|
|
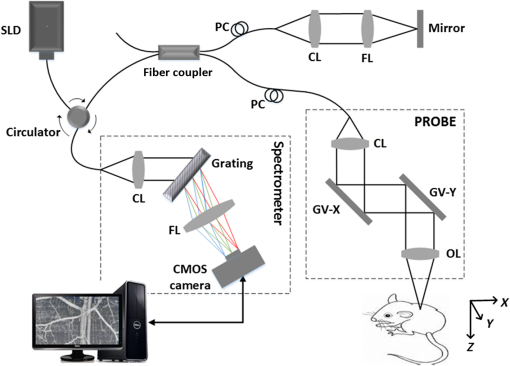
1.IntroductionMainly due to high spatial or temporal resolution, optical imaging techniques have been attractive tools in biomedicine with widespread research and clinical applications when compared with nonoptical methods.1 However, mainly due to the strong scattering in most nontransparent tissues, the strength of the probe light beam attenuates gradually as it penetrates deeper into the tissue. The attenuation effect fundamentally limits the available imaging performances in penetration depth, image contrast, and image resolution.2–4 In particular, cutaneous blood perfusion is highly correlated to a number of peripheral vascular diseases, but the optical imaging of cutaneous blood vessels is quite challenging due to the high scattering property of skin. By matching the refractive index between different tissue components, tissue optical clearing has been found useful for reducing light scattering and improving imaging performance in a wide range of optical techniques, such as laser speckle contrast imaging (LSCI),5,6 optical coherence tomography (OCT),7,8 photoacoustic microscopy,9,10 confocal microscopy,11,12 two-photon microscopy,13,14 and flow cytometry.15 Extensive efforts have been devoted to the screening of high-efficiency and biocompatible optical clearing agents (OCAs) or chemical penetration enhancers for improving the diffusion of OCAs within tissues.3,4 In a previous study, we found a mixture of fructose with PEG-400 and thiazone (FPT) allows in vivo blood flow imaging with higher contrast and resolution.16 Currently, evaluation of a newly developed OCA is usually performed by imaging the blood flow in vivo5,16 or by measuring the optical properties via integration sphere,17 diffuse reflectance spectroscopy,18 and OCT7,19–21 separately. LSCI allows a full-field imaging of the cutaneous blood flow using speckle dynamics and has been used for evaluating the OCA-induced improvement in both the contrast and resolution of blood flow imaging. Due to its lack of depth-resolved information, LSCI fails to measure the optical properties in vivo.22–25 Spectral domain OCT (SDOCT) is capable of high-resolution, real-time, and three-dimensional (3-D) imaging of internal structure of biological tissues in a depth-resolved manner, and the contrast of OCT structure mainly originates from the endogenous differences in optical scattering, eliminating the requirement of tissue-labeling or contrast-enhancing agents.7,19,21,26 The depth-resolved OCT signal offers an approach for measuring optical properties.7,19–21 Thus, the attenuation coefficient, refractive-index mismatching extent, and permeation rate of different tissue compounds can be quantified, which has played a vital role in evaluating the changes of tissue optical properties in previous studies.21 OCA processing, skin optical properties, and blood flow imaging performances are closely correlated, but their relationship has seldom been reported. An approach capable of monitoring the agent-induced changes in skin optical properties and flow imaging performance simultaneously is desirable for a comprehensive understanding of a newly developed OCA. OCT angiography (Angio-OCT) is able to generate structural and angiographic images of skin in parallel. By mathematically analyzing the temporal dynamics of light scattering, Angio-OCT is capable of contrasting the dynamic blood flow against the static tissue bed,27–32 and enables a label-free, motion-contrast 3-D microangiography. Thus, Angio-OCT is quite suitable for OCA characterization by measuring skin optical properties and evaluating blood flow imaging simultaneously. Angio-OCT is used in this study for FPT evaluation. The optical clearing efficacy is quantified by calculating the total attenuation coefficient, refractive-index mismatching extent, and permeation rate. FPT-induced improvement in Angio-OCT imaging performance is presented through imaging resolution, contrast, and imaging depth. Further data analysis is performed to evaluate the correlation between the relative changes in Angio-OCT imaging performance and skin optical properties in vivo. Finally, since the FPT treatment enables the visualization of deep cutaneous blood vessels, dynamic study of flow response to vasoactive drugs is demonstrated by Angio-OCT. 2.Materials and Methods2.1.System SetupAngio-OCT is home-built based on a typical SDOCT configuration, as shown in Fig. 1. Briefly, the light source is a broadband superluminescent diode (SLD; Superlum, Carrigtwohill, Ireland, Broadlighters D855-HP2) with a central wavelength of 850 nm and a full-width-half-maximum bandwidth of 100 nm, theoretically offering a high axial resolution of in air. An objective lens with a focal length of 40 mm was used to focus the probing light beam on the region of interest, yielding a measured lateral-resolution of . The OCT detection unit in our system was a high-speed spectrometer, equipped with a fast line-scan CMOS camera (Basler, Ahrensburg, Germany, Sprint spL4096-140k) providing a 120-kHz line-scan rate and 2048 active pixels. The spectrometer had a designed spectral resolution of 0.062 nm, providing an imaging range of on each side of the zero-delay line in air. The system sensitivity was measured at at the depth position of 0.5 mm, with an incident optical power of 2 mW upon the sample surface. 2.2.Chemical AgentsFPT was used in this study for optical clearing. As described in our previous study,16 FPT was a mixture of saturated fructose (Sinopharm Chemical Reagent Co., Ltd.) solution (78%, w/w) with PEG-400 (Kemiou Reagent Development Corporation) and thiazone (Heming Trading Company Limited) at a volume ratio of . FPT has a refractive index of 1.4801 and a pH value of 6.0. Norepinephrine (NE), purchased from Affiliated Hospital of Huazhong University of Science and Technology (Wuhan, PRC), was used for the dynamic study of cutaneous blood flow. 2.3.Animal PreparationsMale Balb/c mice (, 8 weeks old) were obtained from Shanghai Animal Experimental Center (Shanghai, PRC) and fed under specific pathogen-free conditions. Mice were anesthetized by intraperitoneal injection of a mixture of 10% urethane and 2% -chloralose with a dosage of . The dorsal hair was shaved and the residual hair was thoroughly removed with depilatory cream. Then mice were placed on the experimental platform for OCT imaging. All procedures in the experiments were carried out with the guidelines of the Institutional Animal Care and Use Committee of Zhejiang University, PRC. To evaluate the clearing efficacy of the FPT agent in vivo, the dorsal skin of 15 prepared mice was imaged with Angio-OCT before and after topical application of OCAs. Then six of these mice were injected with of NE () after skin became transparent, and the dynamic response of blood flow to the vasoactive drug was monitored for 25 min with a time interval of 1 min. 2.4.Quantification of Imaging Performance and Skin Optical PropertiesOCT provides depth-resolved images of tissue. Due to the optical scattering and absorption of tissues, the OCT probe light is attenuated as the beam penetrates deeper into the tissue. The OCT depth profile reveals the depth-dependent attenuation coefficient in the tissue. According to Lambert–Beer’s law, the OCT depth profile is related to the attenuation coefficient of tissues as21,33 where and are the detected OCT signal intensity at a reference layer and a certain depth relative to that layer of the tissue, respectively. and refer to the scattering and absorption coefficients, respectively. Here, the factor 2 is due to the light propagating through the tissue twice. The attenuation coefficients can be extracted by fitting an exponential curve to the scattering signal. However, this approach requires accurate segmentation of different skin layers, and it is difficult to provide depth-resolved attenuation coefficients. Recently, Vermeer et al.34 proposed a new method for local attenuation coefficient estimation, which can be expressed as where and refer to the attenuation coefficient and signal intensity for the ’th pixel of the OCT image, respectively, and is the pixel size.The scattering coefficient of tissue is closely dependent on the refractive-index mismatching extent between extracellular fluid () and tissue components (e.g., collagen, fibers, and cell) (). In a simple model of scattering dielectric sphere, can be approximated as35 where , , , and are the volume density of the spheres, the dielectric spherical radius, the scattering anisotropy factor, and the wavelength of the incident light, respectively. , quantifying the refractive-index mismatching extent between extracellular fluid and tissue components. Given that in the near-infrared spectral band, we have , and with the reasonable supposition that the changes of , , and are negligible during the optical clearing process, the OCA-induced relative changes of refractive-index mismatching extent can be evaluated using Eqs. (2) and (3).In addition, the permeability rate () is an important parameter for the evaluation of efficiency of OCAs, which can be estimated by dividing the thickness of the region that OCAs reach by the time of molecular permeation in the monitored region21 The correlation between the relative changes in refractive-index mismatching () and Angio-OCT signal strength can be calculated as where and refer to the signal strength I and refractive index mismatching () at the depth of z, respectively, and are the corresponding average values along the depth.3.Results3.1.Optical Coherence Tomography Structural and Flow Imaging of Mouse Dorsal SkinFigure 2 shows the representative OCT structural and angiographic images of mouse dorsal skin in vivo. Figure 2(a) is a cross-sectional schematic of the layered skin. Figures 2(b1) and 2(c1) show the representative OCT structural cross-sections before and after the topical application of the FPT agent, respectively. The layered structures of skin are quite vague and difficult to distinguish in Fig. 2(b1). In contrast, the imaging contrast and depth are improved greatly in Fig. 2(c1), and the layered structures and features are clearly identified and manually depicted, including the epidermis (ED), dermis (D), hypodermis (HD), and muscles (M), according to cutaneous anatomical features and the obvious boundaries in the Angio-OCT structural cross-section. The ED-D and HD-M boundaries are visualized clearly, and the dermis exhibits a decreased OCT scattering intensity. The hypodermis appears to be an interwoven structure that is correlated to the adipose cells in the layer. The muscle has a sandwich structure. After the topical application of the FPT agent, the thickness of the epidermis and dermis decreases obviously due to agent-induced dehydration. Figures 2(b2) and 2(c2) are the corresponding OCT angiograms before and after FPT treatment, respectively. The blood vessels are isolated from the tissue bed. The big vessels mainly appear at the HD layer, as indicated by the bold arrows, and the small vessels above the HD layer come from the dermis, as indicated by the thin arrows. As shown in Fig. 2(c2), most of the dermal small vessels disappear after the FPT treatment, and the HD blood vessels present an improved visibility, most likely due to the decreased scattering attenuation in the dermis, which can be also observed in the projection view of the 3-D angiography in Figs. 2(b3) and 2(c3). The dermal vasculature can be observed before the FPT treatment in Fig. 2(b3), while the HD big vessels exhibit an improved contrast after the FPT treatment in Fig. 2(c3). Fig. 2Representative OCT structural and angiographic images of mouse dorsal skin in vivo, before (column b) and after (column c) FPT treatment, respectively. (a) Cross-sectional schematic of the layered skin. (b1) and (c1) Structural cross-sections. (b2) and (c2) Cross-sectional angiograms. (b3) and (c3) Projection view of 3-D angiography. ED, epidermis; D, dermis; HD, hypodermis; M, muscles. Thin arrows indicate small vessels. Bold arrows indicate big vessels. 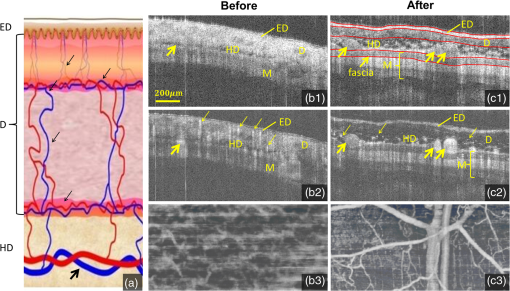 3.2.Quantifications of Skin Optical PropertiesThe skin surface can be identified in the OCT structural cross-sections. The outermost surface was flatted to align all the depth profiles. Then, the OCT depth profile was averaged along the lateral direction so as to reduce effects of the heterogenetic distribution of the cutaneous absorbers and scatters as much as possible. As shown in Fig. 3(a), the solid blue and dashed red curves correspond to the depth profiles before and after the FPT treatment, respectively. After the FPT treatment, OCT intensity strength exhibits a decrease of in the D and HD layers, with an increase of in the M layer. The attenuation coefficient image was calculated according to Eq. (2); thereafter, the outermost surface was flattened and the depth-resolved attenuation coefficient was calculated by averaging the attenuation coefficient image along the lateral direction, as shown in Fig. 3(b). After the FPT treatment, the attenuation coefficients were lower: for the ED, D, and HD layers, and for the M layer. Fig. 3(a) Normalized OCT depth profiles and (b) depth-resolved attenuation coefficients, before (solid blue curves) and after (dashed red curves) FPT treatment, respectively. (c) Relative changes in refractive-index mismatching extent (solid blue curve) and signal intensity (dashed red curve). The vertical dashed lines roughly indicate the skin layers. 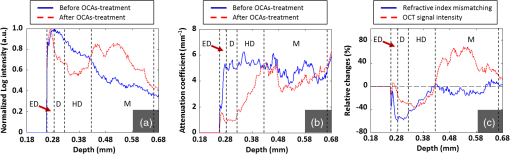 As mentioned before, the relative changes in the refractive-index mismatching extent () between tissue components and extracellular fluid could be quantified using Eq. (3). As shown in Fig. 3(c), the solid blue line represents the relative changes in () after FPT treatment, indicating an average decline of in the ED, D, and HD layers, and in the muscle layer, respectively. The dashed red line is the relative changes in Angio-OCT signal strength. Thereafter, we calculated the correlation between the relative changes in refractive-index mismatching () and Angio-OCT signal strength using Eq. (5), and the results demonstrate that the correlation between the relative changes in refractive-index mismatching () and Angio-OCT signal strength are about 90% and for the superficial (ED + D + HD) layers and the deeper (M) layer, respectively. In addition, the permeability rate is using Eq. (4). 3.3.Blood Flow Dynamic ResponsesWith a combination of optical clearing methods, Angio-OCT exhibits enhanced performance in imaging skin blood flow, allowing cutaneous hemodynamic analysis with high spatiotemporal resolution and contrast. The vasoactive drug-induced changes of vascular diameter and flow flux are estimated over time. The flow flux is measured based on the signal strength of OCT angiograms and normalized with the baseline. Figure 4(a) shows the M-mode Angio-OCT image of the time-lapse blood flow dynamic responses to vasoactive NE after FPT treatment, and the dynamic changes in vessel diameter, which were fitted with red curves, are obvious to see. Figure 4(b) reports the quantifications of the dynamic responses of vessel diameter and normalized blood flow. The baseline of vessel diameter is . After NE injection, the diameter exhibits a gradual decrease of up to 32.8% at the time instant , then returns to of the baseline in 25 min. Along with the dynamic change in diameter, the flow flux exhibits a gradual increase of up to at the time instant , and then returns to of the baseline in 25 min. Fig. 4Blood flow dynamic responses to vasoactive drug monitored by Angio-OCT. (a) The M-mode Angio-OCT image of the time-lapse blood flow dynamic responses (the fitted red curves enhance the visualization of response changes) and (b) the dynamic changes in vessel diameter and normalized blood flow flux. 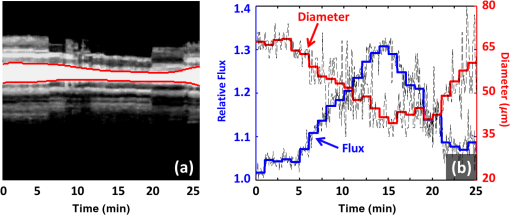 4.Discussion and ConclusionIn this study, a newly developed OCA, FPT, is evaluated with Angio-OCT by quantifying skin optical property and blood flow imaging simultaneously. Angio-OCT offers depth-resolved information about the optical properties, enabling a further understanding of the OCA. Most likely due to the different levels of OCA-induced dehydration and refractive-index matching for each skin layer, there is an evident interface at the ED-D, D-HD, and HD-M junctions, as shown in the Angio-OCT structural cross-sections and depth profiles. As plotted in Figs. 3(b) and 3(c), the D layer presents the largest decrease in scattering coefficient and refractive-index mismatching, while the M layer corresponds to a minimal change. Mainly due to the considerable decrease of attenuation in the superficial layers, more ballistic photons could penetrate into the deeper layers, leading to an improved imaging depth and blood flow contrast. As reported in this study, the application of skin OCAs leads to an improved imaging performance for the deeper tissues (refer to Fig. 2). The imaging performance improvement is most likely caused by the OCA-induced dehydration of skin, and the reduction of attenuation coefficient [refer to Fig. 3(b)] and refractive-index mismatching within the tissue [refer to Fig. 3(c)], as indicated by the high correlation between the relative changes in refractive-index mismatching () and Angio-OCT signal strength [refer to Fig. 3(c)]. These were quite consistent with the conclusions by Ghosn et al.,36 Zhong et al.,37 Xu et al.,38 and Wen et al.39 The permeability rate can also be measured by monitoring the OCT signal slope, the method developed in Ref. 21. The optical clearing rate is an important parameter for OCA evaluation. A fast permeability rate ensures a sufficient optical clearing efficacy within a short time, which is extremely significant for the in vivo study. As discussed above, the FPT is an efficient agent for optical clearing, inducing a significant decrease in attenuation coefficient and refractive-index mismatching, and an improved penetration with a sufficient rate. Combined with optical clearing, Angio-OCT exhibits enhanced performance in imaging skin blood flow, allowing cutaneous hemodynamic analysis with satisfactory spatiotemporal resolution and contrast, and providing a powerful approach for studying skin microcirculation. AcknowledgmentsThe authors acknowledge financial support from National Hi-Tech Research and Development Program of China (2015AA020515), National Natural Science Foundation of China (Grant Nos. 61475143, 11404285, 81171376, 91232710, 31571002, 61335003, 61327007, and 61275196), Zhejiang Provincial Natural Science Foundation of China (LY14F050007), Zhejiang Province Science and Technology Grant (2015C33108), Seed project of Wuhan National Laboratory for Optoelectronics, Science Fund for Creative Research Group (Grant No. 61421064), Fundamental Research Funds for the Central Universities (2014QNA5017), and Scientific Research Foundation for Returned Scholars, Ministry of Education of China. ReferencesS. M. Daly and M. J. Leahy,
“‘Go with the flow’: a review of methods and advancements in blood flow imaging,”
J. Biophotonics, 6
(3), 217
–255
(2013). http://dx.doi.org/10.1002/jbio.201200071 Google Scholar
J. Wang et al.,
“Evaluation of optical clearing with the combined liquid paraffin and glycerol mixture,”
Biomed. Opt. Express, 2
(8), 2329
–2338
(2011). http://dx.doi.org/10.1364/BOE.2.002329 BOEICL 2156-7085 Google Scholar
D. Zhu et al.,
“Recent progress in tissue optical clearing,”
Laser Photonics Rev., 7
(5), 732
–757
(2013). http://dx.doi.org/10.1002/lpor.201200056 Google Scholar
E. A. Genina, A. N. Bashkatov and V. V. Tuchin,
“Tissue optical immersion clearing,”
Expert Rev. Med. Devices, 7
(6), 825
–842
(2010). http://dx.doi.org/10.1586/erd.10.50 1743-4440 Google Scholar
J. Wang, R. Shi and D. Zhu,
“Switchable skin window induced by optical clearing method for dermal blood flow imaging,”
J. Biomed. Opt., 18
(6), 061209
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061209 JBOPFO 1083-3668 Google Scholar
J. Wang et al.,
“Review: tissue optical clearing window for blood flow monitoring,”
IEEE J. Sel. Top. Quantum, 20
(2), 680111
(2014). http://dx.doi.org/10.1109/JSTQE.2013.2286072 IJSQEN 1077-260X Google Scholar
E. A. Genina et al.,
“Optical coherence tomography monitoring of enhanced skin optical clearing in rats in vivo,”
J. Biomed. Opt., 19
(2), 021109
(2014). http://dx.doi.org/10.1117/1.JBO.19.2.021109 JBOPFO 1083-3668 Google Scholar
R. K. Wang et al.,
“Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents,”
J. Opt. Soc. Am. B, 18
(7), 948
–953
(2001). http://dx.doi.org/10.1364/JOSAB.18.000948 JOBPDE 0740-3224 Google Scholar
Y. Liu et al.,
“Optical clearing agents improve photoacoustic imaging in the optical diffusive regime,”
Opt. Lett., 38
(20), 4236
–4239
(2013). http://dx.doi.org/10.1364/OL.38.004236 OPLEDP 0146-9592 Google Scholar
Y. Zhou, J. Yao and L. H. V. Wang,
“Optical clearing aided photoacoustic microscopy with enhanced resolution and imaging depth,”
Opt. Lett., 38
(14), 2592
–2595
(2013). http://dx.doi.org/10.1364/OL.38.002592 OPLEDP 0146-9592 Google Scholar
R. Dickie et al.,
“Three-dimensional visualization of microvessel architecture of whole-mount tissue by confocal microscopy,”
Microvasc. Res., 72
(1–2), 20
–26
(2006). http://dx.doi.org/10.1016/j.mvr.2006.05.003 MIVRA6 0026-2862 Google Scholar
R. Samatham, K. G. Phillips and S. L. Jacques,
“Assessment of optical clearing agents using reflectance-mode confocal scanning laser microscopy,”
J. Innovative Opt. Health Sci., 3
(3), 183
–188
(2010). http://dx.doi.org/10.1142/S1793545810001064 Google Scholar
R. Cicchi and F. S. Pavone,
“Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents,”
Opt. Express, 13
(7), 2337
–2344
(2005). http://dx.doi.org/10.1364/OPEX.13.002337 OPEXFF 1094-4087 Google Scholar
A. Erturk et al.,
“Three-dimensional imaging of solvent-cleared organs using 3DISCO,”
Nat. Protoc., 7
(11), 1983
–1995
(2012). http://dx.doi.org/10.1038/nprot.2012.119 1754-2189 Google Scholar
Y. Ding et al.,
“Signal and depth enhancement for in vivo flow cytometer measurement of ear skin by optical clearing agents,”
Biomed. Opt. Express, 4
(11), 2518
–2526
(2013). http://dx.doi.org/10.1364/BOE.4.002518 BOEICL 2156-7085 Google Scholar
J. Wang et al.,
“Sugars induced skin optical clearing from molecular dynamics simulation to experimental demonstration,”
IEEE J. Sel. Top. Quantum, 20
(2), 7101107
(2014). http://dx.doi.org/10.1109/JSTQE.2013.2289966 IJSQEN 1077-260X Google Scholar
S. A. Prahl, M. J. C. van Gemert and A. J. Welch,
“Determining the optical properties of turbid media by using the adding-doubling method,”
Appl. Opt., 32
(4), 559
–568
(1993). http://dx.doi.org/10.1364/AO.32.000559 Google Scholar
X. Zhong, X. Wen and D. Zhu,
“Lookup-table-based inverse model for human skin reflectance spectroscopy: two-layered Monte Carlo simulations and experiments,”
Opt. Express, 22
(2), 1852
–1864
(2014). http://dx.doi.org/10.1364/OE.22.001852 OPEXFF 1094-4087 Google Scholar
Z. Deng et al.,
“Viscous optical clearing agent for in vivo optical imaging,”
J. Biomed. Opt., 19
(7), 076019
(2014). http://dx.doi.org/10.1117/1.JBO.19.7.076019 JBOPFO 1083-3668 Google Scholar
A. F. Pena et al.,
“Monitoring of interaction of low-frequency electric field with biological tissues upon optical clearing with optical coherence tomography,”
J. Biomed. Opt., 19
(8), 086002
(2014). http://dx.doi.org/10.1117/1.JBO.19.8.086002 JBOPFO 1083-3668 Google Scholar
K. V. Larin et al.,
“Optical clearing for OCT image enhancement and in-depth monitoring of molecular diffusion,”
IEEE J. Sel. Top. Quantum, 18
(3), 1244
–1259
(2012). http://dx.doi.org/10.1109/JSTQE.2011.2181991 IJSQEN 1077-260X Google Scholar
J. Senarathna et al.,
“Laser speckle contrast imaging theory, instrumentation and applications,”
IEEE Rev. Biomed. Eng., 6 99
–110
(2013). http://dx.doi.org/10.1109/RBME.2013.2243140 Google Scholar
D. A. Boas and A. K. Dunn,
“Laser speckle contrast imaging in biomedical optics,”
J. Biomed. Opt., 15
(1), 011109
(2010). http://dx.doi.org/10.1117/1.3285504 JBOPFO 1083-3668 Google Scholar
M. Draijer et al.,
“Review of laser speckle contrast techniques for visualizing tissue perfusion,”
Laser Med. Sci., 24
(4), 639
–651
(2009). http://dx.doi.org/10.1007/s10103-008-0626-3 LMSCEZ 1435-604X Google Scholar
R. Shi et al.,
“Accessing to arteriovenous blood flow dynamics response using combined laser speckle contrast imaging and skin optical clearing,”
Biomed. Opt. Express, 6
(6), 1977
–1989
(2015). http://dx.doi.org/10.1364/BOE.6.001977 BOEICL 2156-7085 Google Scholar
Y. Zhang et al.,
“Time-domain interpolation for Fourier-domain optical coherence tomography,”
Opt. Lett., 34
(12), 1849
–1851
(2009). http://dx.doi.org/10.1364/OL.34.001849 OPLEDP 0146-9592 Google Scholar
Y. Cheng et al.,
“Statistical analysis of motion contrast in optical coherence tomography angiography,”
J. Biomed. Opt., 20
(11), 116004
(2015). http://dx.doi.org/10.1117/1.JBO.20.11.116004 JBOPFO 1083-3668 Google Scholar
M. Roustit and J. L. Cracowski,
“Assessment of endothelial and neurovascular function in human skin microcirculation,”
Trends Pharmacol. Sci., 34
(7), 373
–384
(2013). http://dx.doi.org/10.1016/j.tips.2013.05.007 TPHSDY 0165-6147 Google Scholar
J. Enfield, E. Jonathan and M. Leahy,
“In vivo imaging of the microcirculation of the volar forearm using correlation mapping optical coherence tomography (cmOCT),”
Biomed. Opt. Express, 2
(5), 1184
–1193
(2011). http://dx.doi.org/10.1364/BOE.2.001184 BOEICL 2156-7085 Google Scholar
Y. M. Liew et al.,
“In vivo assessment of human burn scars through automated quantification of vascularity using optical coherence tomography,”
J. Biomed. Opt., 18
(6), 061213
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061213 JBOPFO 1083-3668 Google Scholar
W. J. Choi et al.,
“Improved microcirculation imaging of human skin in vivo using optical microangiography with a correlation mapping mask,”
J. Biomed. Opt., 19
(3), 036010
(2014). http://dx.doi.org/10.1117/1.JBO.19.3.036010 JBOPFO 1083-3668 Google Scholar
L. Guo et al.,
“Improved motion contrast and processing efficiency in OCT angiography using complex-correlation algorithm,”
J. Optics-UK, 18
(2), 025301
(2016). http://dx.doi.org/10.1088/2040-8978/18/2/025301 Google Scholar
R. He,
“Effects of optical clearing agents on noninvasive blood glucose monitoring with optical coherence tomography: a pilot study,”
J. Biomed. Opt., 17
(10), 101513
(2012). http://dx.doi.org/10.1117/1.JBO.17.10.101513 JBOPFO 1083-3668 Google Scholar
K. A. Vermeer et al.,
“Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography,”
Biomed. Opt. Express, 5
(1), 322
–337
(2014). http://dx.doi.org/10.1364/BOE.5.000322 BOEICL 2156-7085 Google Scholar
V. V. Tuchin, Optical Clearing of Tissues and Blood, 1
–129 SPIE Press, Bellingham
(2005). Google Scholar
M. G. Ghosn et al.,
“Monitoring of glucose permeability in monkey skin in vivo using optical coherence tomography,”
J. Biophotonics, 3
(1–2), 25
–33
(2010). http://dx.doi.org/10.1002/jbio.200910075 Google Scholar
H. Zhong et al.,
“Synergistic effect of ultrasound and thiazone-PEG 400 on human skin optical clearing in vivo,”
Photochem. Photobiol., 86
(3), 732
–737
(2010). http://dx.doi.org/10.1111/j.1751-1097.2010.00710.x PHCBAP 0031-8655 Google Scholar
X. Xu, Q. Zhu and C. Sun,
“Assessment of the effects of ultrasound-mediated alcohols on skin optical clearing,”
J. Biomed. Opt., 14
(3), 030442
(2009). http://dx.doi.org/10.1117/1.3156827 JBOPFO 1083-3668 Google Scholar
X. Wen et al.,
“Enhanced optical clearing of skin in vivo and optical coherence tomography in-depth imaging,”
J. Biomed. Opt., 17
(6), 066022
(2012). http://dx.doi.org/10.1117/1.JBO.17.6.066022 JBOPFO 1083-3668 Google Scholar
|