|
|
1.IntroductionBlue light-emitting diodes (LEDs) are semiconductor devices that are commonly used as light sources in LED-backlit liquid crystal displays of various electronic appliances such as smartphones, computer screens, and LED lamps. As compared with a normal lamp, the LED lamp has several advantages, such as less heat, longer life, and good energy efficiency. Blue LEDs also display several other properties apart from being a light source. Blue light has been reported to be lethal to insects.1 Additionally, blue LED light is shown to display a therapeutic effect in seasonal affective disorder.2 However, blue LED emits only short-wavelength high-energy visible light and long-time video display terminal works expose human eyes to blue LED light. Further, its night-time exposure can suppress the secretion of melatonin, resulting in sleep disorders.3–5 Moreover, exposure to blue LED light leads to increased production of reactive oxygen species (ROS).6 Oxidative stress induced by ROS is known to trigger photoreceptor cell7 and retinal pigment epithelium (RPE) cell death.8 Oxidative stress-induced RPE cell death may become a risk factor for age-related macular degeneration (AMD), which is the main cause of blindness in industrialized nations.9,10 Therefore, overexposure to blue light may be a risk for the progression of AMD.8,9 While wet AMD can be treated by several antiangiogenic drugs such as Ranibizumab,11 there is no effective treatment yet for dry AMD. One of the easiest ways to reduce the risks of acquiring blue light exposure-mediated sleep disorder and AMD5,12 is to wear blue light-blocking lenses. A blue light–cutting lens is a yellow lens, which absorbs almost all blue light, or a colorless lens, whose surface is processed to reflect blue light. In this study, the protective effect of light colored lenses on a blue LED light-induced murine photoreceptor-derived cell damage model was investigated by evaluating cell viability, the rate of cell death, and ROS production. Some colored lenses had a protective effect in this model. Notably, our findings showed that the transmittance of blue light is in large correlation with the protective effect of colored lenses in a blue LED light-induced cell damage model. This result strongly suggested that the consideration of an amount of blue light was important in the protection of eyes from light-related eye diseases such as AMD. 2.Materials and Methods2.1.Cell CultureThe murine photoreceptor-derived cell line (661W) was kindly gifted by Dr. Muayyad R. Al-Ubaidi (Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Wako, Osaka, Japan) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, Missouri), penicillin (Meiji Seika Kaisha Ltd., Tokyo, Japan), and streptomycin (Meiji Seika) under 5% atmosphere at 37°C. The cells were passaged every 2 to 3 days by trypsinization. 2.2.Photoreceptor-Derived Cell Damage Induced by Blue Light-Emitting DiodeThe 661W cells were seeded into 96-well plates at a density of cells per well and incubated for 24 h under 5% atmosphere at 37°C. Following this, the cell culture medium was replaced by DMEM containing 1% FBS, and the plates were exposed to 350 to 800 lux blue LED light for 24 h. The wavelength of blue LED light was 464 nm.13 Control groups were shaded by aluminum foil and lens groups were placed on lenses, while exposing experimental groups to blue LED light. 2.3.Colored LensesRETINEX lenses, blue light–reflecting lenses, and Y50, the cutting filter below 500 nm (HOYA, Tokyo, Japan), were used for all experiments. Evaluated colors were Y50 filter, YE, SYD, OO, OG, GN, SY, PN, and blue light–reflecting lenses (Fig. 1). 2.4.Cell Death Analysis by Hoechst 33342 and Propidium Iodide StainingThe 661W cells were seeded in 96-well plates at a density of cells per well and incubated for 24 h in 5% at 37°C. The cell culture medium was replaced by DMEM containing 1% FBS, and the plates were exposed to 350 to 800 lux blue LED light for 24 h. Following LED exposure, the plates were incubated for 12 h in 5% at 37°C, and the cells were stained for 15 min with Hoechst 33342 (Molecular Probes, Eugene, Oregon) and propidium iodide (PI; Molecular Probes). Hoechst 33342 and PI were added in culture wells at final concentrations of 8.1 and , respectively. The stained cells were observed and images were captured by Olympus IX70 inverted epifluorescence microscope (Olympus, Tokyo, Japan). 2.5.Cell Viability AssayCell viability was assayed using CCK-8 (Dojin Kagaku, Kumamoto, Japan). The 661W cells were seeded into 96-well plates at a density of cells per well and incubated for 24 h in 5% at 37°C. Following this, the cell culture medium was replaced by DMEM containing 1% FBS, and the plates were exposed to 350 to 800 lux blue LED light for 24 h. Subsequently, CCK-8 reagents () were added in each well and incubated for 0 to 2 h, after which the optical density at 450 nm was measured with a microplate reader (Varioskan Flash 2.4; Thermo Fisher Scientific, Waltham, Massachusetts). 2.6.Measurement of Reactive Oxygen Species ProductionThe 661W cells were seeded into 96-well plates at a density of cells per well and incubated for 24 h in 5% at 37°C. Following this, the culture medium was replaced by DMEM containing 1% FBS, and the plates were exposed to 350 to 800 lux blue LED light for 24 h. ROS were then measured by 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Eugene, Oregon). CM-H2DCFDA () was added to the wells and incubated for 0 to 1 h in 5% at 37°C. Fluorescence was measured by a microplate reader at 485/535 nm. The number of live cells was counted by Hoechst and PI staining. 3.Results3.1.Rate of Cell Death Was Decreased in Several Lens GroupsThe rate of cell death was calculated based on Hoechst 33342 positive and PI positive cells, and typical fluorescence microscopy images are shown in Fig. 2. The rate of cell death in the no-lens group was observed to increase by 7% to 12% (Fig. 3). The rate of cell death in the Y50 filter, YE, SYD, OO, OG, GN, SY, and PN lens groups was significantly decreased in comparison with the no-lens group [Figs. 3(a)–3(h)]. The rate of cell death in the reflective coating lens group did not show any decrease as compared with the no-lens group [Fig. 3(i)]. Fig. 2Effect of colored lenses on blue LED light-induced damage in 661W cells. Fluorescence microscopy images after staining with Hoechst 33342 (blue) and PI (red). Live cells were stained with Hoechst 33342, and dead cells were stained with Hoechst 33342 and PI. (a) Y50 filter, (b) YE, (c) SYD, (d) OO, (e) OG, (f) GN, (g) SY, and (h) PN lenses decreased the number of dead cells. (i) Reflective coating lenses did not decrease the number of dead cells. . 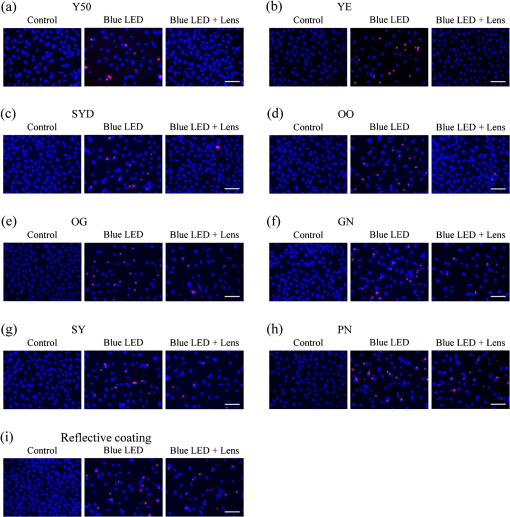 Fig. 3Effect of colored lenses on the rate of cell death upon blue LED light-induced damage in 661W cells. The total number of Hoechst 33342 and PI positive cells was counted, and the rate of cell death was calculated as the percentage of PI positive cells to the number of total cells. Rate of cell death increased upon blue LED light exposure. (a) Y50 filter, (b) YE, (c) SYD, (d) OO, (e) OG, (f) GN, (g) SY, and (h) PN lenses decreased the rate of cell death in comparison with the no-lens group. (i) Reflective coating lenses did not decrease the rate of cell death compared with the no-lens group. Data are expressed as (). ## versus control; * versus blue LED light exposure; and ** versus blue LED light exposure (one-way ANOVA followed by Tukey’s test). 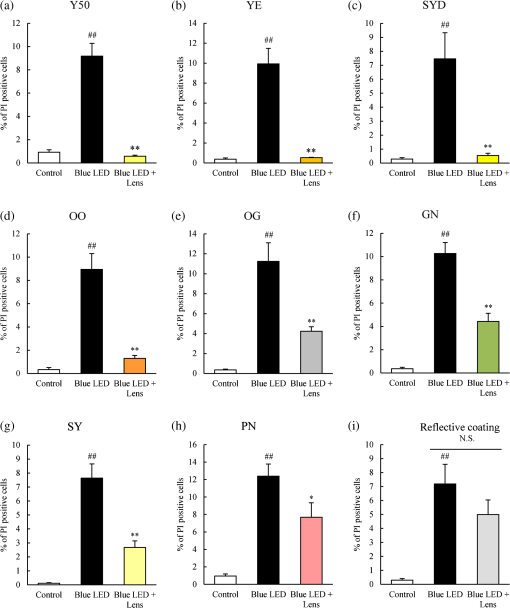 3.2.Several Lens Groups Improved Cell ViabilityCell viability was measured by CCK-8 and was observed to decrease upon blue LED light exposure. In comparison with the control group, the cell viability in the no-lens group decreased by 30% to 50% (Fig. 4). Further, cell viability was significantly improved in the Y50 filter, YE, SYD, OO, OG, and GN lens groups as compared with the no-lens group [Figs. 4(a)–4(f)]. Cell viability was not improved in the SY, PN, and reflective coating lens groups as compared with the no-lens group [Figs. 4(g)–4(i)]. Fig. 4Effect of colored lenses on cell viability upon blue LED light-induced damage in 661W cells. Cell viability was assayed by CCK-8 and was reduced upon blue LED light exposure. (a) Y50 filter, (b) YE, (c) SYD, (d) OO, (e) OG, and (f) GN lenses significantly improved cell viability compared with the no-lens group. (g) SY, (h) PN, and (i) reflective coating lenses did not improve cell viability compared with the no-lens group. Data are expressed as (). ## versus control; and ** versus blue LED light exposure (one-way ANOVA followed by Tukey’s test). 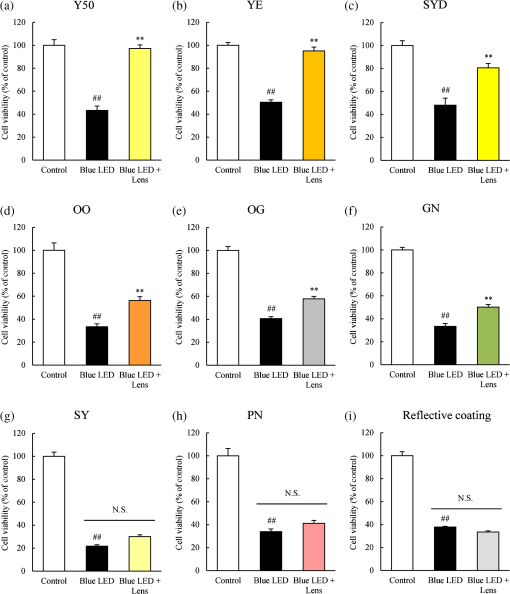 3.3.Reactive Oxygen Species Production Was Decreased in Several Lens GroupsCM-H2DCFDA was used as the fluorescent probe for detecting ROS production. The level of ROS production was increased upon exposure to blue LED light. A 350% to 600% increase in the level of ROS production was observed in the no-lens group as compared with the control group (Fig. 5). Furthermore, compared with the no-lens group, ROS production was significantly decreased in the Y50 filter, YE, SYD, OO, OG, GN, and SY lens groups [Figs. 5(a)–5(g)]. The level of ROS production in the PN and reflective coating lens groups did not decrease as compared with the no-lens group [Figs. 5(h)–5(i)]. Fig. 5Effect of colored lenses on ROS production upon blue LED light-induced damage in 661W cells. ROS production was measured by fluorescence intensity 1 h after the addition of CM-H2DCFDA, and ROS levels increased upon exposure to blue LED light. (a) Y50 filter, (b) YE, (c) SYD, (d) OO, (e) OG, (f) GN, and (g) SY lenses significantly decreased the level of ROS production compared with the no-lens group. (h) PN and (i) reflective coating lenses did not decrease the level of ROS production compared with the no-lens group. Data are expressed as (). ## versus control; and ** versus blue LED light exposure (one-way ANOVA followed by Tukey’s test). 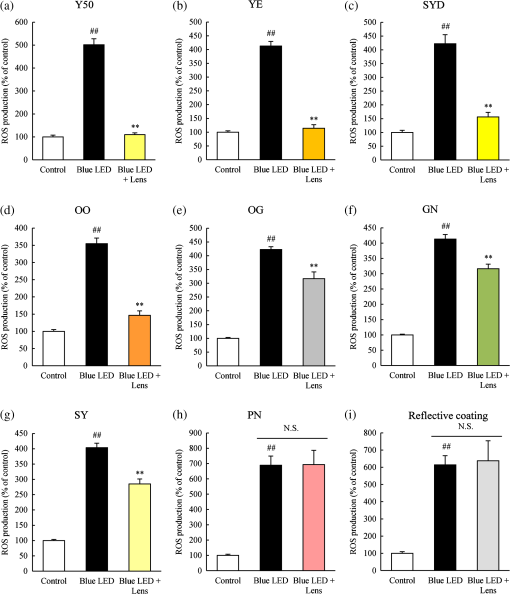 3.4.Protective Effect of Lenses Correlated with TransmittanceThe transmittance of lenses at 400 to 495 nm is shown in Table 1. The protective effects of lenses against blue LED light exposure were evaluated by differences in cell viability, level of ROS production, and the rate of cell death in various groups. Here, the protective effects of various lenses were observed to correlate strongly with their transmittance (Fig. 6). Table 1Transmittance of lenses at 400 to 495 nm. The transmittance of lenses at 464 nm was used for calculating the correlation with their protective effects.
Fig. 6Correlation of protective effects of lenses with transmittance. The correlations between protective levels of lenses and their transmittance were calculated, and the Pearson product–moment correlation coefficients () are shown. (a) Decreased rate of cell death; (b) improved cell viability; and (c) decreased ROS production could be correlated with transmittance of lenses. 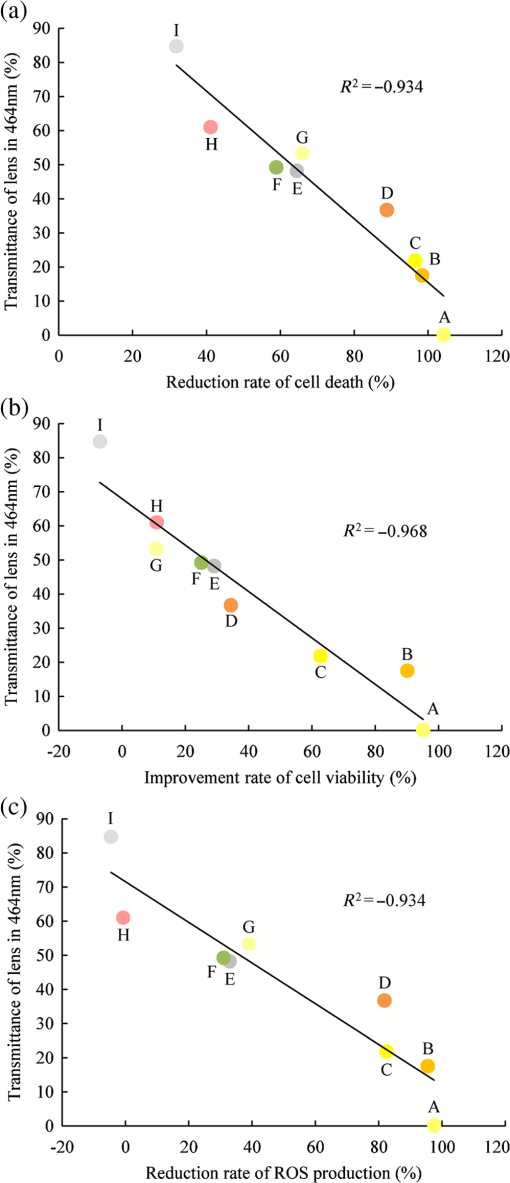 4.DiscussionPrevious in vivo studies have indicated that yellow lenses protect from retinal damage induced by exposure to white or blue LED light.14–16 However, the protective effects of colored lenses have not been investigated in vitro. Recently, we established a blue LED light-induced cell damage model using 661W photoreceptor-derived cells.13 The current study aimed at investigating the protective effects of colored lenses upon exposure to blue LED light using the previously established model. Further, protective effects of colored lenses and their correlation with lens transmittance at 464 nm were also evaluated. ROS levels were found to increase in the no-lens groups, consistent with their reported increase upon blue LED light exposure.13 The 661W cell was damaged by blue LED light exposure in the no-lens group because ROS-induced oxidative stress and oxidative stress caused photoreceptor cell death and apoptosis.17,18 A previous in vivo study has also confirmed photoreceptor cell death and apoptosis induced by blue light exposure.19 Our previous report also showed that blue LED light caused S-opsin aggregation related to endoplasmic reticulum (ER) stress. It should be associated with reduction of the oxidative stress and ER stress by colored lenses. Specifically, colored lenses decreased the cell death through the suppression of ROS production in the present study. The Y50 filter, YE, SYD, OO, OG, and GN lenses displayed highly protective effects against blue LED light exposure, most likely by physically blocking the blue LED light before it arrived at the 661W cells and thereby decreasing the blue LED light exposure levels of the 661W cells. As a result, 661W cell damage was migrated by these lenses. Among these, Y50, the cutting filter below 500 nm, showed the strongest protective effect against 661W cell damage induced by blue LED light exposure. Cutting filters lead to changes of transmittance properties in a particular wavelength. The Y50 filter does not transmit below the wavelength of 500 nm. Subsequently, the Y50 filter has the highest blue light absorptive capacity among the various tested colored lenses. Yellow is known to absorb short wavelengths, consistent with which yellow-colored lenses, namely YE and SYD, also showed highly protective effects against blue LED light exposure. Recent reports have shown that yellow lenses reduce the expression of blue light-induced inflammatory markers in mice16 and have a protective effect against retinal damage induced by blue LED light exposure in rats.14 However, clear lenses showed no protective effects against blue light–induced retinal damage in both models.14,16 Here, the reflective coating lenses showed no protective effect against 661W cell damage owing to their high transmittance at 464 nm, resulting in insufficient blockage and thereby transmittance of blue LED light through these lenses. The lenses with blue light reflective coating showed highest transmittance in the various tested colored lenses and therefore led to cell damage due to direct exposure to blue LED light. Thus, the protective effect of lenses could be correlated with their transmittance at 464 nm. Taken together, these results showed that blue light–induced photoreceptor cell death is correlated with the amount of blue light exposure. Moreover, colored lenses suppressed blue light–induced oxidative stress since the level of ROS production was correlated with lens transmittance at 464 nm. In conclusion, lenses with low transmittance at 464 nm protected the 661W cells from blue LED light-induced damage. These colored lenses were capable of physically absorbing blue LED light and thereby suppressing blue LED light-induced retinal damage. Our findings also showed that the transmittance of blue light is in large correlation with the protective effect of colored lens in a blue LED light-induced cell damage model. This correlation strongly suggested that an amount of blue light was important in the protection of eyes. Moreover, colored lenses (such as gray and green) except for the yellow lens may have a protective effect on blue LED light-induced retinal damage. ReferencesM. Hori et al.,
“Lethal effects of short-wavelength visible light on insects,”
Sci. Rep., 4 7383
(2014). http://dx.doi.org/10.1038/srep07383 Google Scholar
M. C. Gordijn, D. ’t Mannetje and Y. Meesters,
“The effects of blue-enriched light treatment compared to standard light treatment in seasonal affective disorder,”
Br. J. Ophthalmol., 136 72
–80
(2012). http://dx.doi.org/10.1016/j.jad.2011.08.016 Google Scholar
C. Cajochen et al.,
“High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light,”
J. Clin. Endocrinol. Metab., 90
(3), 1311
–1316
(2005). http://dx.doi.org/10.1210/jc.2004-0957 Google Scholar
G. C. Brainard et al.,
“Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor,”
J. Neurosci., 21
(16), 6405
–6412
(2001). JNNUEF Google Scholar
K. E. West et al.,
“Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans,”
J. Appl. Physiol., 110
(3), 619
–626
(2011). http://dx.doi.org/10.1152/japplphysiol.01413.2009 Google Scholar
C. Roehlecke et al.,
“Influence of blue light on photoreceptors in a live retinal explant system,”
Mol. Vision, 17 876
–884
(2011). Google Scholar
N. Sanvicens et al.,
“Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6,”
J. Biol. Chem., 279
(38), 39268
–39278
(2004). http://dx.doi.org/10.1074/jbc.M402202200 Google Scholar
J. Cai et al.,
“Oxidative damage and protection of the RPE,”
Prog. Retinal Eye Res., 19
(2), 205
–221
(2000). http://dx.doi.org/10.1016/S1350-9462(99)00009-9 PRTRES 1350-9462 Google Scholar
F. Q. Liang and B. F. Godley,
“Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration,”
Exp. Eye Res., 76
(4), 397
–403
(2003). http://dx.doi.org/10.1016/S0014-4835(03)00023-X EXERA6 0014-4835 Google Scholar
R. R. Bourne et al.,
“Causes of vision loss worldwide, 1990–2010: a systematic analysis,”
Lancet Global Health, 1
(6), e339
–e349
(2013). http://dx.doi.org/10.1016/S2214-109X(13)70113-X Google Scholar
A. J. Witkin et al.,
“High-speed ultrahigh resolution optical coherence tomography before and after Ranibizumab for age-related macular degeneration,”
Ophthalmology, 116
(5), 956
–963
(2009). http://dx.doi.org/10.1016/j.ophtha.2008.12.018 OPANEW 0743-751X Google Scholar
H. R. Taylor et al.,
“Visible light and risk of age-related macular degeneration,”
Trans. Am. Ophthalmol. Soc., 88 163
–178
(1990). TAOSAT 0065-9533 Google Scholar
Y. Kuse et al.,
“Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light,”
Sci. Rep., 4 5223
(2014). http://dx.doi.org/10.1038/srep05223 Google Scholar
M. Tanito, S. Kaidzu and R. E. Anderson,
“Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats,”
Exp. Eye Res., 83
(6), 1493
–1504
(2006). http://dx.doi.org/10.1016/j.exer.2006.08.006 EXERA6 0014-4835 Google Scholar
T. Kurihara et al.,
“Retinal phototoxicity in a novel murine model of intraocular lens implantation,”
Mol. Vision, 15 2751
–2761
(2009). Google Scholar
T. Narimatsu et al.,
“Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo,”
Exp. Eye Res., 132 48
–51
(2015). http://dx.doi.org/10.1016/j.exer.2015.01.003 EXERA6 0014-4835 Google Scholar
R. R. Krishnamoorthy et al.,
“Photo-oxidative stress down-modulates the activity of nuclear factor- via involvement of caspase-1, leading to apoptosis of photoreceptor cells,”
J. Biol. Chem., 274
(6), 3734
–3743
(1999). http://dx.doi.org/10.1074/jbc.274.6.3734 Google Scholar
K. Tsuruma et al.,
“Role of oxidative stress in retinal photoreceptor cell death in N-methyl-N-nitrosourea-treated mice,”
J. Pharmacol. Sci., 118
(3), 351
–362
(2012). http://dx.doi.org/10.1254/jphs.11110FP Google Scholar
J. Wu et al.,
“Damage of blue light induced apoptosis in rat retina,”
Eye, 13 577
–583
(1999). http://dx.doi.org/10.1038/eye.1999.142 Google Scholar
|