|
|
1.IntroductionOsteoarthritis (OA) is the most commonly found form of arthritis in humans,1 characterized as a degenerative disease that affects the joints as a whole,2 including the articular cartilage, subchondral bone, ligaments, joint capsule, synovial membrane, and periarticular muscles.3 The pathological effects on these structures are manifested by morphological, biochemical, and biomechanical alterations.4 OA is characterized by a plethora of inflammatory mediators and cytokine production beside leukocyte infiltration, especially neutrophils.4,5 Additionally, signs of cartilage degeneration, such as the activation of matrix metalloproteinases (MMPs), are involved in important aspects of tissue damage, leading to the progressive destruction of the joint adjacent structures.4 The standard algorithm for OA treatment is to control symptoms, especially pain, prevent progression of the disease, minimize disability, and improve the quality of life of patients.6 This algorithm can be divided into three main steps: pharmacological treatment, surgery, and nonpharmacological treatments that may be applied alone or in combination.2 Currently, pharmacological treatment is the most commonly used and indicated by clinicians to achieve pain relief in mild to moderate cases of OA.7 However, Bjordal et al.8 clearly demonstrated in a meta-analysis including 13,000 patients that nonsteroidal anti-inflammatory drugs (NSAIDs) and other pharmacological treatments are not different from a placebo concerning OA pain control. In addition, the long-term use of pharmacological treatments causes a wide range of important side-effects.8,9 As an adjunctive treatment for OA, the prescription of moderate exercise is very popular, and the most frequently recommended activities are swimming, dancing, hiking, and cycling. The benefits of this nonpharmacological treatment include the reduction of comorbidities frequently associated with OA, delayed physical and functional limitations, and the preservation of healthy joints.10 On the other hand, the use of photobiomodulation therapy (PBMT) in OA treatment has achieving increasing evidence and power in recent years. PBMT has proven to be an interesting and efficient alternative to pharmacological treatments to OA. It has been demonstrated that PBMT reduces the knee acute inflammation,11,12 reduces cartilage tissue degeneration, and possibly improves tissue repair in OA.12–16 It is believed that the combination of more than one therapy in the treatment of musculoskeletal disorders such as OA would potentiate the beneficial effects observed with each single treatment (e.g., NSAID, exercise, and PBMT). However, to date there are no studies that evaluate the effects of these three important therapies prescribed to treat OA isolated, but especially in combination. It still not known if the use of combined therapy actually results in a better prognostic when compared to a single treatment. In this context, the aim of this study was to evaluate the effects of PBMT, exercise, and topical NSAID alone and as combined therapies, on morphological, inflammatory infiltrate, and related biochemical markers of inflammation and joint degeneration in an experimental model of OA. 2.Material and Methods2.1.AnimalsFifty-four male Wistar rats weighing about 200 to 250 g from the central animal facility of the Institute of Biomedical Sciences, University of São Paulo (USP) were used. The animals were kept in standard conditions of temperature (22 to 24°C), relative humidity (40% to 60%), and light and dark cycles of 12 h, with food and water ad libitum. Experimental protocol was approved by the Ethics Committee for Animal Experimentation of the Institute (number 077, page 130, book 02) of Biomedical Sciences, University of São Paulo (ICB-USP). 2.2.Experimental GroupsThe animals were randomly divided into experimental groups of six animals as follows.
All treatments used to the experimental groups were initiated 21 days after the last injection of papain and occurred once a day, 3 times a week (every other day) for 8 consecutive weeks for a total of 24 therapy sessions. After eight consecutive weeks of treatment, animals were euthanized by cervical dislocation 72 h after the last treatment, in order to collect biological material (intra-articular joint lavage and tissue) for further analysis. 2.3.ProceduresInduction of knee OA: the animals were anesthetized with a mixture of ketamine and xylazine (90 and , respectively; König, Avellaneda, Argentina) intraperitoneally injected. Subsequently, they were submitted to induction of OA in the right knee, according to Murat et al.17 A total of 0.2 mL of papain solution 4%, 0.1 mL of 0.03 M cysteine (activator) were injected intra-articularly with a microsyringe on the right knee of the animal. The needle bevel was positioned medial to the patellar ligament, performing the injection toward the distal articular surface of the femur. This procedure was repeated in the fourth and seventh days after the first application. 2.4.Treatments2.4.1.Isolated therapies
Table 1Exercise protocol.
2.4.2.Association of therapiesAll combined therapies were applied in the same way and with the same parameters used in therapies applied in isolation.
2.5.Collection of Biological MaterialAll animals were anesthetized once intraperitoneally with a mixture of ketamine and xylazine (90 and , respectively), and the biological material (intra-articular lavage and joint tissue) was extracted:
2.6.AnalysisHistology by optical light microscopy: The method used for assembly of the histological slides was based on the study of Murat el al.17 Knee joint samples were washed with saline and fixed in 10% neutral buffered formalin. Subsequently, the samples were decalcified with aqueous 10% formic acid and embedded in paraffin for forming cuts . Once the preparations of the samples were carried out, the sections were placed on glass slides to be stained with hematoxylin and eosin. After staining, the sections were mounted in permanent blades for further examination with the optical microscope (Nikon®, Japan). Subsequently, the sections were photographed by means of a photomicrograph camera. Photographs were taken of all experimental groups using the original magnification . The aspects evaluated were: articular surface, cellularity, and general structure of cartilage. Total cell count: Aliquots of the intra-articular wash were centrifuged at a speed of 1500 rpm for 10 min until a cell pellet formation. The pellet was resuspended in 200 of PBS solution + EDTA + 0.1% BSA. For total cell count, was used intra-articular washed diluted with Turk’s solution () and a Neubauer chamber coupled to an optical microscope (Carl Zeiss) with a magnification. The results were expressed as the total number of leukocytes per joint cavity. Myeloperoxidase (MPO) activity analysis: MPO is a specific neutrophil enzyme used as an indirect indicator of neutrophil infiltration in the tissue. The synovial membrane was homogenized in 0.5% HTAB (Sigma Chemical Co.), using 1 mL buffer per 50 mg of tissue. The homogenate was placed in Eppendorf tubes and heated in an oven for 2 h at 60°C for inactivation of the endogenous catalase activity. Subsequently, the tubes were centrifuged at for 5 min. Then, of supernatant was pipetted (in duplicate) in a microplate of 96 wells and added to of a potassium phosphate buffer solution (pH 6.0) containing o-dianisidine (dihydrochoride) (Sigma Chemical Co.) and 0.0005% hydrogen peroxide (Merck, Germany). The level of absorbance was measured in an ELISA reader (Espectra max plus 384) at 460 nm during 20 min with recordings made at 20-s intervals. Graphs show the function of variation in absorbance time and (maximum reaction speed per second was calculated from these graphs). The time was measured from the absorbance demonstrated linearity of the variation observed in the graph (optical density or absorbance) versus time in seconds, obtaining a value . The MPO activity is defined as the degradation micromol of per minute and was expressed as MPO units (). Analysis of MMP-3 gene expression and MMP-13 by real-time RT-PCR: The sample material was articular cartilage. Total RNA was extracted using Trizol reagent (Gibco BRL) according to manufacturer’s instructions. After treatment with DNase, the synthesis of cDNAs was processed by the method of using the SuperScript reverse transcriptase enzyme (Invitrogen) from of total RNA in the presence of a mixture of random primers and oligo dT. RT-PCR experiments were programmed as follows: an initial one cycle of denaturation for 10 min at 95°C and 40 cycles of amplification (30 s denaturation at 95°C and 1 min annealing and extension at 60°C). The sequences of the primers were the same used by Wang et al.19 and are shown in Table 2. The results were interpreted using the 2-formula (Ct: number of cycles needed to reach the fluorescence threshold above background value — background) that relates the expression of the gene of interest compared the expression of the HPRT gene control. Table 2Following primer.
2.7.Statistical AnalysisInitially, data were tabulated and assessed for normality using the Shapiro-Wilk test, as a normal distribution was obtained and was used for analysis of variance (ANOVA) followed by the Tukey applied for multiple comparisons. Results were expressed as mean and standard error of the mean () and values were considered statistically significant. 3.Results3.1.Histology by Optical Light MicroscopyFigure 1 shows the morphological characteristics of the healthy joint (without OA induction), the joint affected by OA (no treatment), and groups treated with exercise, topical NSAIDs, and PBMT in isolation or associated. We note that the experimental model of OA induction causes morphological changes consistent with the disease, and among treatments, the PBMT is the most effective treatment for reducing these changes. Fig. 1Histologic characterization of the knee joint. (A) Control group: (a) regular articular surface, (b) chondrocytes distributed homogeneously, and (c) zone of resting cartilage; (B) OA group: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) zone of resting cartilage, (d) hypertrophic cartilage, and (e) deep invagination of bone tissue; (C) Exerc group: (a) regular articular surface, (b) chondrocytes distributed heterogeneously, (c) homogeneously, (d) zone of resting cartilage; (D) Diclo group: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) serial cartilage zone, (d) hypertrophic cartilage; (E) PBMT group: (a) regular articular surface, (b) chondrocytes distributed homogeneously, (c) zone of resting cartilage; (F) Exerc + Diclo group: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) zone of resting cartilage; (G) Exerc + PBMT group: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) zone of resting cartilage; (H) Diclo + PBMT: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) zone of resting cartilage, (d) invagination of bone tissue; (I) Exerc + Diclo + PBMT group: (a) irregular articular surface, (b) chondrocytes distributed heterogeneously, (c) zone of resting cartilage, (d) invagination of bone tissue. Magnification 100×. 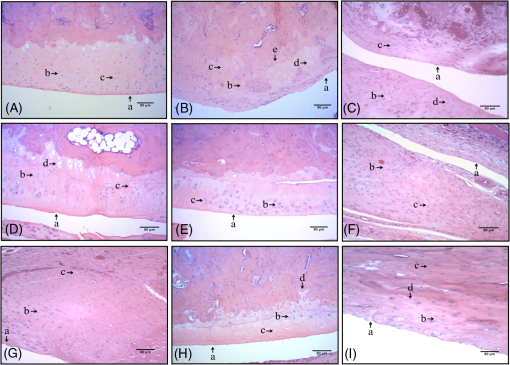 3.2.Total Cell CountFigure 2 shows the total amount of cells in the articular cavity of the animals in all groups. The results demonstrate that the experimental induction model of OA causes an increase in the total number of cells, wherein the PBMT as well as topical NSAID reduce the total number of cells in the inflammatory infiltrate. Fig. 2Total number of cells in the experimental groups. * indicates significant difference compared to the control group. # indicates significant difference compared to the OA group. ** indicates significant difference compared to the Exerc, Exerc + Diclo, Exerc + PBMT, Diclo + PBMT, and Exerc + Diclo + PBMT groups. Error bars indicate SEM. 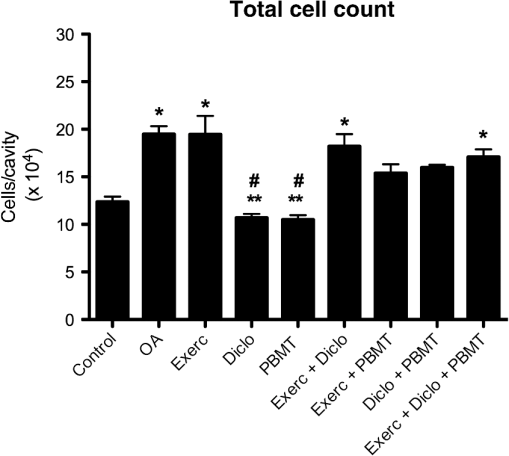 3.3.Myeloperoxidase Activity AnalysisIn Fig. 3, we observed MPO activity in the articular tissue of all groups, it being possible that the experimental model of OA induction caused increased MPO activity, whereas treatment with PBMT was the most effective for reducing the activity of this enzyme. Fig. 3MPO activity in the experimental groups. * indicates significant difference compared to the all experimental groups. # indicates significant difference compared to the control group. ** indicates significant difference compared to the Exerc, Diclo, and Diclo + PBMT groups. Error bars indicate SEM. 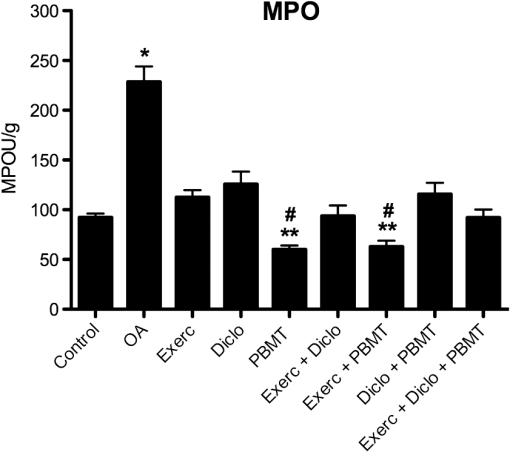 3.4.Gene Expression of MMP-3 and MMP-13 by RT-PCRIn Fig. 4, we find the gene expression levels of MMP-3 and MMP-13 in the joint tissue from all experimental groups, which themselves had increased after induction by our experimental OA model. We observed that both the NSAID and PBMT were effective for reducing the gene expression of MMP-3, however, in relation to the gene expression of MMP-13, PBMT was the most effective treatment. Fig. 4MMP-3 and MMP-13 gene expression in the experimental groups. (a) * indicates significant difference compared to the control group. # indicates significant difference compared to the OA group. (b) * indicates significant difference compared to the control group. # indicates significant difference compared to the OA group. ** indicates significant difference compared to the Diclo + PBMT group. *** indicates significant difference compared to the Exerc + PBMT group. Error bars indicate SEM. 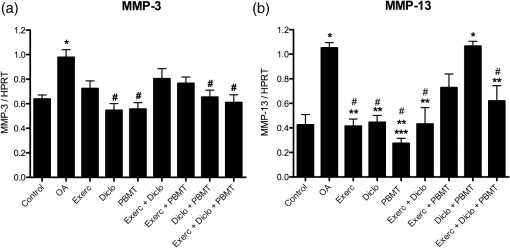 4.DiscussionTo the best of our knowledge, this study is the first to analyze the effects of physical exercise, topical NSAIDs, and PBMT, used in isolation or combination, as the most effective therapy for reducing morphological alterations, degradation signs, and inflammation resulting from OA. Our results demonstrated that the experimental model of OA induction using papain triggered significant morphological alterations, which is consistent with the clinical presentation of OA,13,17 including irregular joint surfaces, fissure areas, heterogeneously distributed chondrocytes, and bone tissue invagination. Furthermore, our experimental model triggered an inflammatory process in the joint, with the release of inflammatory cells to the articular cavity, and increased myeloperoxidase activity. Our results indicated that PBMT was the most effective therapy for controlling the clinical signs and morphological alterations caused by OA, and the outcomes were similar to those observed in a healthy joint, as reported by Alves et al.13 Similarly, PBMT and topical NSAID were the most effective therapies for decreasing the total cell count in the intra-articular lavage, confirming the results obtained in the histological analysis. PBMT and PBMT associated with physical exercises were effective therapies for reducing MPO activity which is an important inflammatory marker.20 Furthermore, our results indicated that PBMT had a major role in the modulation of the inflammation caused by OA by decreasing the important inflammatory markers and possibly contributing to the reduction in disease progression. Confirming our results with those of Da Rosa et al.15 on the effectiveness of PBMT in modulating the inflammatory process, reducing morphological alterations, and decreasing tissue degradation resulting from OA, we observed that PBMT was effective for reducing the inflammatory marker levels and increasing repair. Similarly, Alves et al.13 indicated that PBMT was effective in repairing the joint tissue and decreasing joint degradation. Furthermore, Alves et al.14 observed that PBMT modulated the inflammatory process, and Oshima et al.21 and Dos Santos et al.16 demonstrated that PBMT decreased the inflammatory marker levels. In contrast with our results, Assis et al.22 observed that physical exercise using treadmills decreased MMP-13 levels. Similarly, Milares et al.23 demonstrated that aquatic activities decreased MMP-13 levels and the intensity of the signs of tissue degradation. Although physical exercises are widely prescribed for OA, considerable controversy still exists regarding the best type, intensity, and number of sessions to be performed.24,25 Therefore, we believe that the physical exercise parameters used in this study might contribute to the unfavorable results, considering that Assis et al.22 and Milares et al.23 observed significant alterations with the use of this therapy. We observed that topical NSAID use was neither effective in modulating the inflammatory process nor in decreasing joint tissue degradation. NSAID therapy has been known to be a treatment option for controlling pain in patients with OA.26 However, studies demonstrated that the effectiveness of these drugs for the treatment of this condition is questionable because the results were only slightly better than those obtained with the use of a placebo.8,9,27 We observed that the topical use of diclofenac sodium did not improve morphological alterations triggered by our experimental OA model, demonstrating that drug-alternative therapies are promising, which confirmed the findings of the above-mentioned studies. Furthermore, our results confirmed those reported by Milares et al.23 and Assis et al.22 who demonstrated that the association of therapies did not produce additive effects. Favorable results from the association of therapies in this study are probably caused by the effects of PBMT because their isolated use has been demonstrated to modulate inflammation and decrease tissue degradation, which were not observed with the isolated use of other therapies. Our results suggest that, among the therapies tested, the isolated use of PBMT was the most effective for improving the outcomes, indicating the important role of this therapy in OA treatment. To date, the use of PBMT has not been related to negative side effects or restrictions in musculoskeletal disorders. Therefore, this therapy is considered effective and safe. We believe that the practice of physical exercises for OA should be further investigated, and effective exercise protocols for modulating the inflammatory process and reducing signs of degradation should be established. Furthermore, our results do not support the association between PBMT, physical exercises, and topical NSAID use because no signs of potential beneficial effects are observed in isolated therapies, and its use would increase the duration and cost of treatment. 5.ConclusionOur results indicate that PBMT used alone is the best alternative among the therapies tested in this study, because it reduces the morphological changes and the enzymes that are involved in joint deterioration. However, further studies are required to examine more specifically the role of physical exercise in OA, and to evaluate the association of therapies on pain and loss of functionality. AcknowledgmentsShaiane Silva Tomazoni would like to thank Sao Paulo Research Foundation—FAPESP for PhD scholarship (Grant No. 2011/18785-9). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ReferencesA. D. Woolf and B. Pfleger,
“Burden of major musculoskeletal conditions,”
Bull. World Health Org., 81
(9), 646
–656
(2003). BWHOA6 Google Scholar
Y. Escalante et al.,
“Physical exercise and reduction of pain in adults with lower limb osteoarthritis: a systematic review,”
J. Back Musculoskelet. Rehabil., 23
(4), 175
–186
(2010). http://dx.doi.org/10.3233/BMR-2010-0267 Google Scholar
K. Kuettner, V. M. Goldberg,
“Introduction,”
Osteoarthritic Disorders, American Academy of Orthopaedic Surgeons, Rosemont, Illinois
(1995). Google Scholar
S. Krasnokutsky, J. Samuels and S. B. Abramson,
“Osteoarthritis in 2007,”
Bull. NYU. Hosp. Jt. Dis., 65
(3), 222
–228
(2007). Google Scholar
K. D. Brandt, H. J. Mankin and L. E. Shulman,
“Workshop on etiopathogenesis of osteoarthritis,”
J. Rheumatol., 13 1126
–1160
(1986). JORHE3 Google Scholar
S. M. Seed, K. C. Dunican and A. M. Lynch,
“Treatment options for osteoarthritis: considerations for older adults,”
Hosp. Pract., 39
(1), 62
–73
(2011). http://dx.doi.org/10.3810/hp.2011.02.375 Google Scholar
W. Zhang, A. Jones and M. Doherty,
“Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? a meta-analysis of randomised controlled trials,”
Ann. Rheum. Dis., 63
(8), 901
–907
(2004). http://dx.doi.org/10.1136/ard.2003.018531 ARDIAO 0003-4967 Google Scholar
J. M. Bjordal et al.,
“Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials,”
BMJ, 329
(7478), 1317
(2004). http://dx.doi.org/10.1136/bmj.38273.626655.63 Google Scholar
H. Tannenbaum et al.,
“Third Canadian Consensus Conference Group. An evidence-based approach to prescribing nonsteroidal antiinflammatory drugs. Third Canadian Consensus Conference,”
J. Rheumatol., 33
(1), 140
–157
(2006). JORHE3 Google Scholar
J. M. Hootman et al.,
“Physical activity levels among the general US adult population and in adults with and without arthritis,”
Arthritis Rheum., 49
(1), 129
–135
(2003). http://dx.doi.org/10.1002/(ISSN)1529-0131 Google Scholar
R. C. Pallotta et al.,
“Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation,”
Lasers Med. Sci., 27
(1), 71
–78
(2012). http://dx.doi.org/10.1007/s10103-011-0906-1 Google Scholar
E. S. Pessoa et al.,
“A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals,”
Photomed. Laser Surg., 22
(3), 199
–204
(2004). http://dx.doi.org/10.1089/1549541041438533 Google Scholar
A. C. Alves et al.,
“Effect of low-level laser therapy on metalloproteinase MMP-2 and MMP-9 production and percentage of collagen types I and III in a papain cartilage injury model,”
Lasers Med. Sci., 29
(3), 911
–919
(2013). http://dx.doi.org/10.1007/s10103-013-1427-x Google Scholar
A. C. Alves et al.,
“Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation,”
Arthritis Res. Ther., 15
(5), R116
(2013). http://dx.doi.org/10.1186/ar4296 Google Scholar
A. S. Da Rosa et al.,
“Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis,”
Photochem. Photobiol., 88
(1), 161
–166
(2012). http://dx.doi.org/10.1111/php.2011.88.issue-1 PHCBAP 0031-8655 Google Scholar
S. A. Dos Santos et al.,
“Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and neutrophils and macrophages in acute joint inflammation,”
Lasers Med. Sci., 29
(3), 1051
–1058
(2014). http://dx.doi.org/10.1007/s10103-013-1467-2 Google Scholar
N. Murat et al.,
“Quantification of papain-induced rat osteoarthritis in relation to time with the Mankin score,”
Acta Orthop. Traumatol. Turc., 41
(3), 233
–237
(2007). Google Scholar
W. P. Lima et al.,
“Lipid metabolism in trained rats: effect of guaraná (Paullinia cupana Mart.) supplementation,”
Clin. Nutr., 24
(6), 1019
–1028
(2005). http://dx.doi.org/10.1016/j.clnu.2005.08.004 Google Scholar
X. Wang et al.,
“Overexpression of human matrix metalloproteinase-12 enchances the development of inflammatory arthritis in transgenic rabbits,”
Am. J. Pathol., 165
(4), 1375
–1383
(2004). http://dx.doi.org/10.1016/S0002-9440(10)63395-0 AJPAA4 0002-9440 Google Scholar
M. J. Steinbeck et al.,
“Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease,”
J. Orthop. Res., 25
(9), 1128
–1135
(2007). http://dx.doi.org/10.1002/(ISSN)1554-527X JOREDR 0736-0266 Google Scholar
Y. Oshima et al.,
“Effect of light-emitting diode (LED) therapy on the development of osteoarthritis (OA) in a rabbit model,”
Biomed. Pharmacother., 65 224
–229
(2011). http://dx.doi.org/10.1016/j.biopha.2011.02.011 BIPHEX Google Scholar
L. Assis et al.,
“Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats,”
Osteoarthritis Cartilage, 24 169
–177
(2016). http://dx.doi.org/10.1016/j.joca.2015.07.020 Google Scholar
L. P. Milares et al.,
“Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats,”
Connect Tissue Res., 57
(5), 398
–407
(2016). http://dx.doi.org/10.1080/03008207.2016.1193174 Google Scholar
B. A. Egan and J. C. Mentes,
“Benefits of physical activity for knee osteoarthritis: a brief review,”
J. Gerontol. Nurs., 36
(9), 9
–14
(2010). http://dx.doi.org/10.3928/00989134-20100730-03 Google Scholar
C. Juhl et al.,
“Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials,”
Arthritis. Rheumatol., 66
(3), 622
–636
(2014). http://dx.doi.org/10.1002/art.v66.3 Google Scholar
P. Fuller and S. Roth,
“Diclofenac sodium topical solution with dimethyl sulfoxide, a viable alternative to oral nonsteroidal anti-inflammatories in osteoarthritis: review of current evidence,”
J. Multidiscip. Healthcare, 4 223
–231
(2011). http://dx.doi.org/10.2147/JMDH Google Scholar
J. M. Bjordal,
“Evidence-based medicine turned upside down,”
Photomed. Laser Surg., 33
(8), 391
–392
(2015). http://dx.doi.org/10.1089/pho.2015.3976 Google Scholar
BiographyRodrigo Álvaro Brandão Lopes-Martins is a director of the Center for Technological Research and of the postgraduate program in biomedical engineering at the University of Mogi das Cruzes—UMC. He is a biologist, with a Master in Pharmacology from UNICAMP, and a PhD in cellular and molecular biology from FIOCRUZ. He is a postdoctoral fellow at the USP. He is a visiting associate professor at the University of Bergen, Norway. He was a professor at the University of Sao Paulo from 2005 to 2015 and an associate professor at the Faculty of Medicine at the Universidade Camilo Castelo Branco-UNICASTELO. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

