|
|
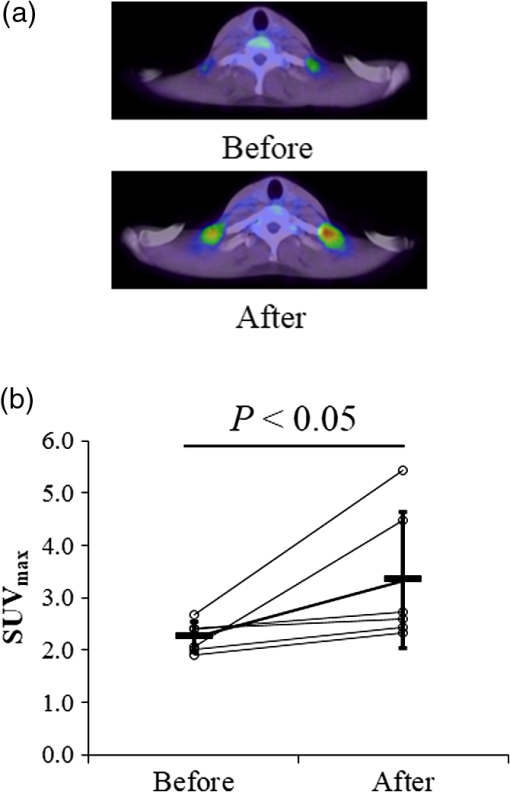
1.IntroductionBrown adipose tissue (BAT) is known to play a critical role in cold-induced nonshivering thermogenesis (CIT) to maintain body temperature.1 In adult humans, metabolically active BAT is potentially identified in the supraclavicular region by using -fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT).2–5 Histological examination confirmed FDG deposits to be BAT. Recent studies using FDG-PET/CT have revealed that BAT is involved in adaptive energy expenditure, thereby contributing to the regulation of body fat.5,6 Moreover, BAT is also suggested to participate in glucose homeostasis7–10 and to improve blood lipid profiles11 in humans. Thus, BAT is expected to be a therapeutic target for obesity and related metabolic disorders in humans.12,13 BAT activity and/or mass can be quantified by FDG-PET/CT after acute cold exposure, which is widely used as a standard method in humans. However, the FDG-PET/CT method has serious limitations, such as its inaccessibility, enormous cost, and patient exposure to ionizing radiation and uncomfortable cold. Particularly, inevitable radiation exposure makes it difficult to evaluate BAT repeatedly in the same subjects/patients in a longitudinal study. Recently, magnetic resonance imaging (MRI)14 and enhanced contrast ultrasonography15 were reported as less-invasive methods for the evaluation of BAT, but these are also limited by inaccessibility, enormous cost, and uncomfortable acute cold exposure. Recently, we demonstrated in healthy humans that total hemoglobin concentration ([total-Hb]), evaluated by near-infrared time-resolved spectroscopy () under thermoneutral conditions (i.e., without cold exposure), is positively correlated with FDG-PET/CT indices only in the supraclavicular region; which potentially contains BAT deposits.16 Considering abundant vascularity of BAT compared with other tissues,16 our results suggest that [total-Hb] estimated by is an index of BAT density. Although the method does not precisely specify an area of BAT location but instead provides an tissue focus, it is noninvasive, simple, inexpensive, and free of radiation exposure for evaluating tissue oxygenation in humans.17,18 Collectively, the method is expected to be suitable for evaluating BAT density in humans, particularly in longitudinal studies. To test this, in the present study, using the method we examined the changes in BAT induced by daily ingestion of thermogenic capsaicin-like compounds, capsinoids, which are known to activate and recruit BAT.13 2.MethodsHealthy volunteer subjects were recruited and given capsinoids every day for 6 to 8 weeks. Before and after the treatment, their BAT activity/density was assessed by FDG-PET/CT (Experiment 1) or (Experiment 2). The study design and protocols were approved by the Institutional Review Board of Ritsumeikan University and Tenshi College, in accordance with the ethical principles contained in the Declaration of Helsinki. Written informed consent was obtained from all participants. These studies were conducted from December 2014 to March 2015, the winter season in Japan. 2.1.SubjectsIn Experiment 1, three healthy males (24- to 30-years old) were recruited by direct contact. In an independent Experiment 2, 10 healthy male and 10 healthy female college students were recruited by advertising on posters or by direct contact (Table 1). The participants were randomly allocated to the capsinoids or placebo group by a third party who did not participate in this study. Table 1Anthropometric parameters and blood pressure before and after the 8-week treatment, and after 8 weeks of follow-up period.
BMI, body mass index; VFA, visceral fat area; SFA, subcutaneous fat area; SBP, systolic blood pressure; DBP, diastolic blood pressure. 2.2.CapsinoidsCapsinoids were extracted from CH-19 Sweet (Capsicum annuum L); consisted of capsiate, dihydrocapsiate, and nordihydrocapsiate in a ratio; and were provided by Ajinomoto Co., Inc. (Tokyo, Japan). Each capsule contained 0 or 1.5 mg of capsinoids and 199 mg of a mixture of rapeseed oil and medium-chain triglycerides.13,19 Participants were instructed to take three capsules in each morning and evening of each day13 for 6 weeks (Experiment 1) or 8 weeks (Experiment 2). 2.3.Study DesignIn Experiment 1, three male subjects were given six capsules containing 1.5 mg of capsinoids each day for 6 weeks. Before and after the treatment, their BAT activity was assessed by FDG-PET/CT. In Experiment 2, 10 male and 10 female subjects were given either capsinoid () or placebo capsules each day for 8 weeks in a double-blind design. Before and after the 8-week treatment, their anthropometric and circulatory parameters were measured. In addition, BAT density was measured every 2 weeks by (Fig. 1). These parameters were measured again 8 weeks after stopping the capsinoid intake (follow-up period). The subjects were instructed to maintain their usual dietary intake and physical activity during the experimental period, and to record a dietary diary during the 16-week period. 2.4.OutcomesThe primary endpoint was the change in BAT density evaluated by [total-Hb] using after 8 weeks of capsinoid-treatment, and the secondary one was that after cessation of the treatment. An additional endpoint was the capsinoid-induced increase in BAT activity assessed by FDG-PET/CT. 2.5.-fluorodeoxyglucose Positron Emission Tomography Combined with Computed TomographyFDG-PET/CT was performed as described previously.2 Briefly, after overnight fasting for , the subjects were exposed to cold by being kept in an air-conditioned room at 19°C with standardized light clothing (a patient’s gown), and intermittently placed their feet on an ice block wrapped in cloth for every 5 min to avoid cooling-associated pain. After 1 h, under these cold conditions, each was given an intravenous injection of -FDG (1.66 to 5.18 megaBecquerel () (MBq)/kg body weight) and kept under the same cold conditions. One hour after the -FDG injection, FDG-PET/CT scans were performed by using a PET/CT system (Aquiduo, Toshiba Medical Systems, Otawara, Japan). BAT activity in the supraclavicular fat deposits was quantified by calculating the maximal standardized uptake value of FDG (), defined as the radioactivity per milliliter within the region of interest divided by the injected dose in MBq/g of body weight. 2.6.Near-Infrared Time-Resolved SpectroscopyThe [total-Hb] was measured using (TRS-20; Hamamatsu Photonics K.K., Hamamatsu, Japan) for 5 min at 27°C by placing the probes on the skin of the supraclavicular region potentially containing BAT deposits; and, as a reference, also in the deltoid muscle region, which is separated from the BAT deposits in the right side. The distance between the emitter and detector was set at 30 mm.16 The tissue was illuminated with a core diameter optical fiber using pulsed light generated from picosecond light pulsers at 760, 800, and 830 nm with 100 ps full width at half-maximum, a 5-MHz repetition rate, and an average power of each wavelength. The emitted photons penetrated the tissue and were reflected to a 3-mm diameter optical bundle fiber, through which they were sent to a photomultiplier tube for single-photon detection and a signal processing circuit for time-resolved measurement. Using the nonlinear least-squares method, the digitized temporal profile data from an in vitro sample or tissue were fitted with a theoretical temporal profile derived from the analytical solution of photon diffusion theory with a semi-infinite homogeneous model in reflectance mode. After convolution with the instrumental response function such that the time response of the instrument itself could be compensated, values for absorption coefficient and reduced scattering coefficient at 760, 800, and 830 nm were obtained. Then the absolute concentrations of [total-Hb] were determined using a least-squares fitting method.18 The system provided data every 10 s. The coefficient of variation for repeated measurements of [total-Hb] was 4.9%.16 2.7.Anthropometric and Circulatory Parameter MeasurementsThe body mass, fat mass, percent body fat, lean body mass, and bone mass were evaluated by a dual-energy x-ray absorptiometry scan (DXA, Lunar Prodigy; GE Healthcare, Buckinghamshire, UK). The visceral fat area (VFA) and subcutaneous fat area (SFA) at the abdominal level of L4–L5 were estimated using 1.5-T MRI (Signa HDxt; GE Healthcare, Buckinghamshire, UK). During DXA measurements, subjects maintained a supine position. Then a series of transaxial MRI scans of abdominal sections were acquired (, , , ). The images were exported and analyzed by the same investigator using an image analysis software program (SliceOmatic 4.3; Tomovision Inc., Magog, Canada). Subcutaneous fat thickness was measured by B-mode ultrasonography (SSD-3500SV; Hitachi Aloka Medical Co., Ltd, Tokyo, Japan) at the supraclavicular region potentially containing BAT and the deltoid muscle region, which is separated from BAT deposits. During ultrasonographic measurements, subjects maintained the same posture as during the measurement. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured using an automated sphygmomanometer (HBP-9020; Omron Corp., Kyoto, Japan) after resting for 10 min. 2.8.Dietary Diary and Records of IntakesDietary habits during the preceding month were assessed using a validated, brief, self-administered diet history questionnaire that contained questions about the consumption frequency of 56 foods and beverages and nine dishes that are commonly consumed in the general Japanese population. Daily intakes of energy, protein, fat, and carbohydrate were calculated20 before and after the 8-week treatment period, and after the 8-week follow-up period. Daily steps and activity energy expenditure were estimated using pedometers (Omron Health Counter HJ-710IT; Omron Healthcare, Kyoto, Japan), and the mean for 7 days was evaluated before and after the 8-week treatment period, and after the 8-week follow-up period. 2.9.Statistical AnalysesData are expressed as . In Experiment 1, to compare the before and after the 6-week period, Wilcoxon signed-rank testing was conducted after results from the Shapiro–Wilk test proved significant. In Experiment 2, a two-way analysis of variance with repeated measures was used to test the interaction () and the main effects (group and time). If there was a significant interaction or main effect, the time or group differences of the variables were analyzed using the paired or unpaired -test, respectively. Values were considered to be statistically significant if was . All statistical analyses were performed using SPSS version 19 (Chicago, Illinois). 3.Results3.1.Experiment 1Three male subjects were given 9 mg of capsinoids every day for 6 weeks, and their BAT activity was assessed by FDG-PET/CT. Figure 2 shows a typical FDG-PET/CT image in the supraclavicular region before and after the 6-week treatment. The calculated in both sides was increased by 48.8% ( versus , ) by the treatment, being consistent with our previous results13 that the daily ingestion of capsinoids recruits BAT. 3.2.Experiment 2Twenty subjects were randomly divided into two groups, and given either 9 mg of capsinoids or placebo for 8 weeks. Before and after the 8-week period, there were no significant changes, either in the anthropometric or circulatory parameters (Table 1) or for the physical activity levels (steps and physical activity, energy expenditure) or dietary intake (energy, fat, protein, and carbohydrate intake) (data not shown). No apparent harmful incidents were observed in any individuals in the present study. Figure 3 shows the [total-Hb] assessed by . There was a significant main effect of group on [total-Hb] in the supraclavicular region close to BAT deposits [Fig. 3(a)], but not in the deltoid muscle region separated from BAT deposits [Fig. 3(b)]. In the supraclavicular region, [total-Hb] increased by 46.4% after the 8-week capsinoid treatment ( versus ; ), despite large interindividual variations [Fig. 3(c)], while it did not change after the placebo treatment ( versus ; ) [Fig. 3(d)]. In contrast, the individual data of the deltoid muscle region separated from BAT deposits were stable in both the capsinoid [Fig. 3(e)] and placebo groups [Fig. 3(f)]. In the supraclavicular region, the change in [total-Hb] during the 8-week period was significantly greater in the capsinoid group than in the placebo group [Fig. 3(g)]. After stopping the capsinoid treatment, [total-Hb] in the supraclavicular region tended to decrease by 12.5%. The change in [total-Hb] during the 8-week follow-up period was insignificantly larger () in the capsinoids group than in the placebo group [Fig. 3(h)]. Fig. 3Total hemoglobin concentration [total-Hb] in (a) the supraclavicular region potentially containing brown adipose tissue and (b) the deltoid muscle region separated from brown adipose tissue deposits. [total-Hb] in the supraclavicular region of individual subjects treated with (c) capsinoids and (d) placebo. [total-Hb] in the deltoid muscle region of individual subjects treated with (e) capsinoids and (f) placebo. (g) Changes in [total-Hb] after the 8-week treatment. (h) Changes in [total-Hb] after stopping the treatment. 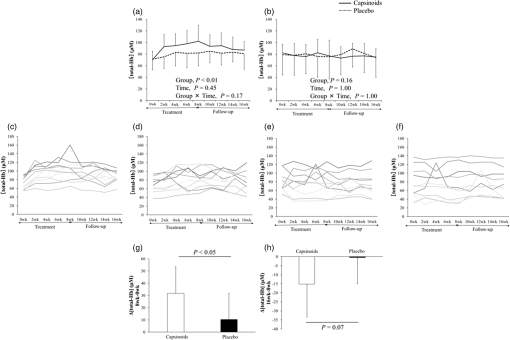 4.DiscussionIn this study, first, we confirmed, by FDG-PET/CT, increased BAT activity after daily ingestion of capsinoids by healthy humans. Then, we found that the capsinoid-induced increase in [total-Hb], a potential parameter for evaluating BAT vascularity, could be continuously monitored by . Capsinoids, such as capsiate, are capsaicin-like compounds found in a nonpungent type of red pepper called “CH-19 Sweet.”13,19,21 Capsinoids are known to have similar physiological effects to capsaicin. Animal studies have shown that capsinoids activate transient potential vanilloid 1 receptors in the gut,22,2324 which in turn increase BAT thermogenesis and body fat mobilization via the sympathetic nervous system.23,24 Similar thermogenic effects of capsinoids were also found in humans: that is, single oral ingestion of capsinoids increases whole-body energy expenditure in subjects with active BAT, but not in those without it.25 Moreover, it was also reported that daily ingestion of capsinoids for 6 weeks resulted in an increased CIT.13 These results suggest that capsinoids not only activate but also recruit BAT in humans. Consistent with these previous findings, in Experiment 1 of the present study, FDG-PET/CT revealed a significant increase in BAT in the supraclavicular region after the 6-week capsinoid treatment. We reported previously a significant relationship between BAT density as evaluated by [total-Hb] in and BAT activity as evaluated by in FDG-PET/CT.16 Thus, it was rational to expect that the capsinoid-induced change in BAT would be detected by . In fact, in Experiment 2 of the present study, we found that [total-Hb] in the supraclavicular region close to BAT deposits increased significantly after the 8-week capsinoid treatment, while it did not change after the placebo treatment. In contrast, no notable change was found in [total-Hb] in the deltoid muscle region separated from BAT deposits. Although the period of capsinoid treatment was different in the two experiments (6 and 8 weeks), the increases in [total-Hb] and were almost similar (48.8% and 46.4%, respectively), supporting again our previous idea that [total-Hb] is an index of BAT density. We also found that [total-Hb] tended to decrease during the 8-week follow-up period after the capsinoid treatment. As there was no notable change in the lifestyle such as food intake or physical activity of the participants during the 16-week test period, the change in [total-Hb] would be attributable to capsinoid ingestion. Taken together, the change in [total-Hb] evaluated by reflects those in BAT density induced by daily ingestion of capsinoids. It is thus evident that is a useful method for evaluating BAT density in humans, particularly in longitudinal intervention studies. There are two distinct types of brown adipocyte: the classical brown adipocyte derived from the Myf-5 cell, and the beige adipocyte transformed from the white adipocyte in response to sympathetic stimulation.26,27 Based on the gene expression pattern, BAT in the supraclavicular region in adult humans was suggested to be mainly composed of beige adipocytes.27 It is to be noted, however, that neither nor FDG-PET/CT can distinguish these two types of adipocyte, and that BAT detected by these methods may contain both types of adipocytes. In the present study, body composition did not change in the capsinoid group, although VFA tended to decrease. This conflicts with previous studies showing a significant reduction in VFA after prolonged ingestion of capsinoids in humans.19,28 This may be due to the difference in the adiposity of participants between the studies: i.e., the participants in the previous studies were obese, while ours were lean. Metabolically, it might be easier to induce a reduction in excess body fat in over-fat participants than it is to induce a reduction in body fat in healthy, lean persons possessing body fat levels within the physiologically healthy range. We reported previously that subcutaneous fat thickness affects NIR signal sensitivity.18 In our present studies, subcutaneous fat thickness in the supraclavicular region did not change during the testing period, supporting the observation that changes in [total-Hb] reflect those in BAT density more than those in subcutaneous fat. 5.ConclusionThe present study demonstrated a parallel change in BAT density, evaluated as [total-Hb] by or BAT activity evaluated as by FDG-PET/CT, after daily ingestion of thermogenic capsinoids in healthy humans, suggesting that is suitable for assessment of human BAT, particularly in longitudinal intervention studies where FDG-PET/CT is difficult to use. Because, in this study, the parameters were obtained from participants who did not undergo FDG-PET/CT, simultaneous assessment by the two methods would be helpful to further confirm our conclusion. AcknowledgmentsThis work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant No. 15H03100). We also thank Ajinomoto Co., Inc. (Tokyo, Japan) for support in funding and for providing the capsinoids used. ReferencesB. Cannon and J. Nedergaard,
“Brown adipose tissue: function and physiological significance,”
Physiol. Rev., 84
(1), 277
–359
(2004). http://dx.doi.org/10.1152/physrev.00015.2003 Google Scholar
M. Saito et al.,
“High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity,”
Diabetes, 58
(7), 1526
–1531
(2009). http://dx.doi.org/10.2337/db09-0530 Google Scholar
A. M. Cypess et al.,
“Identification and importance of brown adipose tissue in adult humans,”
N. Engl. J. Med., 360
(15), 1509
–1517
(2009). http://dx.doi.org/10.1056/NEJMoa0810780 Google Scholar
K. A. Virtanen et al.,
“Functional brown adipose tissue in healthy adults,”
N. Engl. J. Med., 360
(15), 1518
–1525
(2009). http://dx.doi.org/10.1056/NEJMoa0808949 Google Scholar
W. D. van Marken Lichtenbelt et al.,
“Cold-activated brown adipose tissue in healthy men,”
N. Engl. J. Med., 360
(15), 1500
–1508
(2009). http://dx.doi.org/10.1056/NEJMoa0808718 Google Scholar
T. Yoneshiro et al.,
“Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men,”
Obesity, 19
(1), 13
–16
(2011). http://dx.doi.org/10.1038/oby.2010.105 Google Scholar
M. Chondronikola et al.,
“Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans,”
Diabetes, 63
(12), 4089
–4099
(2014). http://dx.doi.org/10.2337/db14-0746 DIAEAZ 0012-1797 Google Scholar
P. Lee et al.,
“Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans,”
Diabetes, 63
(11), 3686
–3698
(2014). http://dx.doi.org/10.2337/db14-0513 DIAEAZ 0012-1797 Google Scholar
M. Matsushita et al.,
“Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans,”
Int. J. Obes., 38
(6), 812
–817
(2014). http://dx.doi.org/10.1038/ijo.2013.206 Google Scholar
M. J. Hanssen et al.,
“Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus,”
Nat. Med., 21
(8), 863
–865
(2015). http://dx.doi.org/10.1038/nm.3891 Google Scholar
S. Ozguven et al.,
“The role of active brown adipose tissue in human metabolism,”
Eur. J. Nucl. Med. Mol. Imaging, 43
(2), 355
–361
(2016). http://dx.doi.org/10.1007/s00259-015-3166-7 CECED9 Google Scholar
T. Yoneshiro and M. Saito,
“Activation and recruitment of brown adipose tissue as anti-obesity regimens in humans,”
Ann. Med., 47
(2), 133
–141
(2015). http://dx.doi.org/10.3109/07853890.2014.911595 Google Scholar
T. Yoneshiro et al.,
“Recruited brown adipose tissue as an antiobesity agent in humans,”
J. Clin. Invest., 123
(8), 3404
–3408
(2013). http://dx.doi.org/10.1172/JCI67803 Google Scholar
H. H. Hu et al.,
“MRI detection of brown adipose tissue with low fat content in newborns with hypothermia,”
Magn. Reson. Imaging, 32
(2), 107
–17
(2014). http://dx.doi.org/10.1016/j.mri.2013.10.003 Google Scholar
A. Flynn et al.,
“Contrast-enhanced ultrasound: A novel noninvasive, nonionizing method for the detection of brown adipose tissue in humans,”
J. Am. Soc. Echocardiogr., 28
(10), 1247
–1254
(2015). http://dx.doi.org/10.1016/j.echo.2015.06.014 Google Scholar
S. Nirengi et al.,
“Human brown adipose tissue assessed by simple noninvasive near-infrared time-resolved spectroscopy,”
Obesity, 23
(5), 973
–980
(2015). http://dx.doi.org/10.1002/oby.21012 Google Scholar
M. Ferrari, M. Muthalib and V. Quaresima,
“The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments,”
Philos. Trans. A Math Phys. Eng. Sci., 369
(1955), 4577
–4590
(2011). http://dx.doi.org/10.1098/rsta.2011.0230 Google Scholar
T. Hamaoka et al.,
“Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans,”
J. Biomed. Opt., 12
(6), 062105
(2007). http://dx.doi.org/10.1117/1.2805437 Google Scholar
N. Inoue et al.,
“Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids),”
Biosci. Biotechnol. Biochem., 71
(2), 380
–389
(2007). http://dx.doi.org/10.1271/bbb.60341 Google Scholar
N. Sugawara et al.,
“Dietary patterns are associated with obesity in Japanese patients with schizophrenia,”
BMC Psychiatry, 14 184
(2014). http://dx.doi.org/10.1186/1471-244X-14-184 Google Scholar
K. Kobata et al.,
“Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, capsicum annuum,”
J. Nat. Prod., 62
(2), 335
–336
(1999). http://dx.doi.org/10.1021/np9803373 Google Scholar
I. Sasahara et al.,
“Assessment of the biological similarity of three capsaicin analogs (Capsinoids) found in non-pungent chili pepper (CH-19 Sweet) fruits,”
Biosci. Biotechnol. Biochem., 74
(2), 274
–278
(2010). http://dx.doi.org/10.1271/bbb.90570 Google Scholar
T. Iida et al.,
“TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate,”
Neuropharmacology, 44
(7), 958
–967
(2003). http://dx.doi.org/10.1016/S0028-3908(03)00100-X NEPHBW 0028-3908 Google Scholar
I. Ono et al.,
“Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses,”
J. Appl. Physiol., 110
(3), 789
–798
(2011). http://dx.doi.org/10.1152/japplphysiol.00128.2010 Google Scholar
T. Yoneshiro et al.,
“Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans,”
Am. J. Clin. Nutr., 95
(4), 845
–850
(2012). http://dx.doi.org/10.3945/ajcn.111.018606 Google Scholar
I. Wu et al.,
“Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human,”
Cell., 150
(2), 366
–376
(2012). http://dx.doi.org/10.1016/j.cell.2012 Google Scholar
L. Z. Sharp et al.,
“Human BAT possesses molecular signatures that resemble beige/brite cells,”
PloS One., 7
(11), e49452
(2012). http://dx.doi.org/10.1371/journal.pone.0049452 POLNCL 1932-6203 Google Scholar
S. Snitker et al.,
“Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications,”
Am. J. Clin. Nutr., 89
(1), 45
–50
(2009). http://dx.doi.org/10.3945/ajcn.2008.26561 Google Scholar
BiographyShinsuke Nirengi is a postdoctoral researcher at the National Hospital Organization Kyoto Medical Center. He received his PhD degree in sport and health science from the Ritsumeikan University in 2015. His research interests include brown adipose tissue, exercise physiology, and biomedical optics. Toshiyuki Homma is an associate professor at the Faculty of Sports and Health Science, Daito Bunka University. He has received a Young Investigators Award, 8th Congress of European College of Sports Science in 2003. He received his PhD degree from the Tokyo Medical University in 2005. His specialization is exercise physiology. His current major research interest is effective training for sports performance and health from a perspective of muscle energy metabolism. Naohiko Inoue received his MSc and PhD degrees in food science and biotechnology from Kyoto University, Japan, in 2001 and 2004, respectively. He is currently working as a researcher at Ajinomoto Co., Inc., which is a food company in Japan. His research interest is functional effects of foods, including enhancement of brown adipose tissue. Hitoshi Sato received his PhD degree in nutrition from Tohoku University, Japan, in 2010. He is currently in charge of academic public relations in Ajinomoto Co., Inc., which is a food company in Japan. His research interest is functional effects of foods, including enhancement of brown adipose tissue. Takeshi Yoneshiro is a postdoctoral fellow at the Hokkaido University Graduate School of Veterinary Medicine. His research interests include the control of energy expenditure and adiposity with special reference to metabolic function of brown adipose tissue in humans. Mami Matsushita received her PhD in nutrition from Tenshi College, Japan, in 2015. She is an assistant professor and registered dietitian in the Department of Nutrition, School of Nursing and Nutrition Tenshi College. Her research interests include brown adipose tissue and obesity-related diseases prevention. Hiroki Sugie received his MD from Asahikawa Medical University in 1983. He is the president of the LSI Sapporo Clinic. Kokoro Tsuzaki has taken the program of the life science specialty in Tottori University Graduate School of Medicine and is now the certified clinical chemist (Japan Society of Clinical Chemistry). Her research interests include lipid metabolism, especially for lipoprotein subfractions, and chrononutrition. Masayuki Saito received his PhD in biochemistry from Osaka University, Japan, in 1970. His main research field is the patho-physiology of energy metabolism and obesity, with special references to brown adipose tissue (BAT). Currently, as an emeritus professor of Hokkaido University, he is working on human BAT, particularly focusing on some food ingredients activating BAT and reducing body fatness. Naoki Sakane received his MD from Jichi Medical School in 1989. He received PhD degrees in medicine from Kyoto Prefectural University of Medicine in 1999. Currently, he is working as a division director at Division of Preventive Medicine, Clinical Research Institute, National Hospital Organization. His research interests include brown adipose tissue, beta3-adrenoceptor polymorphism and beta3-adrenergic agonists, as well as diabetes prevention and diabetes education. Yuko Kurosawa is an assistant professor at Tokyo Medical University. She has received a postdoctoral award at the Cincinnati Translational Neuroscience Symposium 2007 and Best Poster Award of the International Creatine-2015 Conference. Her research expertise is creatine metabolism in brain and skeletal muscles. Takafumi Hamaoka, MD and PhD, has conducted research on muscle oxidative metabolism using near-infrared and phosphorus-magnetic resonance spectroscopies with Prof. Britton Chance. He has received a research awards, such as a Young Investigators Award, 1st Congress of European College of Sports Science in 1996. His research expertise is exercise medicine, control of muscle oxidative metabolism, and evaluation of human adipose tissue. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||