|
|
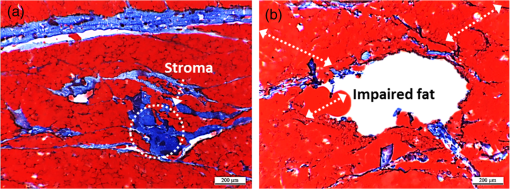
1.IntroductionLaser-assisted lipolysis is a minimally invasive surgical procedure to remove adipose tissue by applying high optical energy.1,2 Based upon absorption spectra, relatively strong light is absorbed by fat or water, resulting in a temperature increase during laser irradiation, which leads to an irreversible thermal decomposition in the tissue.2 Absorption coefficients of the fat are typically associated with volumetric energy deposition in the tissue. Thus, the amount of energy accumulation in the adipose tissue determines the degree of tumefaction, irreversible cellular damage, blood coagulation, and collagen contraction in the dermis.2 Typically, the laser lipolysis procedure is conducted using a 300- or optical fiber with a flat tip (i.e., end-firing), delivered through a 1-mm cannula.3–6 Tumescent liquid is injected through the incision in order to provide local anesthesia to the tissue and reduce any probable side-effects such as blood loss, pain, and bruising.7,8 After inserting the cannula through the skin, plastic surgeons begin to move the optical fiber back and forth in the upper part of the adipose layer in order to melt fat.9 During the laser lipolysis, the interstitial temperature is normally maintained between 38°C and 45°C to ensure safety of the procedure and to achieve favorable clinical outcomes, including neocollagenesis and skin tightening.2 However, high irradiance from the fiber tip, direct tissue contact, and slow fiber movements often cause temperature increase higher than 65°C. The overheating causes excessive thermal injury in the subcutaneous fat layers (i.e., adipocytes and dermal collagen fiber) and even carbonization,10 leading to complications such as swelling, bruising, and bursting of the cell membrane.11,12 A number of clinical studies have evaluated various wavelengths for laser lipolysis, including 980, 1064, and 1320 nm.2,9,13 Initially, the wavelength of 1064 nm was used for clinical lipolysis due to deep optical penetration in tissue. However, the low heat absorption by the adipose tissue often induced severe thermal damage to the collagen in the subcutaneous adipose tissue. On account of higher light absorption than 1064 nm, the 980-nm wavelength was employed instead, but the clinical results demonstrated that the wavelength was less effective for skin tightening.10,14 Lately, the wavelengths ranging from 1320 to 2100 nm were used with flat fibers as both fat and water attain high light absorption.15 As a result, patients experience fewer complications such as burns.15 Recently, diffusing optical applicators were introduced to treat tubular structure tissue in a circumferential manner.16 Laser micromachining creates the predetermined lozenge patterns on the fiber surface, eventually scattering laser light in a radial direction.16,17 In turn, the circumferential irradiation is associated with the decreased irradiance along the 1-cm long diffuser tip, which entails uniform thermal denaturation in the tubular tissue without any carbonization on the tissue–fiber interface. Thus, unlike the flat-cut ended fibers, the diffusing fibers can distribute a wide range of optical energy along the fiber and facilitate photothermal effects in the tissue simultaneously. The previous studies demonstrated that due to low and consistent temperature development, the diffusing applicator would be a minimally invasive device to treat the tubular tissue structure in a safe and efficient manner. The aim of the current study was to evaluate the feasibility of radially diffusing optical applicators for clinical laser lipolysis. It was hypothesized that the optical diffusers with uniform and wide light distribution would entail a low temperature increase and could efficiently remove a large volume of adipose tissue with minimal thermal injury to adjacent tissue. Thus, commercial flat fibers could be replaced by the proposed diffusing applicator due to higher efficacy and safety during the laser lipolysis. Liquefaction threshold and corresponding temperature were initially quantified for the diffusers. Both the flat-cut ended bare fibers and the diffusers were comparatively evaluated in terms of temperature elevation and amount of fat removal on ex vivo porcine adipose tissue. Temporal development of the tissue liquefaction was also monitored using both microscope and thermal cameras. 2.Materials and MethodsFor sample preparation, adipose tissue was harvested from abdominal area in a porcine body and was sliced by 1 to 2 mm thin layers () for laser liquefaction tests. Prior to the tests, all the samples were stored at room temperature (20°C) to minimize any dehydration and structural deformation. A multimode fiber (, FT600EMT, Thorlabs, Newton, New Jersey) was used to prepare a diffusing optical applicator to emit laser light in a radial manner. A 30-W laser (, vi30, Synrad, Mukilteo, Washington) was employed to ablate the surface of the optical fiber. During the laser fabrication, the fiber was moved along the fiber axis to create a series of lozenge patterns (10 mm long) for radial light diffusion. Then, to protect the diffusing tip from any mechanical shocks, a glass cap (17 mm long and 1.5 mm in outer diameter) customized by a local glass company was glued to the fabricated tip by sealing with epoxy. Upon fabrication, a customized goniometer was used to validate the spatial light distribution of the diffusing tip in terms of polar and longitudinal emissions. The measured laser power was normalized to demonstrate the spatial distribution of light intensity (in arbitrary unit) radiating from the diffusing applicator. The detailed description on fiber machining can also be found in a previous study.16 Figure 1 shows an experimental setup for ex vivo laser lipolysis tests. As a conventional wavelength for laser fat removal,2 a 980-nm laser system (S60-980-0, Apollo Instruments, Irvine, California) was employed to liquefy adipose tissue samples at various power levels (8 to 20 W) and irradiation times (0 to 60 s). A flat fiber (, FT600EMT, Thorlabs, Newton, New Jersey) was comparatively evaluated with a diffusing applicator as the flat fibers have clinically been accepted as a standard device for laser lipolysis.5 In turn, both the fibers were tested ex vivo to identify the thermal responses of porcine abdomen adipose tissue during/after laser irradiation. Prior to the lipolysis tests, each fiber was placed above a microscope slide and then completely covered with an adipose sample (Fig. 1). Thus, all the testing conditions confirmed that each fiber made direct contact with the tissue surface to emulate clinical situations.18 A digital microscope camera (AM413ZT, Dino-Lite, New Taipei City, and Taiwan) was situated 5 cm below the microscope slide to record the real-time processes of fat liquefaction. A low-pass filter in a 2.54-cm diameter was placed in front of the microscope camera to filter out the incident wavelength (i.e., 980 nm) during the irradiation. To monitor the spatial-temporal distribution of temperature in the adipose tissue, a thermal IR camera (FLIR A310, FLIR, Wilsonville, Oregon) was also positioned 40 cm above the sample surface during the treatment tests (Fig. 1). The acquired images were transferred to and stored in a computer for postexperimental processing. Fig. 1Experimental setups for ex vivo 980-nm laser lipolysis with diffusing applicator on porcine adipose tissue (, , , , , , , , and ). 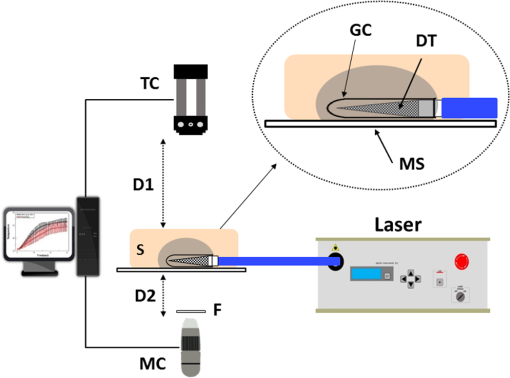 Initially, both threshold time and temperature rise during laser liquefaction were investigated as a function of power level (8, 12, 16, and 20 W) for a diffusing applicator. The irradiation times were varied from 1 to 30 s with an increment of 1 s at each applied power until the fat liquefaction commenced. Both the recorded digital images and IR data validated the onset of the adipose tissue melting (phase transition temperature for porcine to 40°C19), and the corresponding temperature was recorded at the tested power. Each condition was conducted five times (). After the threshold testing, the temporal development of the temperature in the adipose tissue was examined for both the flat fiber and the diffusing applicator at the power level of 20 W. Typically, 60 to 65°C is the desired skin temperature during interstitial laser lipolysis.12 Thus, once the maximum temperature was identified for both the fibers at 20 W, the laser power was adjusted to obtain the identical maximum tissue surface temperature below after 1-min irradiation and eventually to apply the same testing conditions (i.e., 10 W for flat fiber versus 16 W for diffusing applicator). A thermal IR camera recorded the temperature development in the tissue for 60 s. The area of the temperature increase was also measured for comparative analysis by using Image J (National Institute of the Health, Bethesda, Maryland). Five measurements were performed for each condition (). After each testing, the light transmission rate of both the fibers was quantitatively examined to minimize the effect of degradation on the laser liquefaction. Thus, the fibers were immediately replaced with fresh ones when the transmission dropped more than 10%. In an attempt to identify the efficiency of bulky fat removal, both flat fiber (10 W) and diffusing applicator (16 W) delivered 980-nm light to adipose tissue samples for 1 and 5 min. Prior to the testing, each tissue was drilled to create a hole ( in diameter), where each fiber was inserted into. To compare the degree of fat removal between the fibers, a scale was used to measure the weight of each sample before and after the laser lipolysis at two irradiation conditions (1 and 5 min) and to calculate the fat reduction weight for each fiber (). For histological analysis, both control and the tested samples (1-min irradiated) were initially embedded in the optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan) and stored at . Then, each frozen sample was cut into 10-mm thick slides. The prepared slides were fixed in formalin and washed briefly using tap water and 60% isopropanol. The sliced samples were stained with an Oil Red O (Sigma, St. Louis, Missouri) working solution (i.e., 0.15 g of Oil Red O, 30 mL of isopropanol, and 20 mL of distilled water) for 15 min and washed by using 60% isopropanol. The purpose of using the Oil Red O was both lipid detection and fat staining. Typically, paraffin-embedded tissue sectioning requires gradient solvents such as xylene and ethanol, which can dissolve the melted fat (i.e., liquid condition). Thus, the Oil Red O staining on the frozen tissue was instead used to assess the melted adipose tissue after laser irradiation without any morphological change. After counterstaining with hematoxylin, the slides were imaged by using an optical microscope (Olympus BX51, Hamburg, Germany) at the magnification of 100. The degree of the liquefied adipose tissue was measured from the lumen surface by using Image J. For nonparametric statistical analysis, Mann–Whitney U test was performed by using SPSS (Ver 22, IBM Corporation, Armonk, New York), and represents insignificant. 3.ResultsFigure 2(a) shows images of flat fiber and the fabricated glass-capped diffusing applicator for visible observation. Transmission of HeNe laser light () qualitatively confirmed that the flat fiber delivered the light in a forward direction whereas the diffusing applicator emitted in a radial direction. A goniometer system was used to quantify both polar and longitudinal distribution of the normalized laser intensity along the diffuser. According to three-dimensional (3-D) light distribution in Fig. 2(b), the spatial profile was slightly skewed to the distal end of the diffuser, and the mean normalized intensity was estimated to be over the entire diffusing length. The maximum intensity measured from the middle point (5 mm) of the diffusing tip (in red color) was used for normalization. Figure 2(c) shows almost isotropic radiation around the diffuser axis (5 mm from distal end) with less than 5% deviation from the average angular intensities over for the whole diffuser length. Fig. 2Spatial light distribution from diffusing applicator: (a) images of flat fiber (left) and diffusing applicator (right) with HeNe light transmission, (b) normalized 3-D distribution of light irradiation for diffusing applicator, and (c) corresponding polar emissions measured by goniometer ( and ). Note that red color represents the maximum intensity used for normalization (5 mm from distal end). 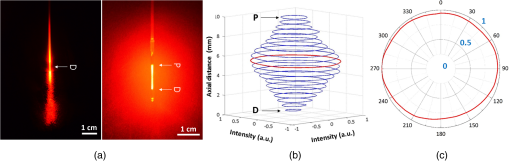 Figure 3 showed liquefaction threshold (left axis) and temperature (right axis) of adipose tissue with a diffusing applicator as a function of laser power (8, 12, 16, and 20 W). For the liquefaction threshold, the higher power precipitated the melting process more rapidly than the lower power (i.e., for 8 W versus for 20 W). Overall, the onset time for the tissue liquefaction linearly decreased with the applied power (simple linear regression, ). However, the corresponding temperature was almost constant and ranged between 29°C and 31°C (), regardless of power. Thus, the adipose tissue commenced to melt at the temperature of around 30°C under the current testing conditions. On the other hand, a flat fiber instantly elevated the temperature over 30°C and melted the tissue sample within 1 s, which was difficult to precisely quantify during the tests. Thus, it was validated that the flat fiber initiated the fat liquefaction considerably faster than the diffusing applicator (e.g., for flat versus 6 s for diffusing at 20 W). Fig. 3Liquefaction threshold and corresponding temperature as function of power with diffusing applicator. 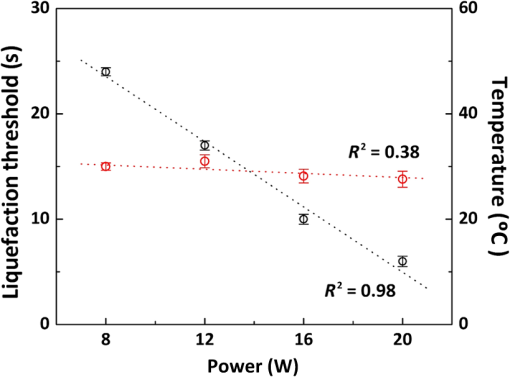 Figure 4 demonstrates temporal developments of temperature in adipose tissue during laser irradiation with a flat fiber and a diffusing applicator. Under the application of the identical power (20 W) in Fig. 4(a), the flat fiber induced a 30% faster temperature increase rate () along with a 25% higher maximum temperature ( in 1 min) than the diffusing applicator ( and in 1 min, respectively; ). Due to the generation of a higher maximum temperature, the flat fiber caused excessive carbonization in the adipose tissue as well as severe fiber deterioration. However, no or minimal thermal damage occurred at the tissue for the diffusing applicator. On the basis of the maximum temperature in Fig. 4(a), the applied power levels for the flat fiber and the diffusing applicator were then regulated to achieve the identical maximum temperature of 60°C to 65°C, which is the favorable temperature for interstitial laser lipolysis.12 Figure 4(b) presents the temperature elevations in the tissue with the flat fiber at 10 W and the diffusing applicator at 16 W. Due to the differences in the power levels, both the fibers accompanied the equivalent temperature increase rate () with the maximum temperature in 1 min (; ). Figure 4(c) demonstrates the corresponding thermal images for spatial temperature distribution in the adipose tissue at various irradiation times (15, 30, and 60 s). It was observed that both the flat fiber and the diffusing applicator entailed the identical maximum temperature. However, the diffusing applicator yielded a twofold wider area of the temperature distribution than the flat fiber (i.e., for flat versus for diffusing at 60 s). Fig. 4Temporal development of temperature in adipose tissue: (a) flat fiber (20 W) and diffusing applicator (20 W), (b) flat fiber (10 W) and diffusing applicator (16 W), and (c) thermal images acquired at various irradiation times (15, 30, and 60 s) with flat fiber (10 W) and diffusing applicator (16 W). Note that the numbers in left upper corners represent the area of temperature distribution (, , ). 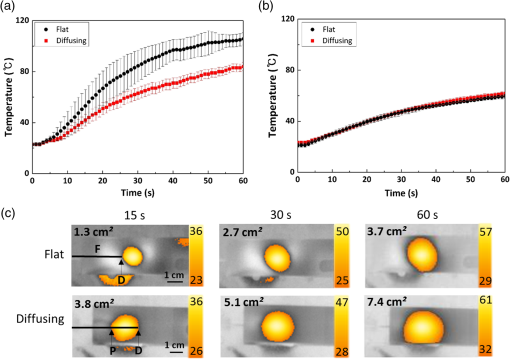 Figure 5 shows a compilation of microscopic images on liquefaction of adipose tissue at various irradiation times with a flat fiber at 10 W in Fig. 5(a) and a diffusing applicator at 16 W in Fig. 5(b). The dotted line represents the total area where the melting occurred in the tissue. The tissue melting was vividly observed at 15 s for both the cases as the threshold was found to be around 10 s or less (Fig. 3). In the case of the flat fiber, the melted area was mostly developed in front of the fiber tip and linearly increased with irradiation times (). On the other hand, the tissue liquefaction occurred around the diffusing applicator in a circumferential manner and showed a more rapid (threefold) increase in the melted area linearly with irradiation times (). Upon the liquefaction, the diffusing applicator melted the adipose tissue in a radial direction at the rate of . For all the recorded times, the diffusing applicator coagulated up to fivefold larger melted tissue area than the flat fiber (e.g., for flat versus for diffusing at 15 s) on account of cylindrical light emission. At 60-s irradiation, both the flat fiber and the diffusing applicator increased the liquefied area even by three- and twofold, respectively (i.e., for flat versus for diffusing) in comparison with the conditions at 15 s (i.e., for flat versus for diffusing). Fig. 5Compilation of microscopic images on tissue liquefaction during laser irradiation with (a) flat fiber (10 W) and (b) diffusing (16 W) applicator ( and ). Note that the numbers in right upper corners represent the area of liquefied tissue. 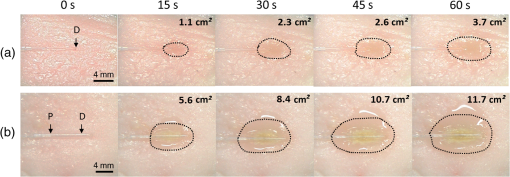 Figure 6 quantitatively compares the degree of fat reduction after 1- and 5-min irradiation with a flat fiber (10 W) and a diffusing applicator (16 W). For the 1-min irradiation, the diffusing applicator removed 63% more tissue than the flat fiber (i.e., for flat versus for diffusing; ). The corresponding removal rates were estimated to be for the flat and for the diffusing. At the 5-min irradiation, the overall fat removal rate proportionally increased for both the cases. The diffusing applicator liquefied () of the adipose tissue whereas the flat fiber did (; ). Thus, the diffusing applicator still facilitated the removal of the adipose tissue by 66%. It was noted that as the irradiation time increased from 1 to 5 min, the removal factors became sixfold for the flat and ninefold for the diffusing. Fig. 6Comparison of tissue removal between flat fiber (10 W) and diffusing applicator (16 W) at two different irradiation times (1 and 5 min; ). 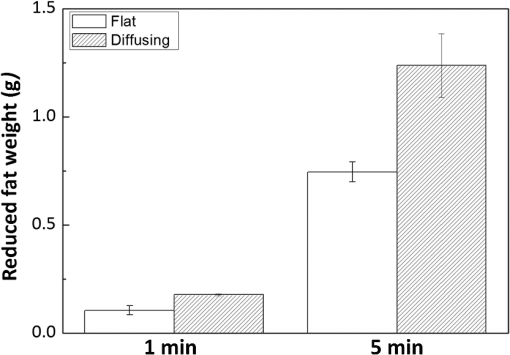 Figure 7 demonstrates Oil Red O-stained histological images of porcine adipose tissue after 1-min irradiation with a diffusing applicator at 16 W. The normal adipose tissue was confirmed by the structures that were enclosed by black lines as shown in Fig. 7(a). In addition, blue lines represent the stroma that supports the structure of the adipose tissue. On the other hand, due to circumferential light irradiation, Fig. 7(b) presented that the distribution of thermal coagulation was almost consistent and circular along the diffusing applicator. White dotted arrows marginally indicate the collapsed/impaired adipose tissue structure in Fig. 7(b). The clear red color in the histology image mirrors the liquefied adipose tissue resulting from thermal decomposition of the tissue structure after the irradiation. In addition, the treated tissue vividly demonstrated the lipid droplets that were slipped down from the stroma (blue lines) due to thermal injury in the adipose tissue structure. The extent of lesion of the impaired adipose tissue was estimated to be around the lumen in Fig. 7(b). 4.DiscussionGiven the identical power (20 W), a diffusing applicator exhibited a 30% lower temperature increase rate with a 25% lower maximum temperature than a flat fiber primarily due to lower irradiance [Fig. 4(a)]. The corresponding irradiances at 1.5 mm away from the tissue surface were estimated to be for the flat fiber and for the diffuser. After power modulation in Fig. 4(b), both the flat fiber (10 W) and the diffusing applicator (16 W) induced rather comparable irradiances (i.e., for flat versus for diffusing). In turn, the adjusted power densities at the tissue surface led to the equivalent temporal development of the tissue temperature for both the fibers as shown in Fig. 4(b). In spite of 60% higher power application, the lower irradiance from the diffuser was associated with steady temperature rises up to 60°C after 1-min interstitial laser lipolysis but simultaneously covered larger areas of the tissue due to wider light distribution ( for flat versus for diffusing after 60-s irradiation). Furthermore, the flat fibers often require fast locomotive movements of a cannula (at 20) during the lipolysis procedure in order to avoid excessive heat accumulation in association with thermal injury. Thus, it is conceived that on account of gradual thermal characteristics, the diffusing applicator may involve relatively slow cannula motions and eventually provide surgeons with more delicate maneuverability and less technique dependence during clinical procedures. On account of radial light distribution, a diffusing applicator coagulated larger areas of adipose tissue, leading to more rapid fat removal than a flat fiber (Figs. 5 and 6). In fact, thermal imaging in Fig. 4(c) validated that circumferential light distribution along the diffuser vividly accompanied wider and longer temperature rises in the adipose tissue during the irradiation. In spite of the comparable total irradiance ( for flat versus for diffusing), a longitudinal distribution of the incident light from the diffusing applicator (Fig. 2) was able to rapidly cover more tissue area by up to sixfold (Fig. 5). Additionally, histological analysis qualitatively confirmed the isotropic liquefaction of the adipose tissue (Fig. 7), implicating that a combination of the polar and the longitudinal emissions contributed to facilitate tissue liquefaction process. It should be noted that the current liquefaction efficiency was comparatively evaluated under static conditions (i.e., no fiber movement) for a short period of irradiation. Conceivably, both dynamic motions of the diffusing light source and long irradiation time could expand the liquefaction coverage during the lipolysis and essentially promote the fat removal process. Therefore, the diffusing applicator with radial distribution of the lower irradiance could help achieve effective laser lipolysis due to a wide range of uniform tissue temperature. Further investigations on dynamic conditions will be performed to elucidate thermal responses of the adipose tissue during the diffuser-assisted laser lipolysis and to identify the optimal therapeutic conditions such as power, cannula velocity, and diffusing length. Although a diffusing optical applicator demonstrated effective adipose liquefaction during laser irradiation, experimental limitations still remained. In spite of radial light distribution, the current emission was still associated with a less homogeneous (i.e., distal end-skewed) profile as presented in Fig. 2(b). Further developments on diffuser fabrication should be pursued to identify the effect of the radial emissions on laser lipolysis, to achieve a flat-top profile from the diffusing surface, and thus to reliably predict temperature distribution in tissue. The current study utilized ex vivo porcine adipose tissue at room temperature to characterize the efficacy of photothermal liquefaction. Thus, due to lower melting point than human body temperature,19 the porcine tissue hardly reflects clinical situations in terms of liquefaction threshold. Moreover, postoperative outcomes still need to be investigated to validate clinical feasibility of the proposed diffuser. Therefore, in vivo mini pig studies with the radial diffusers are currently underway to deliberately emulate clinical conditions for laser liquefaction and to identify preclinical outcomes such as healing response, coagulation of small blood vessels, postoperative skin tightening, and neocollagenesis after the laser lipolysis. Reportedly, a 980-nm wavelength suffers from less effective skin tightening after the laser lipolysis.10 Accordingly, a number of wavelengths (1320, 1470, 1927, and 2100 nm) will be assessed with the diffusing applicator in terms of power, irradiation time, pulse mode (continuous versus pulsed), and cannula velocity to attain the optimal treatment conditions. Finally, to monitor the real-time temperature variations for safe clinical procedures, a thermocouple will also be integrated with the proposed device by adding an extra protective cap. 5.ConclusionThe current study evaluated a radially diffusing optical applicator for feasible liquefaction of adipose tissue in comparison with a flat fiber in terms of temperature development and tissue removal. Both wide light distributions along the diffuser and low irradiance application contributed to achieve gradual temperature elevations and to cover a larger area of tissue liquefaction. Further investigations will test the diffusing applicators with various wavelengths under dynamic conditions in in vivo models to emulate clinical conditions and to optimize therapeutic conditions for diffuser-assisted laser lipolysis. The proposed radially diffusing optical applicator can be a feasible minimally invasive device for thermally treating the adipose tissue in an effective manner. AcknowledgmentsThe research was supported by a grant from Marine Biotechnology Program (20150220) funded by Ministry of Oceans and Fisheries, Korea. ReferencesM. Lukac et al.,
“QCW pulsed Nd: YAG 1064 nm laser lipolysis,”
J. Laser Health Acad., 4
(1), 24
–34
(2009). Google Scholar
S. Tagliolatto, V. B. Medeiros and O. G. Leite,
“Laserlipolysis: update and literature review,”
Surg. Cosmet. Dermatol., 4
(2), 164
–174
(2012). Google Scholar
M. Trelles et al.,
“Laser-assisted lipolysis in the treatment of gynecomastia: a prospective study in 28 patients,”
Lasers Med. Sci., 28
(2), 375
–382
(2013). http://dx.doi.org/10.1007/s10103-011-1043-6 LMSCEZ 1435-604X Google Scholar
S. Mordon et al.,
“Histologic evaluation of laser lipolysis: pulsed 1064-nm Nd: YAG laser versus cw 980-nm diode laser,”
Aesthet. Surg. J., 27
(3), 263
–268
(2007). http://dx.doi.org/10.1016/j.asj.2007.03.005 Google Scholar
J. P. Reynaud et al.,
“Lipolysis using a 980-nm diode laser: a retrospective analysis of 534 procedures,”
Aesthet. Plast. Surg., 33
(1), 28
–36
(2009). http://dx.doi.org/10.1007/s00266-008-9262-3 Google Scholar
J. Dudelzak, M. Hussain and D. J. Goldberg,
“Laser lipolysis of the arm, with and without suction aspiration: clinical and histologic changes,”
J. Cosmet. Laser Ther., 11
(2), 70
–73
(2009). http://dx.doi.org/10.1080/14764170902984895 Google Scholar
J. A. Klein,
“Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction,”
J. Dermatol. Surg. Oncol., 16
(3), 248
–263
(1990). http://dx.doi.org/10.1111/dsu.1990.16.issue-3 Google Scholar
W. Hanke et al.,
“Tumescent liposuction report performance measurement initiative: national survey results,”
Dermatol. Surg., 30
(7), 967
–978
(2004). http://dx.doi.org/10.1111/j.1524-4725.2004.30300.x Google Scholar
R. A. Weiss,
“Laser lipolysis and laser-assisted liposuction,”
Fat Removal: Invasive and Non-Invasive Body Contouring, 120
–136 John Wiley & Sons, New Jersey
(2015). Google Scholar
J. C. McBean and B. E. Katz,
“Laser lipolysis: an update,”
J. Clin. Aesthet. Dermatol., 4
(7), 25
–34
(2011). Google Scholar
B. E. DiBernardo, J. Reyes and B. Chen,
“Evaluation of tissue thermal effects from 1064/1320-nm laser-assisted lipolysis and its clinical implications,”
J. Cosmet. Laser Ther., 11
(2), 62
–69
(2009). http://dx.doi.org/10.1080/14764170902792181 Google Scholar
T. A. Forman and A. Friedman,
“Laser lipolysis with a 980 nm diode laser,”
J. Drugs Dermatol., 9
(5 Suppl ODAC Conf Pt 1), s58
–s61
(2010). Google Scholar
M. D. Palm and M. P. Goldman,
“Laser lipolysis: current practices,”
Semin. Cutan. Med. Surg., 28 212
–219
(2009). http://dx.doi.org/10.1016/j.sder.2009.10.002 Google Scholar
J. G. Khoury et al.,
“Histologic evaluation of interstitial lipolysis comparing a 1064, 1320 and 2100 nm laser in an ex vivo model,”
Lasers Surg. Med., 40
(6), 402
–406
(2008). http://dx.doi.org/10.1002/lsm.v40:6 LSMEDI 0196-8092 Google Scholar
S. Mordon and E. Plot,
“Laser lipolysis versus traditional liposuction for fat removal,”
Expert Rev. Med. Dev., 6
(6), 677
–688
(2009). http://dx.doi.org/10.1586/erd.09.50 1743-4440 Google Scholar
T. H. Nguyen et al.,
“Circumferential irradiation for interstitial coagulation of urethral stricture,”
Opt. Express, 23
(16), 20829
–20840
(2015). http://dx.doi.org/10.1364/OE.23.020829 OPEXFF 1094-4087 Google Scholar
V. V. Volkov et al.,
“Fibreoptic diffuse-light irradiators of biological tissues,”
Quantum Electron., 40
(8), 746
–750
(2010). http://dx.doi.org/10.1070/QE2010v040n08ABEH014338 QUELEZ 1063-7818 Google Scholar
K. Ichikawa et al.,
“Histologic evaluation of the pulsed Nd: YAG laser for laser lipolysis,”
Lasers Surg. Med., 36
(1), 43
–46
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Deman, L. Deman and B. Blackman,
“Melting-point determination of fat products,”
J. Am. Oil Chem. Soc., 60
(1), 91
–94
(1983). http://dx.doi.org/10.1007/BF02540900 Google Scholar
S. R. Mordon et al.,
“Mathematical modeling of laser lipolysis,”
Biomed. Eng. Online, 7
(1), 10
(2008). http://dx.doi.org/10.1186/1475-925X-7-10 Google Scholar
BiographyJieun Hwang is an undergraduate student in the Biomedical Engineering Department, Pukyong National University in the Republic of Korea. Her research interests include laser therapeutics and fiber assisted laser surgery. Nguyen Trung Hau, is a graduate student in the Biomedical Engineering Department, Pukyong National University in the Republic of Korea. He is currently pursuing his PhD at Ubiquitous Sensor Network Laboratory. His research interests include laser-tissue interactions and brain computer interfaces. Sung Yeon Park is an undergraduate student in the Molecular Environmental Biology, University of California, Berkeley. He is interested in studying how to apply biological principles to understand cellular responses and interactions during laser treatment. Yun-Hee Rhee is a research professor in the Beckman Laser Institute Korea, Dankook University in Korea. Her major research is pathology and cell biology, and she currently investigates optical signal cascade. Jin-Chul Ahn is an associate professor in the College of Medicine, Dankook University in the Republic of Korea. His major interest is photo-medicine, and his research fields include photo-modulation using low level light irradiation and laser-assisted cancer treatment. Hyun Wook Kang is an associate professor in the Department of Biomedical Engineering, Pukyong National University in the Republic of Korea. His research interests include laser nano/micromachining, biomedical device design, photonic-phononic interactions, and biomaterial-enhanced photothermal treatment. |