periodontitis using portable LED phototherapy device,”
J. Innovative Opt. Health Sci., 8
(4), 1550017
(2015). http://dx.doi.org/10.1142/S1793545815500170 Google Scholar
E. Tuchina and V. Tuchin,
“Low-intensity LED (625 and 405 nm) and laser (805 nm) killing of Propionibacterium acnes and Staphylococcus epidermidis,”
Proc. SPIE, 7165 71650I
(2009). http://dx.doi.org/10.1117/12.814812 PSISDG 0277-786X Google Scholar
R. Sroka et al.,
“Effects on the mitosis of normal and tumor cells induced by light treatment of different wavelengths,”
Lasers Surg. Med., 25
(3), 263
–271
(1999). http://dx.doi.org/10.1002/(SICI)1096-9101(1999)25:3<263::AID-LSM11>3.0.CO;2-T LSMEDI 0196-8092 Google Scholar
“American Dental Association acceptance program guidelines. Toothbrushes,”
(2015) http://www.ada.org/~/media/ADA/Science%20and%20Research/Files/guide_toothbrushes.ashx September ). 2015). Google Scholar
“WMA declaration of Helsinki—ethical principles for medical research involving human subjects,”
(2015) http://www.wma.net/en/30publications/10policies/b3/index.html September ). 2015). Google Scholar
H. Löe,
“The gingival index, the plaque index and the retention index systems,”
J. Periodontol., 38
(6), 610
–616
(1967). http://dx.doi.org/10.1902/jop.1967.38.6.610 Google Scholar
M. A. B. Rebelo, A. C. de Queiroz, Gingival Diseases—Their Aetiology, Prevention and Treatment, 41
–54 InTech, Rijeka
(2011). Google Scholar
J. Silness and H. Löe,
“Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition,”
Acta Odontol. Scand., 22
(1), 121
–135
(1964). http://dx.doi.org/10.3109/00016356408993968 Google Scholar
C. R. Cowell et al.,
“Testing therapeutic measures for controlling chronic gingivitis in man: a suggested protocol,”
J. Clin. Periodontol., 2
(4), 231
–240
(1975). http://dx.doi.org/10.1111/j.1600-051X.1975.tb01747.x JCPEDZ 0303-6979 Google Scholar
I. Schour and M. Massler,
“Gingival disease in postwar Italy (1945). I. Prevalence of gingivitis in various age groups,”
J. Am. Dent. Assoc., 35
(7), 475
–482
(1947). http://dx.doi.org/10.14219/jada.archive.1947.0266 Google Scholar
S. Turesky et al.,
“Reduced plaque formation by chloromethyl analogue of vitamin C,”
J. Periodontol., 41
(1), 41
–43
(1970). http://dx.doi.org/10.1902/jop.1970.41.1.41 Google Scholar
J. M. de Almeida et al.,
“In vivo effect of photodynamic therapy on periodontal bone loss in dental furcations,”
J. Periodontol., 79
(6), 1081
–1087
(2008). http://dx.doi.org/10.1902/jop.2008.070456 Google Scholar
K. Arakane et al.,
“Singlet oxygen (1Δg) generation from coproporphyrin in Propionibacterium acnes on irradiation,”
Biochem. Biophys. Res. Commun., 223
(3), 578
–582
(1996). http://dx.doi.org/10.1006/bbrc.1996.0937 BBRCA9 0006-291X Google Scholar
Y. Kotoku et al.,
“Bactericidal effect of a 405-nm diode laser on Porphyromonas gingivalis,”
Laser Phys. Lett., 6
(5), 388
–392
(2009). http://dx.doi.org/10.1002/lapl.200910011 1612-2011 Google Scholar
BiographyElina A. Genina is an associate professor in the Departments of Optics and Biophotonics at Saratov State University and a senior researcher in Tomsk State University. She received her PhD in biophysics in 2002. She is authored more than 200 peer-reviewed publications and 7 book chapters on biomedical optics. Her research interests include biomedical optics, laser medicine, and development of methods for control of tissue absorption, and scattering properties for medical optical diagnostics and therapy. Vladimir A. Titorenko graduated from the Dental Faculty at Saratov State Medical University in 1993, now he is a chief doctor and a dentist therapist in Saratov Dental Clinic. He received his PhD in medicine in 2002. He is a coauthor of 52 publications, 1 invention, 2 utility models, and 3 improvement suggestions. Since 2003, he has the highest category of medical specialty dentistry. In 2013, he retrained on the organization of health care and public health. Andrey V. Belikov graduated from Saint-Petersburg Institute of Fine Mechanics and Optics in 1990. From 2013 to 2015 he was a professor in the Laser Technique and Biomedical Optics Department at Saint-Peterburg National Research University of Information Technologies, Mechanics and Optics (ITMO University). From 2015 to present he is a professor of Laser Technologies and Laser Technique Department at ITMO University. His research interests are biomedical optics, and physcis of interaction of light with materials. He has published more than 150 papers. Alexey N. Bashkatov received his PhD in biophysics from Saratov State University in 2002. He has authored 200 publications, 7 book chapters, and over 250 conference presentations. From 2002 to present, he is an associate professor in the Departments of Optics and Biophotonics at Saratov State University and a senior researcher in Tomsk State University. His current research interests include study of optical properties of tissues and modelling of light propagation in turbid media. Valery V. Tuchin is a professor and chairman of the Optics and Biophotonics Department at Saratov State University. He is also the head of laboratory at the Institute of Precision Mechanics and Control, RAS, and the supervisor of Interdisciplinary Laboratory of Biophotonics at Tomsk State University. His research interests include biophotonics, tissue optics, laser medicine, tissue optical clearing, and nanobiophotonics. He is a member of SPIE, OSA, and IEEE, guest professor of HUST (Wuhan) and Tianjin Universities of China, and adjunct professor of the Limerick University (Ireland) and National University of Ireland (Galway). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


