|
|
1.IntroductionNear-infrared (NIR) laser beams have been extensively used in a variety of biophotonic applications, such as laser cutting,1 nonlinear microscopy,2 cell microsurgery,3 Raman spectroscopy,4 and optical tweezers (OTs).4–14 In general, biological systems present low absorption of NIR light, around 700 to 1500 nm, a spectral region localized between high protein absorption (in the visible range) and the increase of water absorption.15 Conversely, lasers in the visible spectral region can induce significant damage to living organisms, even leading to cell death.16 In the biomedical research field, NIR lasers are widely used in optical trapping systems, due to the low NIR light absorption by cells. As the light trapping forces are very small, OTs are employed in the study of cells and particles with dimensions on the order of tens of microns.17 Moreover, the ability to apply and measure forces on the order of piconewtons has also enabled the manipulation and study of molecules, such as DNA18 and chromosome fragments.3 However, OTs have been mostly applied to confine or constrain single cells. The analysis of light scattering from a trapped cell can be used to identify cell size, shape, and internal complexity.19 OTs have also been explored in combination with Raman spectroscopy, allowing the identification of chemical bonds in the trapped cell. This technique can be used to distinguish different bacterial species20 or cancer cells from normal cells.21 Moreover, OTs allow for the transport of cells in homogeneous or heterogeneous liquid media without mechanical contact. Therefore, real-time cell responses to different extracellular environmental conditions can be investigated with OTs.22 Additionally, holographic OTs can be explored to select and place multiple cells in a three-dimensional structure. This technique can be applied to the evaluation of cell proximity in the cellular differentiation process.23 OTs have also been used to study mechanical properties of red blood cells (RBCs).5–13 In particular, mechanical properties of RBCs such as deformability, allow for their passage through capillaries, which is critical to their role of carrying oxygen via blood circulation. Likewise, there is some evidence that RBC deformability is correlated to some pathological conditions,7,24 and, therefore, studies involving this topic are important for the medical and biomedical fields. Raj et al.25 studied the molecular structural changes of a single RBC stretched by optically trapped beads attached to the cell. Nevertheless, as shown in Refs. 4 and 8, the use of Raman microspectroscopy caused considerable, irreversible damage in trapped RBCs due to exposure to a focused, 785-nm optical beam. In particular, damage induced by photochemical and photothermal processes are an important concern when biological cells are trapped. Laser power, irradiation time, and wavelength are main issues governing damage to OT cells.26 In the NIR region, the action spectrum for photodamage on different biological systems, such as Escherichia coli and Chinese hamster ovary cells, has been reported in the literature.27,28 Moreover, Leitz et al.29 have shown that 760-nm laser light can induce photochemical effects in cells of the nematode Caenorhabditis elegans, while 800 nm exposure leads to photothermal damage. Moreover, Liu et al. have shown that NIR laser light of OTs, at 1064 nm, can induce a temperature increase on the order of 10° C/W in Chinese hamster ovary cells.30,31 In this work, we investigated the effect of NIR laser radiation on the biomechanical properties of RBCs by measuring their apparent overall elasticity.7 We exposed RBCs to 10 mW of 785- or 1064-nm laser radiation during 1 or 2 min, subsequent to which elasticity measurements were performed. We show that the analysis of RBC elastic properties can indicate limits for NIR laser application in cell biology. 2.Materials and MethodsOptical damage of NIR-irradiated RBCs was evaluated by deformability analysis through elasticity measurements, exploring 785 nm and 1064 nm OT setups. After trapping the cells with 10 mW of NIR light, the laser power was increased and then the RBC elasticity was evaluated, according to a previously described procedure.5 2.1.Sample PreparationAll blood samples were collected from healthy donors in two 5 mL vacutainer tubes, one containing an ethylenediamine tetraacetic acid (EDTA) anticoagulant solution and another without anticoagulant. After centrifugation, the RBCs obtained from the EDTA blood tube were diluted in serum from the tube without anticoagulant in a proportion of . Large dilutions of the cells in serum were used to avoid floating cells interfering with the measurement. A volume of of this sample was placed in a Neubauer chamber, deep. In the full study, approximately 400 cells were evaluated from four different, healthy donors (report No. 001/2011). 2.2.Irradiation ProcedureRBC samples were exposed to 10 mW of NIR laser power during 1 or 2 min, at 785 or 1064 nm in homemade, optical trapping systems. Neutral density (ND) filters were used to control the laser power on the sample. The 785 nm optical trapping system used a diode laser (XRTA-TOPOPTICA, 785 nm) and a oil immersion objective [UplanSApo, numerical aperture ]. A 1064-nm OT system based on an Ytterbium fiber laser (IPG Photonics, 1064 nm) and oil immersion objective (Achroplan, ) were also used for the cell irradiation procedure. A group of RBCs, the control group, was not irradiated by the 10 mW NIR radiation before elasticity evaluation. 2.3.Elasticity MeasurementElasticity measurements were performed exploring two homemade, optical trapping systems, with a 785-nm and a 1064-nm laser. In both systems, a telescope was used to expand the diameter of the laser beam, allowing use of the nominal NA of the objectives. Figure 1 shows a diagram of the optical trapping systems developed. During the experiments, each RBC was submitted to a set of six drag velocities and its elasticity was obtained by correlating cell elongation with its respective velocity, as described below with more details. Thus, the lower inset of Fig. 1 indicates the drag speeds used in each system. Once trapped, the deformation of the RBCs was obtained by moving a motorized stage in the -direction. In that process, each cell was submitted to six drag speeds (stage velocities). With a CCD camera, connected to a video-capture board (Pinnacle System), videos of the trapped cells were recorded. The video-capture procedure was synchronized with the motorized stage movement. To obtain the cell lengths, we previously calibrated the system by converting pixels to micrometers. An average cell elongation length value was obtained, for each velocity, from 10 frames for each trapped cell. Fig. 1Experimental design of the 785-nm and 1064-nm OT systems. Legend: M, mirror; L, lens; ND, neutral density filter; and BS, beam splitter. 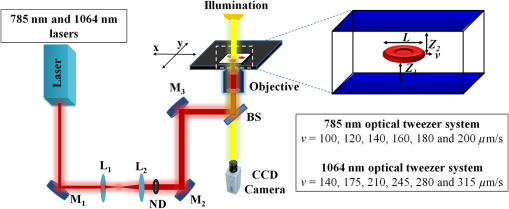 The apparent overall elasticity of the RBC () was associated with the six elongation lengths, , by using a linear fit in accordance with the following expression:7 with being the length of the cell after deformation, is the initial cell length, is the blood serum viscosity, and is the velocity of the cell during elongation. The blood serum viscosity , () cP, was measured previously by using an Ostwald viscometer at 25°C. The cell was located at a distance from the coverslip and from the bottom of the Neubauer chamber. All trapped cells were moved to a fixed height away from the chamber and coverslip surfaces, to avoid changes in the hydrodynamic force due to glass interfaces, so that and, in our measurements . The cell length , , and are indicated in the top right inset of Fig. 1. By automatically measuring the deformed cell length and exploring different drag speeds, the elasticity () of the cell can be determined by linear fitting, according to Eq. (1). For the elasticity measurements, the 785-nm laser power on the sample during elongation measurements was set to approximately 80 mW. A motorized XY stage (Prior Scientific-Prior III) was associated with the 785 nm optical trapping system. Constant drag speeds (100, 120, 140, 160, 180, and ) were used during each individual cell acquisition. The laser power and drag speed values were chosen based on previous work,5 such that the cell was maintained trapped at the maximum velocity applied in order to reach a high elongation, and that reasonable differences of elongation, for each velocity applied, could be measured with confidence.For the elasticity measurements with the 1064 nm OTs system, the laser power on the sample was set to approximately 140 mW. A motorized, computer-controlled XY stage (Prior Scientific-Prior II) was used. Six different predetermined constant drag speeds (140, 175, 210, 245, 280, and ) were set during the acquisition.5 Automatic, real-time evaluation of RBC elasticity by the OT systems and image processing techniques allowed for rapid (20 s) and reproducible evaluation of cell deformability. The whole capture process, as well as the microscope stage movements, was performed by using a Labview platform, in both systems. 3.Results and DiscussionHere RBCs, under optical trapping, were submitted to 10 mW of 785 or 1064-nm irradiation during 1 or 2 min. Approximately 200 RBCs (50 cells per donor) were evaluated under each system. Each one of the 400 cells was measured only once and then discarded. Immediately after irradiation, the laser power was increased by removing the ND filter and the automatic elasticity evaluation procedure was started. The elasticity of the RBCs was obtained from the length deformations in accordance with Eq. (1). Figure 2 shows images of an RBC elongated by the 1064-nm optical trapping system at two different drag speeds (140 and ). The RBC length deformation, of , observed in Fig. 2, is about 10 times greater than the minimal length detectable by the system (). Fig. 2RBC (from the control group) elongations during the automatic elasticity evaluation procedure using the 1064 nm optical trapping system. 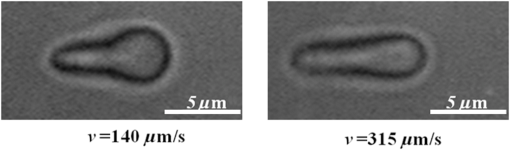 Figure 3 shows the behavior of cell lengths, in relation to the, respectively, applied velocities, during our elasticity measurements. Solid lines were obtained by linear fitting of the experimental data, using Eq. (1). According to Eq. (1), cell elasticity values are determined by the slopes of curves, as shown in Fig 3. One can also notice, in Fig. 3, that the curve slope decreased for cells submitted to NIR irradiation before measurements, indicating an increase of RBC rigidity. The error bars were obtained by evaluating the standard deviation over the 10 greatest lengths, measured at each speed, for each cell. Changes in curve slopes are more significant for cells submitted to the 785 nm than to the 1064-nm laser. Fig. 3Cell length ratios, , in function of drag velocities, for RBCs that were previously exposed to 10 mW of laser radiation at (a) 785 nm and (b) 1064 nm, during 1(red circle) or 2 min (blue triangle), and for RBCs of the control group (black square). 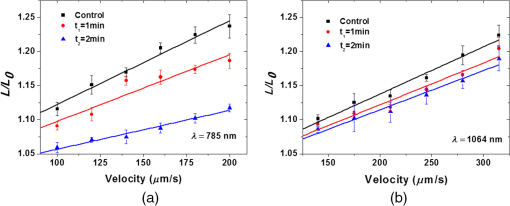 Table 1 shows the elasticity values obtained for the whole cell ensemble involved ( cells). The error values are the standard errors of the mean for each group of cells. For the control samples, with no previous irradiation (), the obtained elasticity values are in accordance with prior studies.5,7,11 For the cells that were exposed to 10 mW of NIR irradiation during 1 min, we observed an increase up to in cell rigidity after 785-nm laser exposure, while stiffness increased after 1064-nm irradiation when compared to the RBCs of the control group. When exposure time was raised to 2 min, an increase of up to on cell rigidity in the 785 nm optical trap was observed, while with the 1064-nm laser it increased by . Table 1Elasticity measurements from the control group and after 1 or 2 min under 10 mW radiation exposure to 785- or 1064-nm laser trapping.
The elasticity evaluation, by the automatic system used,5 takes no longer than 20 s, suggesting that RBCs have no change in their biomechanical properties due to the measurement procedure. To evaluate this hypothesis, control cells (with no previous exposure to laser irradiation) were trapped by 80 mW of 785 and 140 mW of 1064-nm laser light, elongated, and then returned to rest. This procedure was repeated six times with the same drag speed of (for 785-nm laser) and (for 1064-nm laser). The whole process was performed in a time interval of 20 s. Figure 4 shows the behavior of cell elongation under the drag procedure. During the 20 s of evaluation, the cell maintains the same elongation, indicating no change in RBC elasticity. The results shown in Fig. 4(a) (for the 785-nm laser) and Fig. 4(b) (for the 1064-nm laser) were obtained from the evaluation of 10 cells, for each trapping laser wavelength. Figure 4 indicates that the elasticity measurement procedure does not induce optical damage to cells. Therefore, the elasticity changes shown in Table 1 are expressly related to radiation exposure before measurements. During the measurement time using a 785-nm laser, there was a small (not significant) decrease in the length ratio of the cell (), when comparing the initial to the final measurements. In Fig. 4, the solid lines are only visual guides. Fig. 4Elongation measurements for control cells: (a) for the 785 nm optical trapping system at constant velocity and (b) for the 1064 nm optical trapping system at constant velocity. 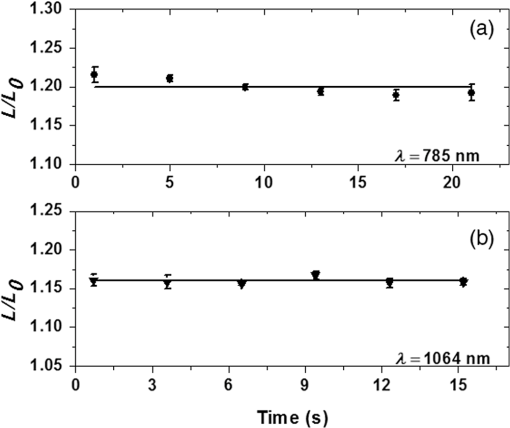 The biomechanics of RBCs is related to the fluid viscosity of their cytoplasm, which is dominated mostly by hemoglobin, and to the viscoelastic properties of the cell membrane, which is associated with the lipid bilayer and cytoskeleton compositions, and also to the interconnections among them with other cellular components.24 Thus, changes related to membrane lipids and proteins, or hemoglobin, e.g., can modify RBC deformability, which can be determined by OT elasticity evaluation. A previous study,4 with RBCs using 10 mW in a 785 nm optical trapping system coupled with Raman spectroscopy, associated optical damage to photochemical changes in the hemoglobin (for the first 500 s of NIR exposure time), followed by an increase in cell-membrane permeability. Moreover, the hemoglobin absorption coefficient at 785 nm is approximately 10 times greater than that at 1064 nm,32 indicating that photodamage can also be determined by the incident laser wavelength, as shown in Table 1. Furthermore, Brandão and coauthors showed that RBCs with hemoglobin S were significantly less elastic than normal ones.7 For the present study, the NIR exposure time was no more than 120 s. In this way, the biomechanical changes observed in this work can be related to, and followed by, initial photo-induced modifications in hemoglobin after irradiation for 1 or 2 min with 785- or 1064-nm laser light. 4.ConclusionsAs far as we know, this is the first work in the literature that evaluates optical trapping damage directly through elasticity measurements. We verify an increase in RBC rigidity by evaluating the apparent overall elasticity of the cells, after exposure to 10 mW of NIR laser radiation at 785 or 1064 nm, during 1 or 2 min. We demonstrated that, in addition to optical damage being associated with laser radiation exposure time, the cell elasticity modifications are also determined by the incident laser wavelength. We observed that the damage caused to the cell is greater at 785 nm than at 1064 nm. We observed that RBCs were up to less deformable after 2 min of 785-nm laser exposure. The 2 min exposure to 10 mW of 1064-nm laser light induced an increase of up to in cell rigidity. We ascribed the differences observed in optical damage to the wavelength absorption by hemoglobin. Moreover, the increase of RBC rigidity could be associated with initial changes caused optically in the hemoglobin after irradiation. Our results establish limits for laser applications in RBCs, by identifying considerable modifications to their elastic properties. AcknowledgmentsThe authors acknowledge the financial support from the Brazilian agencies CAPES/PNPD, FACEPE, CNPq, and the National Institute of Photonics. ReferencesC. Y. Chien and M. C. Gupta,
“Pulse width effect in ultrafast laser processing of materials,”
Appl. Phys. A, 81
(6), 1257
–1263
(2004). http://dx.doi.org/10.1007/s00339-004-2989-z Google Scholar
K. König et al.,
“Cellular response to near-infrared femtosecond laser pulses in two-photon microscopes,”
Opt. Lett., 22 135
–136
(1997). http://dx.doi.org/10.1364/OL.22.000135 OPLEDP 0146-9592 Google Scholar
H. Liang et al.,
“Micromanipulation of chromosomes in ptk2 cells using laser microsurgery (optical scalpel) in combination with laser-induced optical force (optical tweezers),”
Exp. Cell Res., 204
(1), 110
–120
(1993). http://dx.doi.org/10.1006/excr.1993.1015 Google Scholar
M. A. S. de Oliveira et al.,
“Long term Raman spectral study of power-dependent photodamage in red blood cells,”
Appl. Phys. Lett., 104 103702
(2014). http://dx.doi.org/10.1063/1.4868253 APPLAB 0003-6951 Google Scholar
D. S. Moura et al.,
“Automatic real time evaluation of red blood cell elasticity by optical tweezers,”
Rev. Sci. Instrum., 86 053702
(2015). http://dx.doi.org/10.1063/1.4919010 RSINAK 0034-6748 Google Scholar
A. C. De Luca et al.,
“Spectroscopical and mechanical characterization of normal and thalassemic red blood cells by Raman tweezers,”
Opt. Express, 16
(11), 7943
–7957
(2008). http://dx.doi.org/10.1364/OE.16.007943 OPEXFF 1094-4087 Google Scholar
A. Fontes et al.,
“Mechanical and electrical properties of red blood cells using optical tweezers,”
J. Opt., 13
(8), 044012
(2011). http://dx.doi.org/10.1088/2040-8978/13/4/044012 Google Scholar
R. Dasgupta et al.,
“Hemoglobin degradation in human erythrocytes with long-duration near-infrared laser exposure in Raman optical tweezers,”
J. Biomed. Opt., 15 055009
(2010). http://dx.doi.org/10.1117/1.3497048 JBOPFO 1083-3668 Google Scholar
M. L. Barjas-Castro et al.,
“Elastic properties of irradiated RBCs measured by optical tweezers,”
Transfusion, 42 1196
–1199
(2002). http://dx.doi.org/10.1046/j.1537-2995.2002.00201.x TRANAT 0041-1132 Google Scholar
S. Hénon et al.,
“A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers,”
Biophys. J., 76
(2), 1145
–1151
(1999). http://dx.doi.org/10.1016/S0006-3495(99)77279-6 Google Scholar
D. C. N. Silva et al.,
“Optical tweezers as a new biomedical tool to measure zeta potential of stored red blood cells,”
PLoS One, 7
(2), 1
–6
(2012). http://dx.doi.org/10.1371/journal.pone.0031778 POLNCL 1932-6203 Google Scholar
M. M. Haque et al.,
“Stretching of red blood cells using an electro-optics trap,”
Biomed. Opt. Express, 6
(1), 118
–123
(2015). http://dx.doi.org/10.1364/BOE.6.000118 BOEICL 2156-7085 Google Scholar
Y. Kim, K. Kim, Y. Park,
“Measurement techniques for red blood cell deformability: recent advances,”
Blood Cell—An Overview of Studies in Hematology, 167
–194 Intech, New York
(2012). Google Scholar
A. Ashkin, J. Dziedzic and J. M. Yamane,
“Optical trapping and manipulation of single cells using infrared laser beam,”
Nature, 330 769
–771
(1987). http://dx.doi.org/10.1038/330769a0 Google Scholar
K. Svoboda and S. M. Block,
“Biological applications of optical forces,”
Annu. Rev. Biophys. Biomol. Struct., 23 247
–285
(1994). http://dx.doi.org/10.1146/annurev.bb.23.060194.001335 Google Scholar
C. Wang et al.,
“Optical micromanipulation of active cells with minimum perturbations: direct and indirect pushing,”
J. Biomed. Opt., 18
(4), 045001
(2013). http://dx.doi.org/10.1117/1.JBO.18.4.045001 JBOPFO 1083-3668 Google Scholar
K. C. Neuman and S. M. Block,
“Optical trapping,”
Rev. Sci. Instrum., 75
(9), 2787
–2809
(2004). http://dx.doi.org/10.1063/1.1785844 RSINAK 0034-6748 Google Scholar
C. Bustamante, Z. Bryant and S. B. Smith,
“Ten years of tension: single-molecule DNA mechanics,”
Nature, 4216921 423
–427
(2003). http://dx.doi.org/10.1038/nature01405 Google Scholar
D. Watson et al.,
“Elastic light scattering from single cells: orientational dynamics in optical trap,”
Biophys. J., 87 1298
–1306
(2004). http://dx.doi.org/10.1529/biophysj.104.042135 BIOJAU 0006-3495 Google Scholar
C. Xie et al.,
“Identification of single bacterial cells in aqueous solution using confocal laser tweezers Raman spectroscopy,”
Anal. Chem., 77 4390
–4397
(2005). http://dx.doi.org/10.1021/ac0504971 ANCHAM 0003-2700 Google Scholar
F. Zheng, Y. Qin and K. Chen,
“Sensitivity map of laser tweezers Raman spectroscopy for single-cell analysis of colorectal cancer,”
J. Biomed. Opt., 12
(3), 034002
(2007). http://dx.doi.org/10.1117/1.2748060 JBOPFO 1083-3668 Google Scholar
E. Eriksson et al.,
“Optical manipulation and microfluidics for studies of single cell dynamics,”
J. Opt. A, 9
(8), S113
–S121
(2007). http://dx.doi.org/10.1088/1464-4258/9/8/S02 JOAOF8 1464-4258 Google Scholar
P. Jordan et al.,
“Creating permanent 3D arrangements of isolated cells using holographic optical tweezers,”
Lab Chip, 5 1224
–1228
(2005). http://dx.doi.org/10.1039/b509218c LCAHAM 1473-0197 Google Scholar
G. Tomaiuolo,
“Biomechanical properties of red blood cells in health and disease towards microfluidics,”
Biomicrofluidics, 8 051501
(2014). http://dx.doi.org/10.1063/1.4895755 1932-1058 Google Scholar
S. Raj et al.,
“Mechanochemistry of single red blood cells monitored using Raman tweezers,”
Biomed. Opt. Express, 3
(4), 753
–763
(2012). http://dx.doi.org/10.1364/BOE.3.000753 BOEICL 2156-7085 Google Scholar
H. Zhang and K.-K. Liu,
“Optical tweezers for single cells,”
J. R. Soc. Interface, 5 671
–690
(2008). http://dx.doi.org/10.1098/rsif.2008.0052 1742-5689 Google Scholar
K. C. Neuman et al.,
“Characterization of photodamage to Escherichia coli in optical traps,”
Biophys. J., 77
(5), 2856
–2863
(1999). http://dx.doi.org/10.1016/S0006-3495(99)77117-1 BIOJAU 0006-3495 Google Scholar
H. Liang et al.,
“Wavelength dependence of cell cloning efficiency after optical trapping,”
Biophys. J., 70
(3), 1529
–1533
(1996). http://dx.doi.org/10.1016/S0006-3495(96)79716-3 BIOJAU 0006-3495 Google Scholar
G. Leitz et al.,
“Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence,”
Biophys. J., 82 2224
–2231
(2002). http://dx.doi.org/10.1016/S0006-3495(02)75568-9 BIOJAU 0006-3495 Google Scholar
Y. Liu et al.,
“Evidence for localized cell heating induced by infrared optical tweezers,”
Biophys. J., 68
(5), 2137
–2144
(1995). http://dx.doi.org/10.1016/S0006-3495(95)80396-6 BIOJAU 0006-3495 Google Scholar
Y. Liu et al.,
“Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry,”
Biophys. J., 71
(4), 2158
–2167
(1996). http://dx.doi.org/10.1016/S0006-3495(96)79417-1 BIOJAU 0006-3495 Google Scholar
T. G. Phan and A. Bullen,
“Practical intravital two-photon microscopy for immunological research: faster, brighter, deeper,”
Immunol. Cell Biol., 88
(4), 438
–444
(2010). http://dx.doi.org/10.1038/icb.2009.116 ICBIEZ 0818-9641 Google Scholar
BiographyMarcos A. S. de Oliveira earned his doctoral degree in electrical engineering with emphasis on photonics at the Federal University of Pernambuco, Brazil, in 2014. Currently, he occupies the postdoctoral research position in the Biomedical and Imaging Optics Laboratory at the Federal University of Pernambuco. His main current research interests are related to the development of systems using smartphone platforms, Raman microspectroscopy, and optical tweezers (OTs) associated with biomedical optics. Diógenes S. Moura earned his doctoral degree in electrical engineering with emphasis on photonics at the Federal University of Pernambuco, Brazil, in 2016. Currently, he is a teacher at the Application High School of the Federal University of Pernambuco. His main current research interests are related to the development of optical instrumentation, image processing, laser ablation, and OTs in biomedical applications. Adriana Fontes earned her doctoral degree in physics at the State University of Campinas. She is currently a professor of the Biophysics and Radiobiology Department of the Federal University of Pernambuco. Her research lines are OTs for studying biological systems and synthesis and application of quantum dots as fluorescent probes. In 2008, she received the Brazilian L’Oreal Grant for Young Women in Science. She was also an affiliate member of the Brazilian Academy of Sciences from 2009 to 2013. Renato E. de Araujo earned his doctoral degree in physics at the Federal University of Pernambuco, Brazil, in 2001. He did his postdoctoral training in the Robotic Institute at the Carnegie Mellon University, in 2002, and at the Cedars-Sinai Medical Center, Los Angeles, in 2003. He has been a professor at the Federal University of Pernambuco since 2006. His main research interests are biophotonics, nanotechnology, biosensors, optical spectroscopy, and imaging. |
|||||||||||||||

