|
|
1.IntroductionIn the past, studies evaluated the amount of weight and the relation to cardiac output, hypertension, atherosclerosis, angiopathy, vascular reactivity, and endothelial function.1–4 Moreover, it was demonstrated that the human skin is even involved in systemic diseases including hypertension, hypercholesterolemia, cardiovascular disease, diabetes mellitus, and obesity.5–8 However, overweight-associated effects on in vivo cutaneous microcirculation has not been investigated in vivo. Given that adequate microvascular perfusion is essential for cellular integrity, little attention has been given to in vivo histomorphology of the human skin. Hitherto, several noninvasive measuring techniques have been used to assess skin microvascular status including laser Doppler flowmetry, plethysmography, orthogonal polarization spectral imaging, transcutaneous oxygen measurements, electromagnetic flowmetry, or hemoglobin oxygenation.9–14 While all of these monitoring devices have their specific applications and limitations, none of them is able to evaluate both the microvascular function and the local morphological pattern. Both parameters are important, however, to assess restoration of cellular homeostasis. Reflectance-mode confocal microscopy (RCM) is a noninvasive imaging technique to observe the human skin in vivo and in real-time. This high-resolution microscope opens a window in to living tissue and enables investigation of cutaneous microcirculation and histomorphology on cellular and subcellular levels.15–20 The aim of the present study was to evaluate the impact of overweight on in vivo microcirculation and histomorphology of the human skin using RCM. 2.Methods2.1.VolunteersTen normotensive overweight nondiabetic individuals (BMI ) and 10 age- and sex-matched healthy lean controls (BMI ) all with skin type III (Fitzpatrick Classification Scale) were enrolled into the study and assigned to one of the following groups. 2.2.ProtocolIn a standardized session, RCM measurements were performed in both groups on the volar aspect of a randomly selected forearm. All measurements were performed in a fasting state. Thirty minutes prior to measurements, the subjects were recumbent in a room for acclimatization without physical or psychological stress. Prior to the measurements, heart rate, blood pressure, and plasma glucose level were measured as normal. Drugs, creams, ointments, or other cosmetic products were avoided 48 h before starting the study. This study was approved by the local ethics committee and all patients accepted to take part in the study with a written informed consent. In a standardized session, a magnetic tissue ring of the RCM was applied to the skin to stabilize the imaging skin site. The corresponding magnetic objective head of the microscope was positioned over the ring to capture the tissue ring without applying pressure. Moreover, the investigated area in the center of the tissue ring was anytime free of pressure. 2.3.InstrumentRCM was performed using a commercially available confocal microscope (Vivascope1500, Lucid Inc., Rochester, New York). This device enables high resolution and noninvasive imaging of the human skin without the necessity of external fluorescence and without the need to process the tissue by freezing, sectioning, or staining. This confocal model operates with a special gallium-arsenide laser source emitting in a long wavelength band at 830 nm. Since this wavelength is in the “optical window” of the human skin, optical sectioning of the epidermis and dermis, including the upper dermal plexus is achievable up to a controlled depth of . High-resolution imaging on cellular and subcellular levels is feasible owing to a vertical resolution of and a lateral resolution of . By generating 20 frames per second, real-time imaging is achievable. This means the feasibility of observing dynamic processes of microcirculation in real-time such as blood cell flow. Due to the laser’s low power of 30 mW at the skin surface, no tissue damage occurs. The single frame field of view is . 2.4.ParameterIn RCM images, the epidermal–dermal junction reflects strongly due to its melanin content, as bright circles of basal layer with the dark focus corresponding to the dermis. The circles are surrounded by the spinous layer of the epidermis. In the focus of the dermal papillae, the lumina of capillary loops are visible as black holes. Blood cell flow can be clearly observed in real-time imaging as brightly reflecting erythrocytes circulating through the capillary loops (Fig. 1). In vivo RCM was performed to evaluate dermal blood cell flow (DBCF), density of dermal capillaries (DDC), epidermal thickness (ET), and epidermal cell size (ECS).
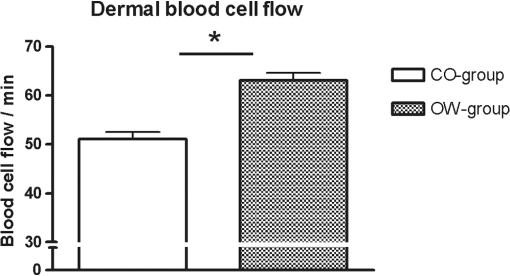
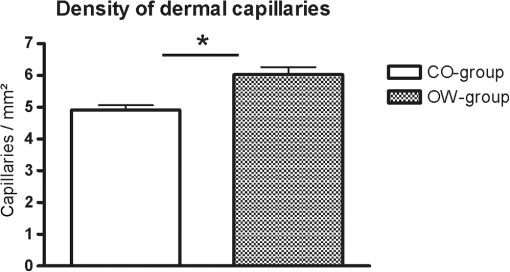
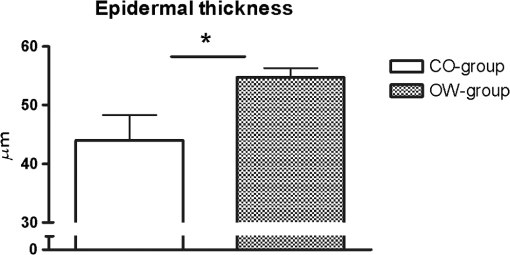
Fig. 1In horizontal, en face virtual sectioning, confocal imaging depicts the epidermal–dermal junction as bright circles of basal layer with the dark focus corresponding to the dermis. In the focus of the circles (dermal papillae), the lumina of capillary loops are visible as black holes (arrows). Blood cell flow can be clearly observed in real-time imaging as brightly reflecting erythrocytes circulating through the capillary loops. The field of vision measures . 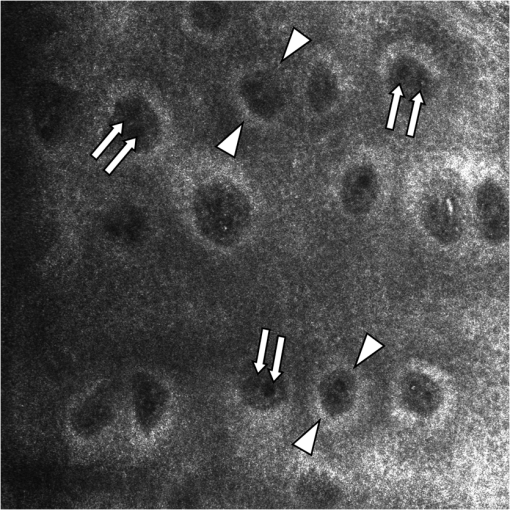 2.5.Statistical AnalysisThe RCM images were evaluated in a blinded manner using a free available image analysis program “ImageTool” (Version 3.0, UTHSCA, San Antonio, Texas).21 This software provides several image analysis tools, such as area or distance measurements. For statistical analysis SPSS version 16.0 (SPSS, SPSS Inc., Chicago, Illinois) software for Windows was used. The distribution of variables was tested for normality using Kolmogorov–Smirnov test. Comparisons between the groups were performed using independent -test (two-sided). -values below 0.05 were considered statistically significant. 3.ResultsBased on more than 4800 s real-time RCM imaging and evaluation of more than 160 offline confocal images following data were obtained. 3.1.Dermal Blood Cell FlowIn real-time RCM imaging, DBCF was counted at in OW-group and thus was over 19% higher compared to the controls at () (Fig. 2). These findings are based on counting more than 4500 blood cells. 3.2.Density of Dermal CapillariesDDC was determined at in OW-group and at in CO-group () (Fig. 3); thus capillary density was reduced over 22% in OW-group compared to the controls. 3.3.Epidermal ThicknessHistometric evaluation of the ET pointed to in OW-group, and thus reveals nearly 20% thicker epidermis in OW-group compared to the CO-group (, ) (Fig. 4). ET results are based on over 160 measurements in over 80 fields of view. 4.DiscussionThe main finding of the present study is that overweight, compared with leans, is characterized by both impaired microvascular function and remodeling of skin histomorphology. Precisely, analysis of in vivo human skin microcirculation with respect to blood flow and capillary density indicate an inversely correlation of overweight and dermal capillary density while blood cell flow was positively related to overweight. In addition, increase of both ET and cell size indicates significant changes of the human skin histomorphology in overweight. Hitherto, a range of modalities have been used to evaluate human skin perfusion focusing on either blood flow or capillary density. RCM, however, enables separate observation of both blood cell flow and functional capillary density by real-time visualization of individual blood cell circulation through dermal capillaries. Hence, effects of vasoconstriction and vasodilatation on blood flow are just as feasible as assessments of total area perfusion. In the past, the amount of weight and the associated microcirculatory disturbances were discussed controversially. Our results obtained in vivo using RCM are consistent with previous studies on impaired microvascular function in obesity,22 however, we could already observe comparable microvascular dysfunction in overweight. Moreover, our findings extend previous reports as we are able to demonstrate morphological changes of the skin in overweight, maybe as a result of the evaluated impaired tissue perfusion. Nestel et al.23 observed blood flow in the forearm microcirculation using plethysmography and found increased local sympathetic neuronal responsiveness and diminished nitric-oxide-mediated dilation in the forearm vasculature with increasing body adiposity. Our RCM results point to an increase of cutaneous blood cell flow of the forearm in accordance with the neuronal and mediator-related vasodilatation described by Nestel et al. In contrast, in a laser Doppler study, blood flow was reported to be reduced in forearm skin of obese nondiabetic individuals.24 However, laser Doppler measures the total local microcirculatory blood perfusion of the tissue and summarizes blood cell flow and density of perfused capillaries. Hence, this does not seem to be a real inconsistency for our findings, as we may confirm reduced total tissue perfusion in terms of capillary density in overweight. By means of intravital video microscopy, Francischetti et al.25 observed skin capillary density at rest and after postocclusive reactive hyperemia. The authors observed structural and functional alterations in skin microcirculation that are proportional to the increase in the degree of obesity. In a similar model, using video microscopy Czernichow et al.22 reported resting capillary density was negatively related with obesity. Hence, the authors suggest that a lower baseline tissue perfusion was evident in obesity. Nailfold capillary density was measured using capillaroscopy and it indicated similar regulatory mechanisms of the dermal capillaries with reduced capillary recruitment in obesity.26 Based on our in vivo RCM observations, we are able to confirm the regulation of dermal capillary density and consequently a lower total tissue perfusion even in overweight. In addition to the impaired skin microcirculation, our results strongly suggest that overweight is associated along with histomorphological remodeling of the skin as we could evaluate significant increase in both ET and cell size. Indeed, this is an interesting finding which has not been reported previously. It can only be speculated that the histomorphological changes observed are an early stage of adiposity-related skin conditions such as dermatitis, callus, corn, or acanthosis nigricans. The pathophysiological mechanism behind the histomorphological adaptation in overweight is probably multifactorial and based on hormonal, neural, and local control mechanisms similar to the cause of microvascular dysfunction. Moreover, it may be the result of a microvascular dysfunction itself. However, it should be emphasized that the design of the present study does not evaluate variables that may explain the relationship among these parameters. Moreover, the evaluated data of lean controls in this study are in accordance with previous reports on RCM measurements concerning skin microcirculation27 and skin morphometry.28 In conclusion, impaired local microvascular function along with histomorphological remodeling of the human skin was evaluated in overweight. Inversely correlation of dermal capillary density and overweight point to reduced total tissue perfusion while positively related blood cell flow indicate vasodilatation. In addition, increase of both ET and cell size reveals remodeling of the human skin histomorphology in overweight, maybe as an early stage of adiposity-related skin conditions. For the first time, the present study indicates the pathophysiological impact of overweight on both cutaneous microcirculation and histomorphology. Further RCM studies could be helpful to improve understanding of adiposity-related pathophysiological interactions by evaluating microcirculation and histomorphology for diverse degree of BMI. ReferencesF. H. Messerli,
“Cardiovascular effects of obesity and hypertension,”
Lancet, 319 1165
–1168
(1982). http://dx.doi.org/10.1016/S0140-6736(82)92234-6 Google Scholar
D. R. Krieger and L. Landsberg,
“Mechanisms in obesity-related hypertension: role of insulin and catecholamines,”
Am. J. Hypertens, 1 84
–90
(1988). http://dx.doi.org/10.1093/ajh/1.1.84 Google Scholar
C. Ferri et al.,
“Early upregulation of endothelial adhesion molecules in obese hypertensive men,”
Hypertension, 34 568
–573
(1999). http://dx.doi.org/10.1161/01.HYP.34.4.568 Google Scholar
R. S. Vasan,
“Cardiac function and obesity,”
Heart, 89 1127
–1129
(2003). http://dx.doi.org/10.1136/heart.89.10.1127 Google Scholar
L. A. Holowatz, C. S. Thompson-Torgerson and W. L. Kenney,
“The human cutaneous circulation as a model of generalized microvascular function,”
J. Appl. Physiol., 105 370
–372
(2008). http://dx.doi.org/10.1152/japplphysiol.00858.2007 Google Scholar
P. A. Carberry, A. M. Shepherd and J. M. Johnson,
“Resting and maximal forearm skin blood flows are reduced in hypertension,”
Hypertension, 20 349
–355
(1992). http://dx.doi.org/10.1161/01.HYP.20.3.349 Google Scholar
F. Khan et al.,
“Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease,”
Vasc. Med., 4 233
–238
(1999). http://dx.doi.org/10.1177/1358836X9900400405 Google Scholar
V. Urbancic-Rovan et al.,
“Macro- and microcirculation in the lower extremities—possible relationship,”
Diabetes Res. Clin. Pract., 73 166
–173
(2006). http://dx.doi.org/10.1016/j.diabres.2006.01.002 Google Scholar
V. Krejci, L. B. Hiltebrand and G. H. Sigurdsson,
“Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis,”
Crit. Care Med., 34 1456
–1463
(2006). http://dx.doi.org/10.1097/01.CCM.0000215834.48023.57 Google Scholar
L. A. Kirschenbaum et al.,
“Microvascular response in patients with cardiogenic shock,”
Crit. Care Med., 28 1290
–1294
(2000). http://dx.doi.org/10.1097/00003246-200005000-00005 Google Scholar
Y. Sakr et al.,
“Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock,”
Crit. Care Med., 32 1825
–1831
(2004). http://dx.doi.org/10.1097/01.CCM.0000138558.16257.3F Google Scholar
L. P. Kamolz et al.,
“Continuous free-flap monitoring with tissue-oxygen measurements: three-year experience,”
J. Reconstr. Microsurg., 18 487
–491
(2002). http://dx.doi.org/10.1055/s-2002-33319 Google Scholar
J. L. Cronenwett and S. M. Lindenauer,
“Direct measurement of arteriovenous anastomotic blood flow in the septic canine hindlimb,”
Surgery, 85 275
–282
(1979). Google Scholar
H. Knotzer et al.,
“Arginine vasopressin does not alter mucosal tissue oxygen tension and oxygen supply in an acute endotoxemic pig model,”
Intensive Care Med., 32 170
–174
(2006). http://dx.doi.org/10.1007/s00134-005-2858-z Google Scholar
M. Rajadhyaksha et al.,
“In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology,”
J. Invest. Dermatol., 113 293
–303
(1999). http://dx.doi.org/10.1046/j.1523-1747.1999.00690.x Google Scholar
S. Gonzalez et al.,
“In vivo reflectance-mode confocal scanning laser microscopy in dermatology,”
Adv. Dermatol., 20 371
–387
(2004). Google Scholar
A. A. Altintas et al.,
“Assessment of microcirculatory influence on cellular morphology in human burn wound healing using reflectance-mode-confocal microscopy,”
Wound Repair Regen., 17 498
–504
(2009). http://dx.doi.org/10.1111/wrr.2009.17.issue-4 Google Scholar
M. A. Altintas et al.,
“Insight in microcirculation and histomorphology during burn shock treatment using in vivo confocal-laser-scanning microscopy,”
J. Crit. Care, 25 173.e1
–173.e7
(2010). http://dx.doi.org/10.1016/j.jcrc.2009.03.003 Google Scholar
A. A. Altintas et al.,
“Histometric and histomorphologic comparison of combustion and ambustion using in vivo reflectance-confocal microscopy,”
Microsc. Res. Tech., 73
(2), 160
–164
(2010). http://dx.doi.org/10.1002/jemt.20770 Google Scholar
A. A. Altintas et al.,
“Local burn versus local cold induced acute effects on in vivo microcirculation and histomorphology of the human skin,”
Microsc. Res. Tech., 74 963
–969
(2011). http://dx.doi.org/10.1002/jemt.v74.10 Google Scholar
D. Wilcox et al.,
“Image Tool Version 3.0,”
(2002) http://ddsdx.uthscsa.edu/dig/itdesc.html February ). 2002). Google Scholar
S. Czernichow et al.,
“Microvascular dysfunction in healthy insulin-sensitive overweight individuals,”
J. Hypertens, 28 325
–332
(2010). http://dx.doi.org/10.1097/HJH.0b013e328333d1fc Google Scholar
P. J. Nestel et al.,
“Control of the forearm microcirculation: interactions with measures of obesity and noradrenaline kinetics,”
Clin. Sci., 95 203
–212
(1998). http://dx.doi.org/10.1042/cs0950203 Google Scholar
J. Doupis et al.,
“Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors,”
Obesity, 19 729
–735
(2010). http://dx.doi.org/10.1038/oby.2010.193 Google Scholar
E. A. Francischetti et al.,
“Skin capillary density and microvascular reactivity in obese subjects with and without metabolic syndrome,”
Microvasc. Res., 81 325
–330
(2011). http://dx.doi.org/10.1016/j.mvr.2011.01.002 Google Scholar
R. T. de Jongh et al.,
“Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance,”
Circulation, 109 2529
–2535
(2004). http://dx.doi.org/10.1161/01.CIR.0000129772.26647.6F Google Scholar
N. Scola, A. Goulioumis and T. Gambichler,
“Non-invasive imaging of mid-dermal elastolysis,”
Clin. Exp. Dermatol., 36
(2), 155
–160
(2011). http://dx.doi.org/10.1111/j.1365-2230.2010.03864.x10.1111/ced.2011.36.issue-2 Google Scholar
M. Huzaira et al.,
“Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy,”
J. Invest Dermatol., 116
(6), 846
–852
(2001). http://dx.doi.org/10.1046/j.0022-202x.2001.01337.x Google Scholar
BiographyAhmet A. Altintas is currently working as plastic, reconstructive, and hand surgeon at the Medical Center Cologne in Cologne, Germany. He studied medicine at Ruhr University of Bochum/Germany with periods in the University of Essen, Germany, and the University of Istanbul, Turkey. He received his medical doctor degree at the University of Heidelberg, Germany. His research interests include noninvasive imaging, content-based image retrieval, and angiogenesis induction. Matthias C. Aust is currently a senior consultant in the Department of Plastic, Reconstructive, and Aesthetic Surgery, Johanniter-Kliniken Bonn, Germany. He passed his medical exam and doctoral thesis with magna cum laude in 2004. He has worked in a number of renowned clinics for plastic and reconstructive surgery in the United States, Switzerland, South Africa, and Germany. His experimental research focuses on skin regeneration and rejuvenation by percutaneous collagen induction therapy and other strategies in antiaging medicine. Robert Krämer is currently working as a senior consultant in the Division of Plastic and Reconstructive Surgery, Universitätsklinikum Schleswig-Holstein, Lübeck, Germany. He passed the German Speciality Boards in plastic surgery in 2014. His scientific interests include microcirculation, in vivo medical imaging, and video processing. |