|
|
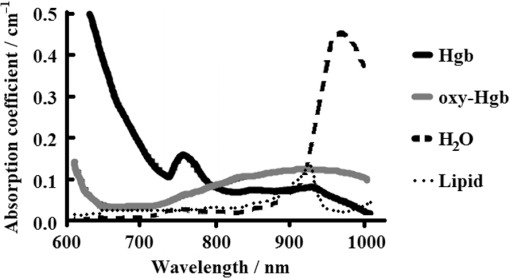
1.IntroductionThe Information Age is rapidly progressing, with more data becoming available to everyone at an ever-increasing pace.1 How one interprets available information and how one reacts to it can be a challenge. More data can potentially be very helpful in decisions that are made, but careful analysis and interpretation is required in order to react properly. This same principle holds true today in modern medicine. Physicians are continuously searching for the ability to gain more quantifiable information about the patients they care for, and this is particularly true in hospital settings where clinical status can change rapidly.2 New techniques are being employed to help determine global tissue perfusion in order to assess physiologic function, and ultimately lead to better outcomes.3 Monitoring splanchnic oximetry through the use of near-infrared spectroscopy (NIRS) represents one such method. Tissue ischemia and hypo-oxygenation can be major contributing factors to patient morbidity and mortality.4 Devices utilizing NIRS have the capability to measure the amount of oxygenated hemoglobin (oxy-Hgb) and deoxygenated hemoglobin (deoxy-Hgb) in human organs and determine regional tissue oxygenation ().5 These values can then be interpreted to provide information about tissue perfusion, oxygenation, and metabolic status both globally and specifically within particular anatomical systems.6 NIRS can safely and noninvasively be used to continuously or periodically check patient oxygenation status and provides insight into the delicate balance between oxygen supply and demand that the body requires to function normally. These devices can be used in surgical, emergency, and inpatient settings and on a variety of patients requiring close monitoring.7 There are also multiple anatomical sites where NIRS sensors can be placed.8 Although placement of sensors on the front of the skull over the brain to measure cerebral tissue oxygenation () has been the site both most studied and used in clinical practice, alternative monitoring locations are becoming increasingly investigated and utilized.9 The reason for this is that while it is important to maintain proper tissue oxygenation in the brain to prevent neurologic injury,10 monitoring other organs either singularly or in conjunction and in relation to is proving effective in providing additional information useful to clinicians.11 Splanchnic tissue oxygenation () is thought to be potentially of high value in the monitoring of critically ill patients because gastrointestinal organs are often the first to suffer ischemic injury.12 It has long been recognized that during low cardiac output states, splanchnic vasoconstriction can be an early physiologic response.13 During conditions of hypovolemia, cardiac dysfunction, and decreased oxygen carrying capacity, blood flow is diverted toward vital organs, such as the brain and the heart at the expense of abdominal organs and peripheral muscle tissue.14 Classical bedside training of medical students includes teaching that the gut is considered the “canary in the coal mine.”15 Circulatory compromise in the splanchnic region is frequently the first step on the way to systemic circulatory collapse or multisystem end organ dysfunction that can ultimately lead to mortality. Even when other parameters such as heart rate, blood pressure, and pulse oximetry are within normal limits, splanchnic tissue oxygenation can already be severely affected, thus making a form of early detection.16 Other modalities have tried to take advantage of this phenomenon, including gastric tonometry and Doppler ultrasound, but none of these techniques have yet proven to be clinically effective, and none are as feasible, operator independent, continuous in capability, or helpful in measuring microcirculation of gastrointestinal organs as measuring with NIRS.17,18 In addition to overall patient assessment, there are also gastrointestinal organ-specific disease processes that can be used to potentially detect or even diagnose. The aim of this review is to describe the basic principles of splanchnic oximetry, demonstrate how has been studied, and examine its use in clinical medicine. Implementation of NIRS into medical practice, and in particular abdominal NIRS, is an emerging area. This paper will show how may provide more than just additional data for physicians and nurses to have to deal with, but rather, truly helpful information that can further fine-tune management of critically ill patients. 2.Background on the Use of Oximetry in Clinical SettingsInfrared radiation was discovered at the beginning of the 19th century by Sir William Herschel, who noticed a type of invisible radiation lying in a spectrum lower in energy than that of red light because of its effect upon a thermometer.19 More than a 100 years later in the 1940s, Dr. Glenn Millikan invented the first practical pulse oximeter utilizing this NIR light that could be used at the bedside to measure changes in oxygen saturation.9 The big breakthrough in the use of NIRS in medicine to measure not just arterial oxygen saturation, but complete tissue oxygenation, came 30 years later when Dr. Frans Jobsis reported his landmark paper in “Science” demonstrating that organs, such as the brain, can be readily accessible to light energy with minimal interference from overlying tissue, and that NIRS can measure the degree of oxy-Hgb and deoxy-Hgb in such tissue.20 Soon after, in 1985, another pioneer in the field of medical NIRS, Dr. Marco Ferrari, published studies showing that NIRS could effectively be used to measure cerebral oxygenation in human adults.21 That same year, Dr. Jane Brazy published her work in “Pediatrics,” demonstrating the ability of NIRS to also measure brain oxygenation in the smallest of human patients, preterm infants.22 Since then, there has been a multitude of human research studies conducted and a plethora of case reports demonstrating the clinical utility of NIRS in medical settings. However, to date, there have not been the large randomized control trials required to fully evaluate the impact that monitoring tissue oxygenation has on improving outcomes in either adults or children.23 However, because there is little risk involved when monitoring with NIRS, it has been determined that class II, level B evidence supports its use as a likely effective and beneficial hemodynamic monitor for the care of patients at risk of critical conditions.7 This has led to the growing and now widespread use of NIRS by anesthesiologists, surgeons, and critical care physicians working in a variety of settings. In fact, recent studies have reported routine use of NIRS technology by certain types of specialized critical care units to be between 70% and 90%.24,25 Many tout NIRS technology for its ability to noninvasively provide crucial information on a tissue level that previously could only be obtained with invasive techniques, such as measuring mixed venous oxygen saturation () through the use of inline catheters.26 This property of NIRS is especially useful in patients that may not have access to these critical care techniques or in patients where this type of monitoring is not feasible because of patient size, such as in infants and preterm neonates. As more manufacturers begin making NIRS devices and the technology becomes more affordable and accessible, its presence in hospitals will likely only continue to expand. 3.Basis for the Use of Near-Infrared Spectroscopy in MedicineMonitoring tissue oxygenation in patients with NIRS is based on three principles: (1) biological tissue is relatively transparent to NIR light,27 (2) oxy-Hgb and deoxy-Hgb are the primary chromophores that significantly absorb light in the NIR spectrum;28 and (3) oxy-Hgb and deoxy-Hgb have specific absorption properties that make them distinguishable29 (see Fig. 1). By utilizing these principles, and then applying a form of the Beer–Lambert Law, amounts of oxy-Hgb and deoxy-Hgb can be determined.11 NIRS probes transmit light beneath the skin surface.30 The two main types of NIRS devices used clinically either are saturation monitors [i.e., INVOS 5100 (Covidien, Mansfield, Massachusetts)] or concentration monitors [i.e., NIRO 500 (Hamamatsu Photonics, Shizuoka, Japan)].31 While the saturation monitor type is most commonly used, similar values are obtained when the devices have been compared,32–34 and most believe that commercial NIRS devices are highly correlated.16 NIRS can also determine some information about other chromophores, including cytochrome aa3. However, this review focuses only on its ability to measure the difference between Hgb in an oxygenated state and a deoxygenated state, reported as .35 Clinicians think of as representing the balance of oxygen that is delivered minus what is extracted at the tissue level because blood in the microcirculation that NIRS measures is heavily venous weighted.36 A majority of the circulation being monitored by NIRS comes from venules (70%), while arterioles (20%), and capillaries (10%) make up the remaining components.37 Thus, can act as a proxy for and provide a good indication of oxygen balance.23 Measuring can be complimentary to monitoring pulse oxygen saturation (). Fractional tissue oxygen extraction is a measurement of the amount of oxygen extracted by tissue. It is calculated with the following formula: [] and provides an estimate of the balance between oxygenation and perfusion compared with metabolic demand.38 In addition to adding supplemental clinical information to , can serve solely to provide oxygen level data when pulse oximetry fails or is impossible. Because is venous weighted, pulsatile blood flow is not required for NIRS to function. values can, therefore, be detected even in the most critical states when cardiac output may have ceased or complete circulatory collapse is occurring.31 4.Anatomical Sites Monitored with Near-Infrared SpectroscopyThere are multiple anatomical sites that NIRS sensors can be placed in humans to measure . NIRS devices have the capability to monitor and display multiple values simultaneously (see Fig. 2). The location that has been vastly more studied and utilized since the inception of clinically capable NIRS is the forehead.29 This location allows for direct monitoring of the frontal cortex region, providing values.39 In adults, two sensors are generally placed, one on each side of the forehead, to measure either the right or left frontal cortex, whereas in preterm neonates, because of space limitations, generally one NIRS sensor is placed in the midline of the forehead.40 The brain is not only a most crucial organ but also it lacks fuel stores and, therefore, requires a continuous supply of oxygen and glucose. Continuous cerebral blood flow, cerebral oxygen tension and delivery, and mitochondrial activity are critically important to maintain normal brain function and tissue viability.41 Any change of condition leading to hypoxemia can quickly lead to brain damage, and is why monitoring during times of critical illness or during surgical procedures can be important.42,43 Fig. 2Typical NIRS device screen showing multiple regional tissue oxygenation () values simultaneously. 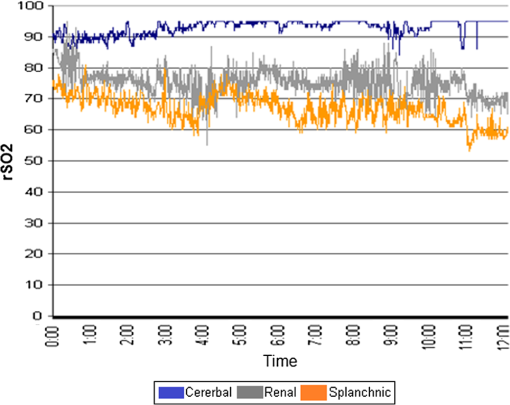 While the brain was the site first studied, not long after, NIRS began to be utilized to research muscle metabolism.9 Many muscle systems are located just underneath the skin. NIRS can be used to understand muscle oxygenation during normal health at rest, during various degrees of exercise, and during pathologic states. It has been utilized by sports medicine physicians, but also by critical care physicians.44 This is because blood flow can be diverted away from peripheral muscle tissue during times of decreased oxygen delivery, as in the case of anemia or shock.45,46 Of perhaps more significance to physicians caring for patients in the hospital setting is another site where NIRS has been utilized, over the flank measuring renal tissue oxygenation (). The kidneys receive of cardiac output and play a major role in regulating blood pressure and global tissue perfusion.47 The thinner the patient, the more easily accessible the kidneys are to the skin surface, and much utilization of values has been performed in pediatric populations.48 As an example, in some institutions, it is now part of routine protocol during cardiac surgeries to monitor .16 Physicians have found renal oximetry to be helpful in diagnosing low cardiac output conditions that may not be picked up by monitoring alone, because of the strong autoregulatory mechanisms of the cerebral circulation.49 In our own experience, we have found to be easily measured in term infants and able to demonstrate shifts in somatic blood flow that occur during the transition from intrauterine to extrauterine life, as hemodynamics changes take place over the first few days after birth.50 Splanchnic oximetry is now gaining much increased interest. One of the main reasons is that because of the nature of the splanchnic circulatory system, ischemic injury to abdominal organs, such as the gut or liver, may take place in patients that would appear otherwise stable whether by physical examination or commonly used clinical monitoring tools.41 The remainder of this article will review the benefits of monitoring, its limitations, and potential uses in clinical care. 5.Interest in Splanchnic OximetryThe primary interest of clinicians in measuring is due to the well-known phenomenon that splanchnic circulation vasoconstriction can occur during any time of metabolic or circulatory stress. This self-preserving mechanism has evolved as an attempt to maintain both coronary artery blood flow and cerebral blood flow at the expense of less vital organs that do not rely on constant oxygen delivery.51 Therefore, it is felt that monitoring devices can serve as an early alert system of future complications.52 If declines in splanchnic oxygenation values occur, this may mean that further circulatory compromise is imminent, despite vital signs that could suggest stability. For example, Price et al.14 demonstrated in healthy adult humans that simply a 15% reduction in total circulating blood volume resulted in an estimated forty percent reduction in blood volume of the splanchnic circulatory system despite having no discernable effect on heart rate or blood pressure measurements. This notion is applicable to many different conditions that can affect patients, including, but not limited to, sepsis, hemorrhage, allergic reactions, and anemia. All of these conditions have the same common pathway: they all ultimately lead to a shock-like state, with subsequent decreased perfusion and oxygen delivery. In these cases, it is the abdominal organs that often take the “first hit,” especially in the microcirculation of the gastrointestinal tract.53 One can, therefore, see that there are many potential applications for monitoring to track patient condition or progress through different procedures or disease states. Regional tissue oxygen saturation, including , can be measured as a value by itself or in conjunction with other values (see Fig. 3). Both methods have been shown to demonstrate potential merit.54,45 However, even with more established NIRS monitoring modalities, such as , experts still caution about interpreting any absolute values, and most often recommend following trends in patients from their baseline values when making medical decisions.55 Therefore, it is important to take into context any splanchnic oximetry measurement in and of itself. However, we suggest that if one takes two trends, for example, and , and compares them with each other in an individual patient, it may be more than just a trend; it could quite possibly tell you “absolutely” about what is happening clinically to that particular patient. Fig. 3Model of an infant patient depicting typical locations that NIRS sensors are placed in clinical practice.  Splanchnic oximetry differs from or in that it can be obtained from more than one organ, depending on the NIRS sensor location. Focus thus far has been on the liver and the intestines. It has not been established which location is ideal to gather a sense of overall splanchnic circulation perfusion, but research and clinical usage is trending toward monitoring the gut.56 Hepatic tissue may provide a steadier signal due to its more uniform tissue consistency, but monitoring of the intestines may provide more clinical insight.36 Either way, multiple organ site capability shows how splanchnic oximetry also has the ability to be somewhat particular, rather than generalized, when being used to diagnose organ specific disease processes, such as liver injury or necrotizing enterocolitis (NEC). Using NIRS to measure is a much simpler way to determine the perfusion status of splanchnic organs than other techniques that have been investigated. NIRS appears to be superior to gastric tonometry, as it can detect oxygenation issues at an early stage, before acidosis occurs and pH levels drop, and also has less potential usage complications.57,58 NIRS does not take specialized training to utilize and can be incorporated into a continuous monitoring model, in comparison to Doppler flow velocimetry, which has also shown ability to detect conditions such as NEC but requires specialized operators and has only intermittent monitoring capabilities.59 Finally, is likely advantageous to commonly used biomarkers that can signify poor splanchnic perfusion, such as lactic acid. One reason for this is that lactic acidosis indicates ischemia and tissue damage which has already taken place, whereas splanchnic oximetry may help a physician see changes occurring in perfusion status before tissue damage sets in.60 6.Limitations of Splanchnic OximetryWhile monitoring tissue oxygenation status in splanchnic organs has several potential benefits, there are certainly limitations to this technology and these techniques at present. One of the most prominent is that there is a much greater degree of intrapatient signal variability in than with other oximetry values.61 This was well studied in a neonatal population by Mintzer et al.,62 who found by measuring coefficients of variation for different NIRS values that there can be a multifold increase in the degree of variability seen in , as compared with and . They discuss how this may result either from real-time changes in blood flow to the splanchnic tissue beds or from momentary alterations in splanchnic organ oxygen consumption. Of particular importance, these researchers concluded that this increased variability in at baseline makes it more difficult to determine when has significantly changed in a monitored patient that would signify some clinical concern. We have seen this in our research and own clinical practice as well.50 However, Mintzer et al.62 describe methods that can be used by clinicians to overcome the variability issue. First, they recommend utilizing relatively short (5 to 15 min) epoch periods as a preferred data averaging interval for measurements when establishing clinical trend monitoring. Second, they recommend that clinicians evaluate over longer periods of time, while using these brief data sampling epochs, than they would with values when making medical decisions based on NIRS monitoring.62 In addition to an increased variability in signal, the literature reports that there also can be more momentary losses of NIRS signal when measuring and longer segments of very low readings as compared to other values.13,54,61 This information needs to be taken into account before using NIRS as part of clinical decision-making processes. It emphasizes that , at this point, should likely not be used solely, but rather only in context with other physiologic measurements, as well as the probable need to monitor over relatively longer periods of time than with other parameters, such as , to make an effective assessment. Another limitation of splanchnic oximetry is that it can often be hard to determine the exact tissue that one is monitoring with splanchnic NIRS. The intestine is not a fixed organ, and movement can occur. It is also important that the NIRS sensor be placed in a position above where the bladder could interfere with the signal in cases of urinary retention.63 In addition, abdominal wall thickness can begin to exceed the penetration depth of currently used commercially available NIRS sensors based on patient age, weight, and percentage of body fat. At a certain point, NIRS sensors may not have the capability to actually measure . Balaguru et al.64 found that in most patients, this point occurs around 6 years of age or 30 kg in weight. Essentially, the intestines may be located too deep beneath the surface of the skin in most adult patients for splanchnic oximetry to be of clinical value and is a likely reason why a majority of research and medical usage has been in pediatrics. There is also concern about the tissue itself that is being monitored. Many speculate that because the intestine is a hollow organ that can be filled with air, fluid, or solids with changing gas fluid surfaces, that values may be too unreliable.65 Peristalsis occurs and stool that contains biliverdin and bilirubin can move under the NIRS sensor. However, the spectral absorption capability of biliverdin and bilirubin is below that which most NIRS devices use.11 The liver is more homogeneous in nature and may not present as many issues in this regard. But that points out another limitation of splanchnic NIRS: that splanchnic oximetry has location specific characteristics. measurements taken over the liver versus those taken over the intestines can correlate but should not be substituted for one another.66 A final concern about splanchnic oximetry is the proprietary algorithms that are used by the various devices.34 To begin with, each manufacturer uses their own, and these slight computational differences may contribute to the interpatient variability that can be seen with .5 However, this is also true with pulse oximetry, and this has not been seen as a major problem clinically, as pulse oximetry obtained by any device has now become a standard of care.67,68 Another potential issue with the algorithms used is that they were mostly intended to calculate cerebral oximetry and may not necessarily be ideal to measure tissue oxygenation in other organ systems. Most clinicians are using “universal sensors” that were originally designed for the brain to also monitor abdominal contents. 7.Animal StudiesAs with most medical technology, studies examining the utility of splanchnic oximetry were first conducted in animal models. Research mainly has focused on hepatic injury or intestinal injury, both being conditions that represent splanchnic ischemic states. Using a large pig model, El-Desoky et al.69 measured total hepatic blood flow and directly on the surface of the liver using a laparotomy technique. They demonstrated that measurement correlated well with either hepatic vascular inflow occlusion or reduced inspired oxygen that led to reduced levels. This group then went on to demonstrate in a similar model that splanchnic oximetry was superior to hepatic vein oxygen partial pressure measurements in predicting splanchnic ischemia.70 Finally, they demonstrated in a rabbit model that splanchnic oximetry could not only detect hepatic ischemia but could actually help determine the severity of injury to the liver that occurred as a result of under perfusion–reperfusion injury.71 Vanderhaegen et al.72 looked at a rabbit model to demonstrate that measured at the skin surface, as opposed to directly on the liver, could also demonstrate splanchnic ischemia. They occluded the superior mesenteric artery, while monitoring and simultaneously. The study results showed that noninvasive NIRS could detect a significant decrease in signifying ischemic injury to the liver and intestines despite peripherally obtained remaining stable throughout. Animal models have also demonstrated that shock states due to endotoxin release and subsequent vascular vasodilation that occurs with sepsis can be monitored with . Using piglets, Nahum et al.73 induced septic shock with E. coli bacteria and found that decreasing trends in correlated well with the degree of gastrointestinal ischemia monitored by NIRS sensors whether placed on the skin overlying the liver or directly on the liver. Gay et al.74 used a premature piglet model to determine if abdominally positioned NIRS sensors placed over the bowel could predict which animals would develop NEC. They found that readings were significantly lower soon after birth in animals that went on to develop NEC many days later. During the experiments, they also determined that splanchnic oximetry readings also correlated well with apneic episodes that occurred in these preterm piglets. Another group of researchers demonstrated that could also monitor for and diagnose NEC using another piglet model. In their study, Zamora et al.75 found that values consistently predicted NEC with 97% sensitivity and 97% specificity, and that increased variability during initial stages of feeding was also highly predictive of NEC. One group of researchers examined the utility of placing NIRS sensors directly in the stomach using a nasogastric tube to measure .76 Beilman et al.76 in this adult pig model demonstrated that a minimally invasive gastric splanchnic oximetry technique was capable of monitoring decreases in that correlated well with increasing degrees of hemorrhagic shock. 8.Pediatric StudiesThere have been many fewer human studies examining splanchnic oximetry compared with other NIRS anatomical sites, but those that have been conducted have mostly been done in pediatric patients. As previously mentioned, children are ideally suited to have monitored, as splanchnic organs are more easily accessible to NIRS sensors with an average penetration depth of 2 cm.30 The two commercial NIRS devices primarily available, INVOS 5100 (Covidien) and NIRO 500 (Hamamatsu Photonics), have been used when conducting this splanchnic oximetry research (see Table 1). Studies vary, but most focus on a few populations: children with congenital heart disease (CHD), preterm and term infants, and children with critical illnesses being monitored in an intensive care setting. Table 1NIRS devices used in human splanchnic oximetry studies.
8.1.Normal Value StudiesMcNeill et al.77 studied baseline values in stable preterm infants during their first weeks of life using infraumbilical sensor placement. They found that baseline values ranged between 32% and 66% and gradually increased with gestational age. They also found that there was a high degree of variability as compared with or , but that this variability decreased over time. Cortez et al.61 confirmed these results, finding a similar range of normal values in a study examining splanchnic oximetry patterns of preterm infants up to day of life 14. In a study examining healthy full-term infants, Bailey et al.50 reported mean values of on the first day of life and on the second day. This is in line with the preterm normative studies that showed values increasing with gestational age. These studies demonstrate values, but not splanchnic-cerebral oxygenation ratio (SCOR) values that assess splanchnic perfusion and oxygenation in context with subject cerebral oximetry. Bailey et al.78 did report on SCOR values in healthy term babies taking regular feedings, and found normal SCOR to be on the first day of life, which increased to by the second day. This shows how in normal pediatric subjects, splanchnic oximetry values should be similar to cerebral oximetry values in states of standard health. It is important to note that in all of these studies, there were some subjects who had periods of low values during complete clinical stability. This again emphasizes the point that when using splanchnic oximetry, trend monitoring from baseline values and observing over time is likely required to maximize the diagnostic capabilities of abdominal NIRS. 8.2.Universal Monitoring During Intensive CareIn an early case series demonstrating the utility of splanchnic NIRS, Petros et al.79 reported that episodes of apnea may have a more significant and long lasting effect on splanchnic circulation than on other organ systems in the body. In the immediate postoperative period, Li et al.80 showed how can correlate with oxygen levels measured directly from the blood as well as central blood pressure monitoring values. They concluded that could be helpful during the intensive care required after CHD surgery, but emphasized the importance of trending in individual patients rather than comparing to a normal range. Mintzer et al.81 showed the effects of administering sodium bicarbonate on . This is a common therapy used during intensive care.82 A result of their study was that sodium bicarbonate did decrease blood base deficit and increase pH but had no significant effect on splanchnic oxygenation. It, therefore, suggested the potential futility of such intervention. Recently, Bozzetti et al.83 looked at SCOR values to monitor subjects who were severely growth restricted at birth. They found that splanchnic oximetry, used in conjunction with cerebral oximetry, could help determine if patients are ready for enteral feedings to begin, or if splanchnic blood flow and perfusion remains impaired. This may be very helpful for surgeons and intensivists if determining in any postoperative patient when feedings may first be attempted. 8.3.FeedingDave et al.84 have reported that values increase after feedings in stable preterm infants tolerating bolus feedings while values remain constant. They also examined the SCOR and showed that this too increases after feedings, all accounted for by an increase in intestinal tissue oxygenation. Dani et al.85 examined preterm infants during their first days of life receiving continuous feeds. These researchers did not find that correlated with the time needed to achieve full enteral feedings. This perhaps could be because these subjects were receiving continuous feeds rather than bolus feeds. Another group studied the correlation of with superior mesenteric after velocities in infants before and after feedings.86 Gillam-Krakauer et al.86 found that splanchnic blood flow increased after feedings and that reflected the degree of this change. 8.4.Necrotizing EnterocolitisThe first clinical investigators to report that splanchnic oximetry could help detect and diagnose NEC were Fortune et al.87 In their prospective, observational cohort study, they looked at infants with acute abdomens, who were referred for surgery and infants receiving intensive care only for medical reasons. They monitored and , and calculated a SCOR. They found that a SCOR of had a sensitivity of 90% and positive predictive value of 75% to diagnose NEC and a negative predictive value of 96% to exclude it. DeWitt et al.54 prospectively studied infants with CHD, who had single ventricle palliation surgery and were known to be at risk for postoperative NEC. They found that patients who developed NEC had lower mean values at baseline and more times when intermittently dropped to very low levels. In addition to CHD, red blood cell transfusion possibly also places infants at risk for NEC.88 In a study of preterm infants who were either fed or not fed during transfusions, Marin et al.89 found that those subjects who had fed experienced significantly lower values for at least 15 h after the transfusion completed. They speculated that this placed them at increased risk for mesenteric ischemia, which could then lead to NEC. 8.5.TransfusionIn regards to blood transfusion, splanchnic oximetry has been investigated in two ways. Researchers have sought to observe what happens to splanchnic blood flow and as a result of a blood transfusion, and also what is occurring in terms of splanchnic oxygenation and perfusion during anemic states prior to any transfusion. The information could be clinically relevant by determining if splanchnic oximetry has utility in monitoring transfusions for complications, and perhaps more importantly, to see if splanchnic oximetry could be used as a tool to determine red blood cell transfusion need. Bailey et al.90 looked at symptomatic anemic preterm infants and examined during and after the transfusion period. They found that increased from before the transfusion to immediately after. began to decline again after the transfusion was completed over the subsequent 12 h, although not to pretransfusion levels. Dani et al.91 likewise found that increased significantly during blood transfusion. Recently, Banerjee et al.92 examined not only but also mesenteric blood flow utilizing Doppler ultrasound. This group also found an increase in during the transfusion period, but without a significant alteration in blood flow velocity through the splanchnic circulatory tree. Bailey et al.93 later published another paper demonstrating the possible usefulness of splanchnic oximetry in helping to determine red blood cell transfusion needs. The study included 34 symptomatic preterm infants who were given blood transfusions, and 18 asymptomatic infants with low Hgb levels found on routine weekly laboratory sampling who were not transfused. The researchers found that baseline SCOR values were significantly lower in neonates who improved with transfusion (0.61) when compared to those without improvement (0.75) as well as those asymptomatic subjects with equally low Hgb levels (0.77). They concluded that a baseline SCOR had a 74% sensitivity and 73% specificity, and a positive predictive value of 78% in determining if a patient would improve after transfusion. 9.Adult StudiesThere are very few studies with adult subjects examining the use of splanchnic oximetry in clinical medicine. As mentioned previously, this is almost certainly because splanchnic organs are difficult to monitor using current NIRS sensor technology that is only capable of measuring tissue oxygenation beneath the skin surface.94 In most adults, muscle and fat layers beneath the skin are too thick to allow consistent access to splanchnic organs. Despite this, recently, a research group did examine values in adult patients using NIRS sensors placed on the abdomen. Kalkan et al.95 evaluated adults who suffered cardiac arrest outside of the hospital and were brought to the emergency room. These researchers placed two abdominal NIRS sensors, one over the liver and one in the mid-abdomen over the navel, prior to cardiopulmonary resuscitation (CPR) and monitored tissue oximetry values throughout the procedure. They found that patients who began CPR with higher values upon arrival to the emergency room, were more likely to be successfully resuscitated. They also saw that the degree to which increased during CPR was highly correlated with survival. In another study, Widder et al.96 placed NIRS sensors on the skin surface of the lower abdomen in order to monitor intra-abdominal hypertension. Their sensors were possibly picking up signal from the bladder as supposed to intestinal tissue, but they did demonstrate that this oximetry technique was able to monitor increases in intra-abdominal hypertension. The more elevated the pressure became, the lower the values went. Other researchers examining the clinical use of in adult patients have bypassed the problem of abdominal wall thickness by placing sensors directly on the organs of interest, rather than on the skin surface above. Crerar-Gilbert et al.97 built miniature NIRS sensors and used these in patients undergoing laparoscopic surgeries in order to obtain recordings directly from the bowel and liver. They were able to demonstrate strong splanchnic NIRS signals and show the potential for this technique to monitor during laparoscopic surgery. 10.SummaryIn summary, while far from proven, this review demonstrates that monitoring splanchnic oximetry holds promise in its ability to judge physiological status and detect disease states in humans, especially in the most vulnerable population, neonates. Although there are yet to be randomized control trials proving the effectiveness of monitoring as part of patient care, this is also true with other tissue oximetry values and other different physiologic parameters that have been incorporated into patient care practices. Over the last 20 years, there have been hundreds of studies examining the use of NIRS in clinical medicine. However, only a small number of these have examined splanchnic oximetry in conjunction with the more established anatomical sites. Even fewer have focused solely on splanchnic NIRS monitoring. Clearly, there is much more work in this area that needs to take place. With further research and improvements in technology, it seems quite reasonable to think that splanchnic oximetry can become a valuable tool for clinicians caring for patients. At this time, it is unclear if splanchnic NIRS monitoring will become helpful in all patient populations. However, for smaller children and infants, splanchnic oximetry can likely provide great physiologic insight. This is especially true in children when at risk of shock or who require intensive care. has the capability to become a useful clinical parameter, and not just ever-increasing extra information that clinicians have to filter through when caring for patients. Of all the somatic tissue oxygenation values that can be monitored using NIRS, splanchnic oxygenation likely has the most clinical potential, but also the most questions that remain regarding how it can become an established part of medical care methodology. AcknowledgmentsNeither author has any conflicts of interests to report. Some of our previous research described in this review had been funded in part by a research grant from Covidien. The oximeters used in our previously described research were funded by a KIDS of NYU research grant. ReferencesM. Hilbert and P. Lopez,
“The world’s technological capacity to store, communicate, and compute information,”
Science, 332
(6025), 60
–65
(2011). http://dx.doi.org/10.1126/science.1200970 SCIEAS 0036-8075 Google Scholar
L. Dijoy et al.,
“The history of goal-directed therapy and relevance to cardiopulmonary bypass,”
J. Extra Corpor. Technol., 47
(2), 90
–94
(2015). Google Scholar
N. J. Ekbal et al.,
“Monitoring tissue perfusion, oxygenation, and metabolism in critically ill patients,”
Chest, 143
(6), 1799
–1808
(2013). http://dx.doi.org/10.1378/chest.12-1849 CHETBF 0012-3692 Google Scholar
T. W. L. Scheeren, P. Schober and L. A. Schwarte,
“Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications,”
J. Clin. Monit. Comput., 26 279
–287
(2012). http://dx.doi.org/10.1007/s10877-012-9348-y Google Scholar
F. Van Bel, P. Lemmers and G. Naulers,
“Monitoring neonatal regional cerebral saturation in clinical practice: value and pitfalls,”
Neonatology, 94 237
–244
(2008). http://dx.doi.org/10.1159/000151642 Google Scholar
J. Steppan and C. W. Hogue,
“Cerebral and tissue oximetry,”
Best Pract. Res. Clin. Anaesthesiol., 28
(4), 429
–439
(2014). http://dx.doi.org/10.1016/j.bpa.2014.09.002 Google Scholar
N. S. Ghanayem, G. Wernovsky and G. M. Hoffman,
“Near-infrared spectroscopy as a hemodynamic monitor in critical illness,”
Pediatr. Crit. Care Med., 12 S27
–S32
(2011). http://dx.doi.org/10.1097/PCC.0b013e318221173a Google Scholar
G. Greisen, T. Leung and M. Wolf,
“Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care?,”
Philos. Trans. A: Math. Phys. Eng. Sci., 369
(1955), 4440
–4451
(2011). http://dx.doi.org/10.1098/rsta.2011.0261 Google Scholar
M. Ferrari and V. Quaresima,
“Near infrared brain and muscle oximetry: from the discovery to current applications,”
J. Near Infrared Spectrosc., 20 1
–14
(2012). http://dx.doi.org/10.1255/jnirs.973 Google Scholar
N. P. Blockley et al.,
“A review of calibrated blood oxygenation level-dependent methods for the measurement of task-induced changes in brain oxygen metabolism,”
NMR Biomed., 26
(8), 987
–1003
(2013). http://dx.doi.org/10.1002/nbm.2847 Google Scholar
B. G. Sood, K. McLaughlin and J. Cortez,
“Near-infrared spectroscopy: applications in neonates,”
Semin. Fetal Neonatal. Med., 20 164
–172
(2015). http://dx.doi.org/10.1016/j.siny.2015.03.008 Google Scholar
K. M. Reber, C. A. Nankervis and P. T. Nowicki,
“Newborn intestinal circulation. Physiology and pathophysiology,”
Clin. Perinatol., 29
(1), 23
–39
(2002). Google Scholar
U. S. Bhalala et al.,
“Change in regional (somatic) near-infrared spectroscopy is not a useful indicator of clinically detectable low cardiac output in children after surgery for congenital defects,”
Pediatr. Crit. Care Med., 13
(5), 529
–534
(2012). http://dx.doi.org/10.1097/PCC.0b013e3182389531 Google Scholar
H. L. Price et al.,
“Hemodynamic and metabolic effects of hemorrhage in man, with particular reference to the splanchnic circulation,”
Circ. Res., 18 469
–474
(1966). http://dx.doi.org/10.1161/01.RES.18.5.469 Google Scholar
D. R. Dantzker,
“The gastrointestinal tract. The canary of the body?,”
JAMA, 270 1247
–1248
(1993). http://dx.doi.org/10.1001/jama.1993.03510100097040 JAMAAP 0098-7484 Google Scholar
J. P. Scott and G. M. Hoffman,
“Near-infrared spectroscopy: exposing the dark (venous) side of the circulation,”
Pediatr. Anesth., 24 74
–88
(2014). http://dx.doi.org/10.1111/pan.2013.24.issue-1 Google Scholar
A. B. Groeneveld and J. J. Kolkman,
“Splanchnic tonometry: a review of physiology, methodology, and clinical applications,”
J. Crit. Care, 9
(3), 198
–210
(1994). http://dx.doi.org/10.1016/0883-9441(94)90016-7 Google Scholar
J. J. Kolkman et al.,
“Diagnosis and management of splanchnic ischemia,”
World J. Gastroenterol., 14
(48), 7309
–7320
(2008). http://dx.doi.org/10.3748/wjg.14.7309 Google Scholar
C. Downs,
“Progress in infrared photodetectors since 2000,”
Sensors, 13
(4), 5054
–5098
(2013). http://dx.doi.org/10.3390/s130405054 SNSRES 0746-9462 Google Scholar
F. F. Jobsis,
“Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,”
Science, 198 1264
–1267
(1977). http://dx.doi.org/10.1126/science.929199 SCIEAS 0036-8075 Google Scholar
M. Ferrari et al.,
“Continuous non invasive monitoring of human brain by near infrared spectroscopy,”
Adv. Exp. Med. Biol., 191 873
–882
(1985). http://dx.doi.org/10.1007/978-1-4684-3291-6 AEMBAP 0065-2598 Google Scholar
J. E. Brazy et al.,
“Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations,”
Pediatrics, 75
(2), 217
–225
(1985). PEDIAU 0031-4005 Google Scholar
R. Bronicki,
“Near-infrared spectroscopy oximetry: part science, part art,”
Pediatr. Crit. Care Med., 17
(1), 89
–90
(2016). http://dx.doi.org/10.1097/PCC.0000000000000565 Google Scholar
A. U. Hoskote et al.,
“A cross-sectional survey of near-infrared spectroscopy use in paediatric cardiac ICUs in the United Kingdom and Ireland, Italy, and Germany,”
Pediatr. Crit. Care Med., 17 36
–44
(2016). http://dx.doi.org/10.1097/PCC.0000000000000564 Google Scholar
A. F. Rossi et al.,
“Web based survey of current trends in hemodynamic monitoring after congenital heart surgery,”
World J. Pediatr. Congenital Heart Surg., 3 301
–309
(2012). http://dx.doi.org/10.1177/2150135111433472 Google Scholar
T. A. Tortotiello et al.,
“A noninvasive estimation of mixed venous oxygen saturation using near-infrared spectroscopy by cerebral oximetry in pediatric cardiac surgery patients,”
Pediatr. Anesth., 15
(6), 495
–503
(2005). http://dx.doi.org/10.1111/pan.2005.15.issue-6 Google Scholar
A. J. Wolfberg and A. J. du Plessis,
“Near-infrared spectroscopy in the fetus and neonate,”
Clin. Perinatol., 33
(3), 707
–728
(2006). http://dx.doi.org/10.1016/j.clp.2006.06.010 Google Scholar
L. Hoofd, W. Colier and B. Oeseburg,
“A modeling investigation to the possible role of myoglobin in human muscle in near infrared spectroscopy (NIRS) measurements,”
Adv. Exp. Med. Biol., 530 637
–643
(2003). http://dx.doi.org/10.1007/978-1-4615-0075-9 Google Scholar
M. Ferrari, L. Mottola and V. Quaresima,
“Principles, techniques, and limitations of near infrared spectroscopy,”
Can. J. Appl. Physiol., 29
(4), 463
–487
(2004). http://dx.doi.org/10.1139/h04-031 Google Scholar
A. Pellicer and C. Bravo,
“Near-infrared spectroscopy: a methodology-focused review,”
Semin. Fetal Neonatal Med., 16 42
–49
(2011). http://dx.doi.org/10.1016/j.siny.2010.05.003 Google Scholar
J. D. Tobias,
“Cerebral oxygenation monitoring: near-infrared spectroscopy,”
Expert Rev. Med. Devices, 3
(2), 235
–243
(2006). http://dx.doi.org/10.1586/17434440.3.2.235 Google Scholar
M. Thavasothy et al.,
“A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS near-infrared spectrophotometers,”
Anaesthesia, 57
(10), 999
–1006
(2002). http://dx.doi.org/10.1046/j.1365-2044.2002.02826.x Google Scholar
N. Nagdyman et al.,
“Comparison of different near-infrared spectroscopic cerebral oxygenation indices with central venous and jugular venous oxygenation saturations in children,”
Pediatr. Anesth., 18 160
–166
(2008). http://dx.doi.org/10.1111/j.1460-9592.2007.02365.x Google Scholar
L. M. Dix et al.,
“Comparing near-infrared spectroscopy devices and their sensors for monitoring regional tissue oxygen saturation in the neonate,”
Pediatr. Res., 74 557
–563
(2013). http://dx.doi.org/10.1038/pr.2013.133 Google Scholar
J. M. Murkin et al.,
“Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study,”
Anesth. Analg., 104
(1), 51
–58
(2007). http://dx.doi.org/10.1213/01.ane.0000246814.29362.f4 Google Scholar
T. Marin and J. Moore,
“Understanding near-infrared spectroscopy,”
Adv. Neonatal Care, 11
(6), 382
–388
(2011). http://dx.doi.org/10.1097/ANC.0b013e3182337ebb Google Scholar
J. Menke et al.,
“Reproducibility of cerebral near infrared spectroscopy in neonates,”
Neonatology, 83
(1), 6
–11
(2003). http://dx.doi.org/10.1159/000067006 Google Scholar
C. M. Kissack et al.,
“Cerebral fractional oxygen extraction is inversely correlated with oxygen delivery in the sick, newborn, preterm infant,”
J. Cereb. Blood Flow Metab., 25
(5), 545
–553
(2005). http://dx.doi.org/10.1038/sj.jcbfm.9600046 Google Scholar
G. Vretzakis et al.,
“Cerebral oximetry in cardiac anesthesia,”
J. Thorac. Dis., 6
(Suppl. 1), S60
–S69
(2014). http://dx.doi.org/10.3978/j.issn.2072-1439.2013.10.22 Google Scholar
K. Kiski et al.,
“Influence of patient variables and sensor location on regional cerarbal oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers,”
J. Neurosurg. Anesthesiol., 15 302
–306
(2003). http://dx.doi.org/10.1097/00008506-200310000-00002 Google Scholar
B. H. Verweij, G. J. Amelink and J. P. Muizelarr,
“Current concepts of cerebral oxygen transport and energy metabolism after severe traumatic brain injury,”
Prog. Brain Res., 161 111
–124
(2007). http://dx.doi.org/10.1016/S0079-6123(06)61008-X Google Scholar
H. Orbig and A. Villringer,
“Beyond the visible– imaging the human brain with light,”
J. Cereb. Blood Flow Metab., 23
(1), 1
–18
(2003). http://dx.doi.org/10.1097/01.WCB.0000043472.45775.29 Google Scholar
M. Banaji et al.,
“A model of brain ciruculation and metabolism: NIRS signal changes during physiological challenges,”
Comput. Biol., 4
(11), e1000212
(2008). http://dx.doi.org/10.1371/journal.pcbi.1000212 Google Scholar
V. Quaresima, R. Lepanto and M. Ferrari,
“The use of near infrared spectroscopy in sports medicine,”
J. Sports Med. Phys. Fitness, 43
(1), 1
–13
(2003). Google Scholar
S. P. Wardle and M. Weindling,
“Peripheral fractional oxygen extraction and other measures of tissue oxygenation to guide blood transfusions in preterm infants,”
Semin. Perinatol., 25 60
–64
(2001). http://dx.doi.org/10.1053/sper.2001.23200 Google Scholar
A. Lima et al.,
“Low tissue oxygen saturation at the end of early goal-directed therapy is associated with worse outcome in critically ill patients,”
Crit. Care, 13
(Suppl 5), S13
(2009). http://dx.doi.org/10.1186/cc8011 Google Scholar
M. R. Choi et al.,
“Renal dopameniergic system: pathophysiological implications and clinical perspectives,”
World J. Nephrol., 4
(2), 196
–212
(2015). http://dx.doi.org/10.5527/wjn.v4.i2.196 Google Scholar
R. M. Cerbo et al.,
“Global perfusion assessment and tissue oxygen saturation in preterm infants: where are we?,”
Early Hum. Dev., 89 S44
–S46
(2013). http://dx.doi.org/10.1016/S0378-3782(13)70014-8 Google Scholar
J. Li et al.,
“The influence of systemic hemodynamics and oxygen transport on cerebral oxygen saturation in neonates after the Norwood procedure,”
J. Thorac. Cardiovasc. Surg., 135 83
–90
(2008). http://dx.doi.org/10.1016/j.jtcvs.2007.07.036 Google Scholar
S. M. Bailey, K. D. Hendricks-Munoz and P. Mally,
“Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns,”
Am. J. Perinatol., 31 339
–344
(2014). http://dx.doi.org/10.1055/s-0033-1349894 Google Scholar
A. Kraut, E. Barbiro-Michaely and A. Mayevsky,
“Differential effects of norepinephrine on brain and other less vital organs detected by a multisite multiparametric monitoring system,”
Med. Sci. Monit. Basic Res., 10
(7), BR215
–BR220
(2004). Google Scholar
S. M. Cohn et al.,
“Splanchnic perfusion evaluation during hemorrhage and resuscitation with gastric near-infrared spectroscopy,”
J. Trauma: Inj. Infect. Crit. Care, 50
(4), 629
–635
(2001). http://dx.doi.org/10.1097/00005373-200104000-00006 Google Scholar
C. Y. Wu et al.,
“Effects of different types of fluid resuscitation for hemorrhagic shock on splanchnic organ microcirculation and renal reactive oxygen species formation,”
Crit. Care, 19
(1), 434
(2015). http://dx.doi.org/10.1186/s13054-015-1135-y Google Scholar
A. G. DeWitt et al.,
“Splanchnic near-infrared spectroscopy and risk of necrotizing enterocolitis after neonatal heart surgery,”
Pediatr. Cardiol., 35
(7), 1286
–1294
(2014). http://dx.doi.org/10.1007/s00246-014-0931-5 Google Scholar
S. M. Liao and J. P. Culver,
“Near infrared optical technologies to illuminate the status of the neonatal brain,”
Curr. Pediatr. Rev., 10
(1), 73
–86
(2014). http://dx.doi.org/10.2174/157339631001140408121507 Google Scholar
M. Weiss et al.,
“Transcutaneuosly measured near-infrared spectroscopic liver tissue oxygenation does not correlate with hepatic venous oxygenation in children,”
Can. J. Anesth., 49
(8), 824
–829
(2002). http://dx.doi.org/10.1007/BF03017416 Google Scholar
X. Zhang et al.,
“Gastric tonometry guided therapy in critical patients: a systematic review and meta-analysis,”
Crit. Care, 19 22
(2015). http://dx.doi.org/10.1186/s13054-015-0739-6 Google Scholar
P. M. Fortune, A. Pierro and A. Petros,
“Splanchnic oxygen delivery in exomphalos detected with near infrared spectroscopy,”
Eur. J. Pediatr., 160
(2), 144
–145
(2001). http://dx.doi.org/10.1007/s004310000648 Google Scholar
A. B. Bhatt et al.,
“Abnormal Doppler flow velocimetry in the growth restricted fetus as a predictor for necrotizing enterocolitis,”
J. Postgrad. Med., 48
(3), 182
–185
(2002). Google Scholar
F. M. van Haren et al.,
“Gastrointestinal perfusion in septic shock,”
Anaesth. Intensive Care, 35
(5), 679
–694
(2007). Google Scholar
J. Cortez et al.,
“Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates,”
J. Matern. Fetal Neonatal Med., 24
(4), 574
–582
(2011). http://dx.doi.org/10.3109/14767058.2010.511335 Google Scholar
J. P. Mintzer et al.,
“Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates,”
J. Neonatal Perinatal. Med., 7
(3), 199
–206
(2014). http://dx.doi.org/10.3233/NPM-14814035 Google Scholar
F. F. Farag et al.,
“Near-infrared spectroscopy of the urinary bladder during voiding in men with lower urinary tract symptoms: a preliminary study,”
BioMed. Res. Int., 2013 452857
(2013). http://dx.doi.org/10.1155/2013/452857 Google Scholar
D. Balaguru et al.,
“Computed tomography scan measurement of abdominal wall thickness for application of near-infrared spectroscopy probes to monitor regional oxygen saturation index of gastrointestinal and renal circulations in children,”
Pediatr. Crit. Care Med., 12
(3), e145
–e148
(2011). http://dx.doi.org/10.1097/PCC.0b013e3181e8b430 Google Scholar
B. G. Sood et al.,
“Near infrared spectroscopy as a biomarker for necrotizing enterocolitis following red blood cell transfusion,”
J. Near Infrared Spectrosc., 22
(6), 375
–388
(2014). http://dx.doi.org/10.1255/jnirs.1135 Google Scholar
T. E. Schat et al.,
“Abdomial near-infrared spectroscopy in preterm infants: a comparison of splanchnic oxygen saturation measurements at two abdominal locations,”
Early Hum. Dev., 90 371
–375
(2014). http://dx.doi.org/10.1016/j.earlhumdev.2014.04.008 Google Scholar
N. Shah et al.,
“Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers,”
J. Clin. Anesth., 24
(5), 385
–391
(2012). http://dx.doi.org/10.1016/j.jclinane.2011.10.012 Google Scholar
S. J. Barker,
“‘Motion-resistant’ pulse oximetry: a comparison of new and old models,”
Anesth. Analg., 95
(4), 967
–972
(2002). http://dx.doi.org/10.1213/00000539-200210000-00033 Google Scholar
A. E. El-Desoky et al.,
“Change in tissue oxygenation of the porcine liver measured by near-infrared spectroscopy,”
Liver Transplant. Surg., 5
(3), 219
–226
(1999). http://dx.doi.org/10.1002/(ISSN)1527-6473a Google Scholar
A. E. El-Desoky et al.,
“Measurement of hepatic tissue hypoxia using near infrared spectroscopy; comparison with hepatic oxygen partial pressure,”
Eur. Surg. Res., 32
(4), 207
–214
(2000). http://dx.doi.org/10.1159/000008766 Google Scholar
A. E. El-Desoky et al.,
“Assessment of hepatic ischaemia reperfusion injury by measuring intracellular tissue oxygenation using near infrared spectroscopy,”
Liver Int., 21
(1), 37
–44
(2001). http://dx.doi.org/10.1034/j.1600-0676.2001.210106.x LIVEDR 0106-9543 Google Scholar
J. Vanderhaegen et al.,
“Use of the liver tissue oxygenation index as a noninvasive parameter of intestinal ischemia in rabbits,”
World J. Surg., 31 2359
–2362
(2007). http://dx.doi.org/10.1007/s00268-007-9269-y Google Scholar
E. Nahum et al.,
“Correlation of transcutaneous hepatic near-infrared spectroscopy readings with liver surface readings and perfusion parameters in a piglet endotoxemic shock model,”
Liver Int., 26 1277
–1282
(2006). http://dx.doi.org/10.1111/liv.2006.26.issue-10 Google Scholar
A. N. Gay et al.,
“Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a usedful indicator of intestinal blood flow and necrtotizing enterocolitis in premature piglets,”
J. Pediatr. Surg., 46 1034
–1040
(2011). http://dx.doi.org/10.1016/j.jpedsurg.2011.03.025 Google Scholar
I. J. Zamora et al.,
“Low abdominal NIRS values and elevated plasma intestinal fatty acid-binding protein in a premature piglet model of necrotizing eneterocolitis,”
PLoS One, 10
(6), e0125437
(2015). http://dx.doi.org/10.1371/journal.pone.0125437 POLNCL 1932-6203 Google Scholar
G. J. Beilman et al.,
“Near-infrared spectroscopy measurement of regional tissue oxyhemoglobin saturation during hemorrhagic shock,”
Shock, 12
(3), 196
–200
(1999). Google Scholar
S. Mcneill et al.,
“Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants,”
J. Perinatol., 31 51
–57
(2011). http://dx.doi.org/10.1038/jp.2010.71 Google Scholar
S. M. Bailey et al.,
“Splanchnic-cerebral oxygenation ratio (SCOR) values in healthy term infants as measured by near-infrared spectroscopy (NIRS),”
Pediatr. Surg. Int., 29 591
–595
(2013). http://dx.doi.org/10.1007/s00383-013-3285-9 Google Scholar
A. J. Petros et al.,
“Near infrared spectroscopy can detect changes in splanchnic oxygen delivery in neonates during apneic episodes,”
Eur. J. Pediatr., 158
(2), 173
–174
(1999). http://dx.doi.org/10.1007/s004310051046 Google Scholar
J. Li et al.,
“Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic hemodynamic variables and oxygen transport after the Norwood procedure,”
Heart, 92
(11), 1678
–1685
(2006). http://dx.doi.org/10.1136/hrt.2005.087270 Google Scholar
J. P. Mintzer et al.,
“Gamma. Effects of sodium bicarbonate correction of metabolic acidosis on regional tissue oxygenation in very low birth weight neonates,”
J. Perinatol., 35 601
–606
(2015). http://dx.doi.org/10.1038/jp.2015.37 Google Scholar
J. L. Aschner and R. L. Poland,
“Sodium bicarbonate: basically useless therapy,”
Pediatrics, 122 831
–835
(2008). http://dx.doi.org/10.1542/peds.2007-2400 PEDIAU 0031-4005 Google Scholar
V. Bozzetti et al.,
“Cerebral and somatic NIRS-determined oxygenation in IUGR preterm infants during transition,”
J. Matern. Fetal Neonatal Med., 29
(3), 443
–446
(2016). http://dx.doi.org/10.3109/14767058.2014.1003539 Google Scholar
V. Dave et al.,
“Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding,”
J. Perinatol., 29 213
–218
(2009). http://dx.doi.org/10.1038/jp.2008.189 Google Scholar
C. Dani et al.,
“Splanchnic tissue oxygenation for predicting feeding tolerance in preterm infants,”
J. Parenter. Enteral Nutr., 39
(8), 935
–940
(2015). http://dx.doi.org/10.1177/0148607114538671 Google Scholar
M. Gillam-Krakauer et al.,
“Correlation of abdominal with superior mesenteric artery velocities in preterm infants,”
J. Perinatol., 33
(8), 609
–612
(2013). http://dx.doi.org/10.1038/jp.2013.3 Google Scholar
P. M. Fortune, M. Wagstaff and A. J. Petros,
“Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates,”
Intensive Care Med., 27 1401
–1407
(2001). http://dx.doi.org/10.1007/s001340100994 ICMED9 0342-4642 Google Scholar
P. Mally et al.,
“Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates,”
Am. J. Perinatol., 23 451
–458
(2006). http://dx.doi.org/10.1055/s-2006-951300 Google Scholar
T. Marin et al.,
“Feeding preterm infants during red blood cell transfusion is associated with a decline in postprandial mesenteric oxygenation,”
J. Pediatr., 165
(3), 464
–471
(2014). http://dx.doi.org/10.1016/j.jpeds.2014.05.009 Google Scholar
S. M. Bailey et al.,
“Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants,”
Am. J. Perinatol., 27 445
–453
(2010). http://dx.doi.org/10.1055/s-0030-1247598 Google Scholar
C. Dani et al.,
“Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants,”
Transfusion, 50 1220
–1226
(2010). http://dx.doi.org/10.1111/trf.2010.50.issue-6 TRANAT 0041-1132 Google Scholar
J. Banerjee, T. Leung and N. Aladangady,
“Effect of blood transfusion on intestinal blood flow and oxygenation in extremely preterm infants during first week of life,”
Transfusion, 56
(4), 808
–815
(2016). http://dx.doi.org/10.1111/trf.2016.56.issue-4 Google Scholar
S. M. Bailey, K. D. Hendricks-Munoz and P. Mally,
“Splanchnic-cerebral oxygenation ratio as a marker of preterm infant blood transfusion needs,”
Transfusion, 52 252
–260
(2012). http://dx.doi.org/10.1111/trf.2012.52.issue-2 TRANAT 0041-1132 Google Scholar
K. R. Ward et al.,
“Near infrared spectroscopy for evaluation of the trauma patient: a technology review,”
Resuscitation, 68 27
–44
(2006). http://dx.doi.org/10.1016/j.resuscitation.2005.06.022 RSUSBS 0300-9572 Google Scholar
A. Kalkan et al.,
“Abdominal oxygen saturation for monitoring return of sponataneous circulation in out-of-hospital cardiac arrest using near infrared spectrophometry,”
Am. J. Emerg. Med., 33 344
–348
(2015). http://dx.doi.org/10.1016/j.ajem.2014.11.029 Google Scholar
S. Widder et al.,
“Use of near-infrared spectroscopy as a physiologic monitor for intra-abdominal hypertension,”
J. Trauma: Inj. Inf. Crit. Care, 64
(5), 1165
–1168
(2008). http://dx.doi.org/10.1097/TA.0b013e31814695dd Google Scholar
A. J. Crerar-Gilbert et al.,
“Assessment of photoplethysmographic signals for the determination of splanchnic oxygen saturation in humans,”
Anaesthesia, 57 442
–445
(2002). http://dx.doi.org/10.1046/j.0003-2409.2001.02453.x Google Scholar
BiographySean M. Bailey is an assistant professor of pediatrics in the Division of Neonatology at New York University School of Medicine. He is the medical director of the Neonatal Intensive Care Unit at NYU Langone Medical Center as well as the director of the Neonatal Simulation Training Program. In addition to overseeing and providing direct clinical care, he also spends much time conducting research pertaining to critically ill infants. Pradeep V. Mally is an associate professor of pediatrics in the Division of Neonatology at New York University School of Medicine. He is the Chief of the Division and also the director of the NYU Neonatology Fellowship Program. He oversees the care provided in the neonatal intensive care units at both NYU Langone Medical Center as well as Bellevue Hospital Center. In addition, he directs clinical research activities for the division. |