|
|
|
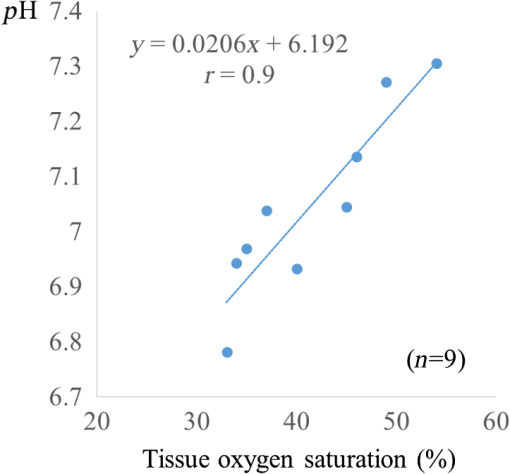
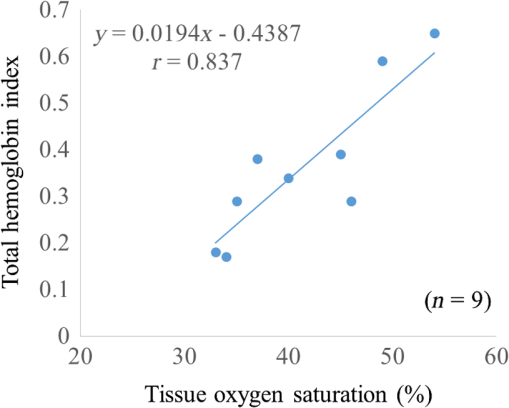
There are only a few methods for fetal assessment during intrapartum, including fetal heart rate (FHR) monitoring, ultrasound, and fetal scalp blood sampling (FSBS). FHR monitoring is the gold standard to evaluate fetal well-being because this examination is noninvasive for the fetus and pregnant woman. However, FHR monitoring has been shown to have a high number of false positive readings.1 This increases the rate of cesarean sections and instrumental labor, despite the fact that positive FHR monitoring may not be associated with fetal hypoxemia. To cover the drawbacks of FHR monitoring, obstetricians have performed FSBS during the intrapartum period. In 1962, Saling2 reported a fetal blood gas analysis used for assisting the evaluation of fetal well-being intrapartumally. A fetal pH value of is regarded as normoxemic status.3 A pH value of in the second stage of labor means hypoxemia, which requires an emergency intervention for rescuing from neonatal asphyxia.3 A meta-analysis reported that both FSBS and FHR monitoring during the intrapartum period resulted in avoiding unnecessary operative deliveries.4 The National Institute for Health and Care Excellence recommends FSBS to assess fetal status when FHR monitoring yields a positive result.5 Although FSBS is a good test for the management of labor, it is infrequently performed in clinical settings. Jørgensen and Weber4 revealed that the FSBS procedure was performed for 3.8% and 6% of cases in 2005 and 2011, respectively, for all full- and post-term deliveries in Denmark. Usually, FSBS for pH measurement requires 30 to of blood. Because FSBS is invasive for the fetus, many cases of complications have been reported.6–9 According to a recent case report, one serious complication, cerebrospinal fluid leakage from the incision site, occurred during FSBS.9 Kanayama and Niwayama10 have developed a smaller oximetry with the sensor attached to the examiner’s finger (KN-15, ASTEM, Kwasaki, Japan). This medical instrument consists of a probe with near-infrared spectroscopy (NIRS) and a numeral display device [Figs. 1(a) and 1(b)]. The size of the sensor in the probe is (Fig. 2). This probe is attached to the examiner’s finger [Fig. 1(a)]. Examiners can attempt to measure any sites of the human body, even the organs within a body cavity during surgery, because the device is small in size and portable. This oximetry can measure tissue oxygen saturation () and the absolute value of hemoglobin concentration. We have previously investigated the characteristics of this oximetry technique and clinical data obtained during labor.11 We concluded that the mean fetal for the fetal head during labor significantly correlated with umbilical cord arterial pH immediately after delivery. This result could potentially indicate that the measurement of fetal head can be substituted for an FSBS pH assessment. Therefore, using this noninvasive oximetry might dramatically change the management of labor. In the present study, we further investigated whether craniofacial using the oximetry with the sensor attached to the examiner’s finger is associated with blood pH in an animal model. Fig. 1Examiner’s finger-mounted tissue oximetry: (a) the measurement of the newborn and (b) the device displays in the upper area and T-HbI in the lower area. 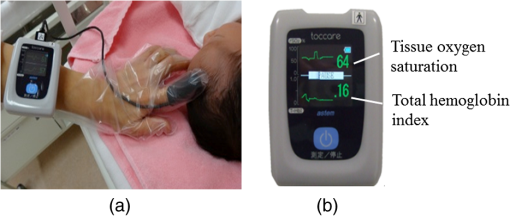 Fig. 2Flexible substrate mounted with two LEDs, and two photodiodes adhered to a black rubber sheet. 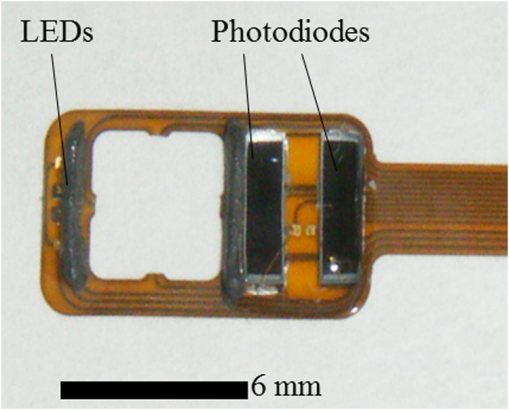 The wavelengths of the light sources were 770 and 810 nm, and the sensitivity of the photodiodes (PD2501, Epitex Co., Ltd., Kyoto, Japan) was high in the near-infrared band. The bare chips of the light-emitting diodes (LEDs) and photodiodes were mounted on the substrate with a wire bonding. The detectors were located 6 and 8 mm away from the LEDs to determine the absolute value of hemoglobin concentration using the laboratory-made spatially resolved NIRS (Fig. 2). This study has been conducted in compliance with the Animal Research Committee, Hamamatsu University School of Medicine, and the principles of the Guide for the Care and Use of Laboratory Animals: 8-week-old ICR female mice, weighing 29 to 31 g, were obtained from Japan SLC Inc. (Hamamatsu, Japan) for this study. They were housed individually in a specific pathogen-free facility. The hair on the forehead of the mice was cut short to decrease the influence of hair in oximetry measurement. At first, every mouse was measured at the craniofacial site by the oximetry at 21% of oxygen concentration (indoor oxygen content) and in the range of 24.0°C to 24.5°C. One mouse was placed in a cage (CL-0113-4; width 136 mm, depth 208 mm, height 115 mm, CLEA Japan Inc., Tokyo, Japan), with some blocks of dry ice (solidified carbon dioxide), and an oxygen concentration meter (XP-3180E, New Cosmos Electric Co., Ltd., Osaka, Japan) was enclosed in a polyethylene bag (width 65 cm and depth 80 cm). Examiners detected the decrease in the oxygen concentration and measured the and total hemoglobin index (T-HbI) at the craniofacial site in the polyethylene bag, preventing the entry of any outside air as much as possible. After a mouse was measured one to four times in the polyethylene bag, examiners immediately cut the facial vessel using a lancet and collected the blood using a capillary tube (at least three drops). Immediately after blood collection, a sample for pH measurement was evaluated using the handheld i-STAT®1 Analyzer (Abbot Laboratories, East Windsor, New Jersey) and EG6+ cartridge (Abbott Laboratories, East Windsor, New Jersey). Examiners did not investigate the and T-HbI using oximetry again once the blood sample was collected. The value of the and T-HbI was investigated three times until the numeric data stabilized. A median value from all the measurement data was selected as a numeric value for the data under the conditions of oxygen concentration. Statistical significance was calculated using a Spearman’s correlation coefficient. A value of was considered statistically significant. A total of 11 mice underwent measurement using the oximetry and their blood was collected. Eleven mice were measured for the and T-HbI under the changing oxygen concentrations. A total of nine blood samples were collected and their pH was analyzed; two samples could not be measured because of insufficient blood volume. The range of the oxygen gas concentration in the polyethylene bag was 16.7% to 21%, detected in the blood of the analyzed mice using an oxygen concentration meter (). The oxygen concentration and were positively correlated (Fig. 3; , , ). A correlation was strongly exhibited between the and pH in mice (Fig. 4; , , ). The and T-HbI also had a strong positive correlation (Fig. 5; , , ). Fig. 3There is a significant correlation between oxygen concentration and in the closed polyethylene bag. 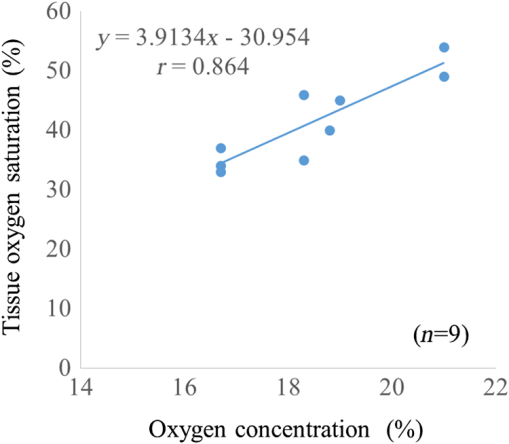 The present study is the first report of an animal experiment using this small, portable device with NIRS. The existing oximeters for human are attached to the head or muscle and are relatively large in size. This new oximeter can measure considerably small sites, such as the forehead and face in mice if they remain immobile. After examiners with the sensor probe had touched the targets, the numerical data on the monitor screen stabilized within 10 s. Because the power source of this device is supplied by two dry batteries, wiring with an external power source is unnecessary. Using this oximeter, we have previously reported that our fetal reflects the contributions of approximately two-thirds of the fetal brain and one-third of the scalp in the measurement of the fetus during labor.7 This source–detector distance provides a depth of 3.0 to 4.0 mm. Because it is relatively easy to obtain blood samples from facial vessels in a mouse, we attempted to attach the probe to the mouse’s facial site in the present study. We believed that this source–detector distance would provide the thickness of the mouse’s facial skin (0.5 mm) and subcutaneous tissue (2.5 mm). Craniofacial tissue consists of skin, muscles (masseters and temporalizes), brain with skull, and connective tissues. Because the length of the cutting edge in the lancet that was used in this study was 3.0 mm, we thought that the depth of tissue measured using this oximetry and the depth of tissue at which blood was collected with a lancet were similar. The mouse has a very narrow face, and the data obtained from our oximetry might provide mixed information regarding facial tissue and cerebral tissue. Because the values of and T-HbI are slightly reduced without cutting the hair, we cut the hair on the foreheads of the mice in the present study. One limitation of this study was that we could not analyze two blood samples. One blood sample was thick and viscous; this abnormal blood coagulation might have occurred because of 12.4% oxygen concentration. A technical mistake occurred while collecting the other blood sample. No matter how excellent the examinations for fetal evaluations are, invasive tests such as FSBS are not extensively popular worldwide.4 The results of this study demonstrate that the and tissue blood pH in mice exhibit a strong positive correlation. Thus, the results of the present study support the conclusion of the previous report that during labor is associated with umbilical arterial blood pH. We assume that the correlation between the and tissue blood pH can be explained by the Bohr effect (i.e., hemoglobin easily releases oxygen when the blood pH decreases).12 Furthermore, T-HbI is useful for determining tissue ischemia and congestion,13 and correlated with in the present study. Under low oxygen concentrations in mice, the absolute volume of the blood might decrease in the craniofacial section because blood perfusion decreased due to the hypometabolic state. Obstetricians have managed to take care of the fetus with a few limited examinations. This oximetry would be a breakthrough device for the early detection of fetal asphyxia. Obstetricians hesitate to perform FSBS in most cases because FSBS is an invasive procedure. If this oximeter is used during labor, obstetricians can attempt to check the status of fetal acidosis at several time intervals. Because the present study evaluated only a small number of animal cases, the validity of these conclusions is another limitation. However, we demonstrate that craniofacial is associated with blood pH using an examiner’s finger-mounted tissue oximetry in mice. Larger clinical trials using this oximetry are required to confirm our findings. We are confident that our small oximetry has the potential to become a new fetal monitoring approach in a clinical setting. AcknowledgmentsThe authors thank the following for their excellent technical support: Mr. Hikaru Suzuki, Mr. Yutaka Yokoyama, Yukio Chitose, and Ms. Aya Takiguchi (ASTEM Co., Ltd., Kawasaki, Japan) and Ms. Miuta Sawai and Ms. Urmi Jeenat Ferdous (Department of Obstetrics and Gynecology, Hamamatsu University School of Medicine). We acknowledge the financial support provided by MEXT KAKENHI, Grant No. 15K10667. ReferencesC. Signore, R. K. Freeman and C. Y. Spong,
“Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop,”
Obstet. Gynecol., 113
(3), 687
–701
(2009). http://dx.doi.org/10.1097/AOG.0b013e318197bd8a Google Scholar
E. Saling,
“A new method for examination of the child during labor. Introduction, technique and principles,”
Arch. Gynakol., 197 108
–122
(1962). http://dx.doi.org/10.1007/BF02590014 Google Scholar
A. Huch et al.,
“Guidelines for blood sampling and measurement of pH and blood gas values in obstetrics. Based upon a workshop held in Zurich, Switzerland, March 19, 1993 by an Ad Hoc Committee,”
Eur. J. Obstet. Gynecol. Reprod. Biol., 54
(3), 165
–175
(1994). http://dx.doi.org/10.1016/0028-2243(94)90277-1 Google Scholar
J. S. Jørgensen and T. Weber,
“Fetal scalp blood sampling in labor—a review,”
Acta Obstet. Gynecol. Scand., 93
(6), 548
–555
(2014). http://dx.doi.org/10.1111/aogs.12421 Google Scholar
“Intrapartum care for healthy women and babies,”
National Institute for Health and Care Excellence, Guidance, RCOG Press, London
(2014). Google Scholar
G. H. Nelson et al.,
“A complication of fetal scalp blood sampling,”
Am. J. Obstet. Gynecol., 110
(5), 737
–738
(1971). Google Scholar
H. D. Modanlou and E. M. Linzey,
“An unusual complication of fetal blood sampling during labor,”
Obstet. Gynecol., 51
(Suppl. 1), 7s
–8s
(1978). Google Scholar
L. L. Reti et al.,
“Excessive bleeding from fetal scalp blood sampling,”
Aust. N. Z. J. Obstet. Gynaecol., 20
(1), 55
–57
(1980). http://dx.doi.org/10.1111/j.1479-828X.1980.tb00897.x Google Scholar
T. P. Schaap et al.,
“Cerebrospinal fluid leakage, an uncommon complication of fetal blood sampling: a case report and review of the literature,”
Obstet. Gynecol. Surv., 66
(1), 42
–46
(2011). http://dx.doi.org/10.1097/OGX.0b013e318213e644 Google Scholar
N. Kanayama and M. Niwayama,
“Examiner’s finger-mounted fetal tissue oximetry,”
J. Biomed. Opt., 19
(6), 067008
(2014). http://dx.doi.org/10.1117/1.JBO.19.6.067008 Google Scholar
T. Uchida et al.,
“Examiner’s finger-mounted fetal tissue oximetry: a preliminary report on 30 cases,”
J. Perinat. Med.,
(2015). http://dx.doi.org/10.1515/jpm-2014-0297 Google Scholar
Y. Sakai et al.,
“A novel system for transcutaneous application of carbon dioxide causing an “artificial Bohr effect” in the human body,”
PLoS One, 6
(9), e24137
(2011). http://dx.doi.org/10.1371/journal.pone.0024137 Google Scholar
A. Ebihara et al.,
“Detection of cerebral ischemia using the power spectrum of the pulse wave measured by near-infrared spectroscopy,”
J. Biomed. Opt., 18
(10), 106001
(2013). http://dx.doi.org/10.1117/1.JBO.18.10.106001 Google Scholar
|