|
|
1.IntroductionPhotodynamic therapy (PDT) is based on light-induced death of cells stained with a photosensitizing dye in the presence of oxygen. Photoexcitation of the sensitizer molecules leads to generation of singlet oxygen and other reactive oxygen species that induce oxidative stress and kill the cells. PDT is used to treat cancer, including brain tumors.1–3 Not only tumor cells, but also normal nerve and glial cells are damaged during brain PDT. Thus, the study of signaling mechanisms regulating photodynamic damage or protection of normal neurons and glial cells are of importance.4 However, in the mammalian brain, numerous neurons interact with hundreds of other neurons. It is difficult to identify them and determine which glial cells interact with a given neuron. The use of a simpler neuroglial system such as the crayfish stretch receptor (CSR) that consists of a single mechanoreceptor neuron surrounded by the glial envelope5,6 is preferable. It has been used as a simple but informative experimental model in the studies of signaling mechanisms of photodynamic effect on neurons and glial cells.4,7–9 Nitric oxide (NO) is a second messenger, involved in the regulation of various cellular functions. It participates in neurotransmission, cell responses to stress, and neurodegeneration.10,11 PDT has been shown to induce NO production in different cell lines.12–14 Some authors have also demonstrated the involvement of NO in PDT-induced apoptosis.12,15 On the other hand, it was shown that NO can protect tumor cells from PDT-induced death either through inhibition of lipid peroxidation in the cellular membranes16,17 or through cGMP-dependent signaling pathway.18 Some authors did not find any correlations between NO production and PDT-induced cell damage.19 Thus, the role of NO in cell death is not clearly understood yet. The role of NO in PDT-induced death of neuronal and glial cells is also insufficiently studied. As recently shown, NO enhances PDT-induced apoptosis of glial cells in the CSR. At the same time, it protects crayfish glial cells from PDT-induced necrosis.7 The dynamics of NO production during PDT has not been well examined. Some authors studied NO production at different post-PDT time points, but not during the treatment.14 Another important problem is the mechanism of neuroglial interactions under stress conditions. The crayfish mechanoreceptor neuron is known to protect satellite glial cells from PDT-induced apoptosis but not necrosis.20 These neuroglial interactions may be mediated by a intercellular signaling messenger such as neurotrophic factors.21,22 or NO. Is NO involved in the neuroglial interactions in the photosensitized CSR? What is the source of NO, the neuron, or glial cells? What is the dynamics of NO production? In this work, we addressed these questions using the NO-sensitive fluorescence probe 4,5-diaminofluorescein diacetate that provides NO visualization and accurate and fast measurement of the dynamics of its production. 2.Materials and MethodsThe following chemicals were used in this work: the NO-sensitive fluorescent probe 4,5-diaminofluorescein diacetate (DAF-2DA), NO generator DEA NONOate (), NO synthase (NOS) substrate L-arginine hydrochloride (L-arg HCl) (), NO scavenger PTIO (), nonspecific NO synthase (NOS) inhibitors L-NG-nitro arginine methyl ester (L-NAME) (1 mM) and -nitro-L-arginine (L-NNA) (1 mM), inhibitor of the inducible NOS isoform (iNOS) 5-methylisothiourea hemisulfate (SMT) (, 1 mM, 10 mM), and the fluorescent dye Hoechst-33342. All chemicals were purchased from Sigma-Aldrich Rus (Moscow, Russia). Photosensitizer photosens, a mixture of sulphonated alumophthalocyanines , where mean , was obtained from NIOPIK (Moscow, Russia). The abdominal stretch receptors of the crayfish Astacus leptodactylus that contain single sensory neurons, surrounded by the multilayer glial envelope,6 were isolated as described in Ref. 5. The isolated receptors were placed into the chamber filled with van Harreveld’s saline for cold-blooded animals (mM: NaCl, 205; KCl, 5.4; , 0.24; , 5.4; , 13.5; pH 7.2 to 7.4). Spikes were recorded extracellularly from axons by glass suction electrodes and processed by a personal computer using the home-made software providing continuous firing monitoring and registration of firing rates. The initial firing level was set at 6 to 10 Hz by application of the appropriate receptor muscle extension. The NO production was examined using the fluorescent probe 4,5-diaminofluorescein diacetate (DAF-2DA). This cell-permeable and highly sensitive NO probe forms intensely fluorescing 4,5-diaminofluorescein triazole (DAF-2T) upon direct reaction with NO inside the cell.23 This technique has been used to study the real-time changes in intracellular NO level in various conditions in different animal models, including invertebrates.24,25 After 30-min control recording of the neuronal activity, the preparations were incubated 60 min in the saline containing DAF-2DA (). Then the samples were washed out five times with the dye-free saline to remove the excess of the dye. Photosens (10 nM) was added into the chamber, and after a 30-min incubation, the preparations were irradiated by the diode laser (670 nm, , 3-mm beam diameter). Then the stretch receptor preparations were replaced from the electrophysiological apparatus to the Axio Lab. A1 microscope (Carl Zeiss), equipped with the AxioCam ERc 5s camera, which registered the fluorescence of DAF-2DA before PDT and 1, 4, 7, 10, 15, 20, 25, 30, and 40 min during PDT. The images were acquired with ZEN 2012 (Carl Zeiss) software on the personal computer (Intel Core i3, 3.10 GHz, RAM 4 Gb, Windows 7 Enterprise 64 bit) and processed using Image-Pro Plus Version 6.0 (Media Cybernetics) software. The mean fluorescence intensity was measured within the circle () around the neuronal soma [Fig. 1(a)] that contained the neuronal nucleus and 30 to 40 nuclei of surrounding glial cells. It was then normalized relatively to the fluorescence intensity of the distant stretch receptor region, where the fluorescence intensity did not change. Application of NO generator DEA NONOate (), NOS substrate L-arginine hydrochloride (), NO scavenger PTIO (), NOS inhibitors L-NAME (1 mM), L-NNA (1 mM), and iNOS inhibitor SMT (, 1 mM, 10 mM) were used as a positive or negative control of NO production. All experiments were performed at . Statistical evaluation of the difference between independent experimental groups was performed using one-way ANOVA. Results are presented as . Fig. 1The distribution of NO in the mechanoreceptor neuron and satellite glia of the CSR. (a) Fluorescent image of the CSR stained with NO-sensitive fluorescent probe DAF-2DA; (b) double fluorochrome staining of the same preparation with NO probe DAF-2DA (green) and Hoechst-33342 that visualizes the nuclei of neurons and glial cells (blue); (c) brightfield image of the same stretch receptor; and (d) the scheme of the CSR morphology. a, axon; D, dendrites; g, glial cells; N, neuronal nucleus; P, perikaryon; RM, receptor muscle. Scale bar . 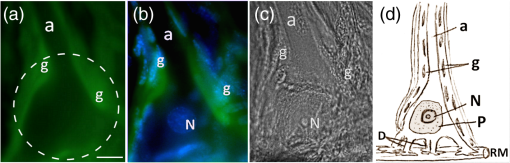 3.ResultsThe morphology of the isolated stretch receptor in the control preparations is shown in Figure 1. The dendrites of the single mechanoreceptor neuron are known to attach to the receptor muscle and ramify between muscle fibers, whereas the axon goes to the ventral cord ganglion.5,6 Satellite glial cells form the roulette-like envelope around the neuronal soma and processes.6 Due to such morphology, the cytoplasm of separate glial cells cannot be distinguished at the optical level, but the nuclei of different glial cells fluorochromed by Hoechst-33342 are clearly seen in Fig. 1(b) and Ref. 6. The distribution and dynamics of DAF-2DA fluorescence that displays the NO location and production in the CSR preparation is shown in Figs. 1(a) and 1(b). The mean fluorescence intensity in control glial cells before PDT was relative units (Table 1, at ). The mean levels of DAF-2DA fluorescence in the neuronal axon, soma, and dendrites [points a, s, and d in Fig. 2(a)] were 3.3, 2.9, and 1.8 times lower than in the glial envelope (point g), respectively (Table 1, at ). Table 1The mean fluorescence intensity of DAF-2DA (relative units±SEM) in glial cells, neuronal soma, receptor muscle, and axon at different time points during PDT (n=9).
Fig. 2The dynamics of NO production in the isolated CSR during PDT evaluated with NO-sensitive fluorescence probe DAF-2DA. (a) DAF-2DA fluorescence in the CSR before and at different time intervals during PDT. The dashed circle indicates the region of interest. Points a, s, d, and g show the regions of local measurements of DAF-2A fluorescence in small squares () within the neuronal axon, soma, dendrite region, and in glial cells, respectively. (b) The dynamics of the mean fluorescence intensity of DAF-2DA: squares, laser only treatment (); triangles, photosensitizer only treatment (); circles, PDT effect (). Asterisks indicate the significant difference from initial fluorescence level (**). Scale bar . 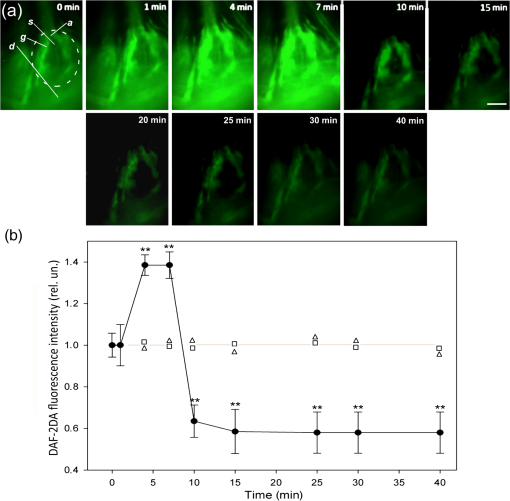 The glial envelope around the sensory neuron can be observed in the overlay of DAF-2DA fluorescence image [Fig. 1(a)] and the image of Hoechst-33342 staining, which shows the nuclei of glial cells and neurons [Fig. 1(b)]. To check if the changes in the mean intensity of DAF-2DA fluorescence were due to the changes in NO level, we applied NO generator DEA NONOate () or NOS substrate L-arginine hydrochloride (), NO scavenger PTIO () and NOS-inhibitors L-NAME (1 mM), L-NNA (1 mM), and SMT (, 1 mM, 10 mM) in the absence of photosensitizer (Table 2). All these modulators did not change neuronal activity during the experiment. Table 2The changes in the mean fluorescence intensity of DAF-2DA in the presence of NO-generator DEA NONOate (25 μM), NOS substrate L-arginine hydrochloride (500 μM), NO scavenger PTIO (500 μM), nNOS inhibitors L-NAME (1 mM), L-NNA (1 mM), and SMT (500 μM) in the absence of PDT relatively to the initial fluorescence level (t=0). Significant difference from the mean fluorescence intensity in the control group: **p<0.01 or ***p<0.001. The number of experiments is indicated in brackets.
DEA NONOate, applied after DAF-2DA, increased the mean intensity of DAF-2DA fluorescence [measured within the circle shown in Fig. 1(a)] by 1.4 and 2.3 times for 5 and 10 min, respectively (Table 2). During next 50 min, it gradually decreased by 1.7 times as compared with the maximal level and did not change during the next 60 min. L-arginine increased the mean intensity of DAF-2DA fluorescence by 1.9 times for 60 min. This level was maintained during the next 3 h, and then slightly decreased (Table 2). NO scavenger PTIO, applied 1 h before DAF-2DA, almost completely blocked the DAF-2DA fluorescence (Table 2). The nonspecific NOS inhibitors L-NAME and L-NNA, applied 1 h before DAF-2DA, decreased the mean intensity of DAF-2DA fluorescence by 1.7 to 1.8 times (Table 2), whereas iNOS inhibitor SMT did not influence DAF-2DA fluorescence. The dynamics of DAF-2DA fluorescence in the CSR under laser irradiation, or photosensitizer application, or under their combined action (PDT) are shown in Fig. 2. Laser irradiation or photosensitizer application acting separately did not influence the mean intensity of DAF-2DA fluorescence in the CSR [Fig. 2(b)]. PDT transiently increased DAF-2DA fluorescence by 1.4 times at 4 to 7 min after PDT start [Figs. 2(a) and 2(b)]. However, at 10 min, the mean intensity of DAF-2DA fluorescence decreased by 40% relatively to the initial level and did not change further during next 30 min [Fig. 2(b)]. We also compared the dynamics of PDT-induced NO production in different parts of the CSR: neuronal axon, soma, dendrite region, and glial envelope (points a, s, d, and g, respectively, shown in [Fig. 2(a)]. In this experiment, we measured DAF-2A fluorescence in small squares () within these regions. The distribution of NO during PDT was similar to that in the control preparations. The highest DAF-2A fluorescence was observed in the glial envelope around the neuronal body [Fig. 2(a) and Table 1]. The redistribution of the NO probe fluorescence was not observed. 4.DiscussionThe presented experiments confirmed the specificity of DAF-2DA to detect the intracellular NO level23–25 in crayfish neurons and glial cells. DAF-2DA fluorescence increased in the presence of the exogenous (DEA NONOate), or endogenous (L-arginine hydrochloride) NO generators, or decreased under application of NO scavenger (PTIO), or NO synthase inhibitors (L-NAME, L-NNA). The separate application of the photosensitizer or laser irradiation did not influence DAF-2DA fluorescence. Although some authors assume that changes in the DAF-2DA fluorescence can be associated not only with NO production,26 it is commonly accepted that the DAF probes have a detection limit for NO as low as 5 nM and show no fluorescence in the presence of various interferents such as , , , and peroxynitrite ().23,27 It should be noted that we observed the delay in the increase of DAF-2DA fluorescence in the presence of NONOate or L-arginine: 10 and 60 min, respectively, and then a slow decrease (Table 2). This nonlinear kinetics should be taken into account in the prolonged NO measurements. Nevertheless, DAF-2DA fluorescence is a relevant method for registration of NO production dynamics, although quick measurements do not reach the maximal NO sensitivity. Both inducible and neuronal isoforms of NO synthase (iNOS and nNOS, respectively) have been found in neurons and glial cells.28,29 These were involved in the PDT-induced death of crayfish neurons and glia,7 as well as in death of other cells.29,30 NO scavenger PTIO suppressed DAF-2DA fluorescence almost completely, whereas nonselective NOS inhibitors, L-NAME and L-NNA, reduced it only by 1.7 to 1.8 times. One can hypothesize the presence of additional NO sources, other than nNOS and iNOS, which are not affected by L-NAME and L-NNA. It may be mitochondrial NO synthase incorporated into the inner mitochondrial membrane and performing some functions of neuronal NOS.31 PTIO can also scavenge NO, formed during the nonenzymatic destruction of nitrites or nitrates. These inert anions can be important alternative source of NO, in particular in the hypoxic state.32 In our experiments, PDT rapidly increased for 4 min the NO level which was followed by its decrease after 7 min (Fig. 2). Similarly, the transient activation of NO production in the cancer cell lines was followed by inhibition, possibly due to nNOS destruction by reactive oxygen species.25 One can suggest that the fast PDT-induced increase in NO production in the CSR preparation could be due to activation of nNOS rather than iNOS. In fact, a 4-min interval is too short to express iNOS. Additionally, iNOS inhibitor SMT did not influence the NO level (Table 2). nNOS is known to be activated by intracellular and PDT rapidly increases the cytosolic level.33,34 Finally, the correlation between the cytosolic calcium level and NO production during photodynamic treatment has been reported.35,36 We showed previously that NO mediates PDT-induced apoptosis of glial cells.7 This raised the question: what is the source of NO in this neuroglial preparation, the neuron or glia? The present experiments showed that NO production was concentrated mainly in the glial envelope around the neuron soma, and, to a lesser extent, in the proximal dendrite zone. (However, in this location, we could not separate possible NO sources between the proximal parts of dendrites, or the glial envelope, or both.) NO levels inside the neuronal soma, nucleus, and axon of the mechanoreceptor neuron were much lower. One can suggest that the proapoptotic effect of NO on the photosensitized crayfish glial cells observed in our previous work7 was due to NO production in the glial cells rather than in the mechanoreceptor neuron. Additionally, taking into the account that the glial area of NO production was much higher than that in dendrites, one can suggest the glial envelope to be the main NO source. The increase of NO level in the beginning of PDT action could initiate the proapoptotic cascades in glial cells. As we showed previously, the neuronal soma protects satellite glial cells from PDT-induced apoptosis.20 This function could be performed by intercellular messengers such as neurotrophic factors NGF and GDNF.21,22 The role of another intercellular messenger NO was unknown. The present data showed that NO could be involved in neuroglial interactions during PDT. However, it played the proapoptotic rather than antiapoptotic role.9 On the other hand, NO produced in the glial envelope could protect neurons from PDT-induced necrosis and impairment of neuronal activity.7 AcknowledgmentsThis work was supported by the Russian Foundation for Basic Research (Grant No. 15-04-05367); control experiments with NOS inhibitors were supported by the Russian Science Foundation (Grant No. 14-15-00068). The work of A. B. Uzdensky was supported by the Ministry of Education and Science of Russian Federation (Research organization Grant No. 790). ReferencesS. S. Stylli and A. H. Kaye,
“Photodynamic therapy of cerebral glioma—a review. Part I. A biological basis,”
J. Clin. Neurosci., 13 615
–625
(2006). http://dx.doi.org/10.1016/j.jocn.2005.11.014 Google Scholar
B. J. Quirk et al.,
“Photodynamic therapy (PDT) for malignant brain tumors–where do we stand?,”
Photodiagn. Photodyn. Ther., 12 530
–544
(2015). http://dx.doi.org/10.1016/j.pdpdt.2015.04.009 Google Scholar
H. Kostron,
“Photodynamic diagnosis and therapy and the brain,”
Photodynamic Therapy: Methods and Protocols, Methods in Molecular Biology, 635 Springer Science + Business Media, Berlin
(2010). Google Scholar
A. B. Uzdensky, Cellular and Molecular Mechanisms of Photodynamic Therapy, Nauka, Sankt-Petersburg
(2010). Google Scholar
E. FloreyE. Florey,
“Microanatomy of the abdominal stretch receptors of the crayfish (Astacus fluviatilis L.),”
J. Gen. Physiol., 39 69
–85
(1955). http://dx.doi.org/10.1085/jgp.39.1.69 JGPLAD 0022-1295 Google Scholar
G. M. Fedorenko and A. B. Uzdensky,
“Ultrastructure of neuroglial contacts in crayfish stretch receptor,”
Cell Tissue Res., 337 477
–490
(2009). http://dx.doi.org/10.1007/s00441-009-0825-7 Google Scholar
V. D. Kovaleva et al.,
“Involvement of nitric oxide in photodynamic injury of neurons and glial cells,”
Nitric Oxide, 29 46
–52
(2013). http://dx.doi.org/10.1016/j.niox.2012.12.006 Google Scholar
A. B. Uzdensky et al.,
“The response of neurons and glial cells of crayfish to photodynamic treatment: transcription factors and epigenetic regulation,”
Biochem. Suppl. Ser. A, 9 329
–336
(2015). http://dx.doi.org/10.1134/S1990747815050190 Google Scholar
A. B. Uzdensky et al.,
“Protection of the crayfish mechanoreceptor neuron and glial cells from photooxidative injury by modulators of diverse signal transduction pathways,”
Mol. Neurobiol., 52
(2), 811
–825
(2015). http://dx.doi.org/10.1007/s12035-015-9237-8 MONBEW 0893-7648 Google Scholar
S. Moncada and J. P. Bolanos,
“Nitric oxide, cell bioenergetics and neurodegeneration,”
J. Neurochem., 97 1676
–1689
(2006). http://dx.doi.org/10.1111/jnc.2006.97.issue-6 JONRA9 0022-3042 Google Scholar
G. C. Brown,
“Nitric oxide and neuronal death,”
Nitric Oxide, 23 153
–165
(2010). http://dx.doi.org/10.1016/j.niox.2010.06.001 Google Scholar
S. Gupta, N. Ahmad and H. Mukhtar,
“Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis,”
Cancer Res., 58 1785
–1788
(1998). CNREA8 0008-5472 Google Scholar
S. Coutier et al.,
“Foscan (mTHPC) photosensitized macrophage activation: enhancement of phagocytosis, nitric oxide release and tumour necrosis factor-alpha-mediated cytolytic activity,”
Br. J. Cancer, 81 37
–42
(1999). http://dx.doi.org/10.1038/sj.bjc.6690648 BJCAAI 0007-0920 Google Scholar
S. M. Ali and M. Olivo,
“Nitric oxide mediated photo-induced cell death in human malignant cells,”
Int. J. Oncol., 22 751
–756
(2003). http://dx.doi.org/10.3892/ijo.22.4.751 IJONES 1019-6439 Google Scholar
Z. Lu et al.,
“Mitochondrial reactive oxygen species and nitric oxide-mediated cancer cell apoptosis in 2- butylamino-2-demethoxyhypocrellin B photodynamic treatment,”
Free Radical Biol. Med., 41 1590
–1605
(2006). http://dx.doi.org/10.1016/j.freeradbiomed.2006.08.021 FRBMEH 0891-5849 Google Scholar
M. Niziolek, W. Korytowski and A. W. Girotti,
“Chain-breaking antioxidant and cytoprotective action of nitric oxide on photo photodynamically stressed tumor cells,”
J. Photochem. Photobiol., 78 262
–270
(2003). http://dx.doi.org/10.1562/0031-8655(2003)078<0262:CAACAO>2.0.CO;2 Google Scholar
M. Niziolek, W. Korytowski and A. W. Girotti,
“Nitric oxide-induced resistance to lethal photooxidative damage in a breast tumor cell line,”
Free Radic. Biol. Med., 40 1323
–1331
(2006). http://dx.doi.org/10.1016/j.freeradbiomed.2005.11.022 FRBMEH 0891-5849 Google Scholar
E. R. Gomes et al.,
“Nitric oxide modulates tumor cell death induced by photodynamic therapy through a cGMP-dependent mechanism,”
J. Photochem. Photobiol., 76 423
–430
(2002). http://dx.doi.org/10.1562/0031-8655(2002)076<0423:NOMTCD>2.0.CO;2 Google Scholar
G. Di Venosa et al.,
“Sensitivity to ALA-PDT of cell lines with different nitric oxide production and resistance to NO cytotoxicity,”
J. Photochem. Photobiol., 80 195
–202
(2005). http://dx.doi.org/10.1016/j.jphotobiol.2005.05.001 Google Scholar
M. Kolosov and A. Uzdensky,
“Crayfish mechanoreceptor neuron prevents photoinduced apoptosis of satellite glial cells,”
Brain Res. Bull., 69 495
–500
(2006). http://dx.doi.org/10.1016/j.brainresbull.2006.02.018 BRBUDU 0361-9230 Google Scholar
A. V. Lobanov and A. B. Uzdensky,
“Protection of crayfish glial cells but not neurons from photodynamic Injury by nerve growth factor,”
J. Mol. Neurosci., 39 308
–319
(2009). http://dx.doi.org/10.1007/s12031-009-9199-2 JMNEES 0895-8696 Google Scholar
A. B. Uzdensky et al.,
“Protection effect of GDNF and neurturin on photosensitized crayfish neurons and glial cells,”
J. Mol. Neurosci., 49 480
–490
(2013). http://dx.doi.org/10.1007/s12031-012-9858-6 JMNEES 0895-8696 Google Scholar
H. Kojima et al.,
“Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins,”
Anal. Chem., 70
(13), 2446
–2453
(1998). http://dx.doi.org/10.1021/ac9801723 ANCHAM 0003-2700 Google Scholar
X. Ye et al.,
“Production of nitric oxide within the aplysia californica nervous system,”
ACS Chem. Neurosci., 1 182
–193
(2010). http://dx.doi.org/10.1021/cn900016z Google Scholar
R. Bhowmick and A. W. Girotti,
“Rapid upregulation of cytoprotective nitric oxide in breast tumor cells subjected to a photodynamic therapy-like oxidative challenge,”
Photochem. Photobiol., 87
(2), 378
–386
(2011). http://dx.doi.org/10.1111/j.1751-1097.2010.00877.x PHCBAP 0031-8655 Google Scholar
C. Csonka et al.,
“Measurement of NO in biological samples,”
Br. J. Pharmacol., 172 1620
–1632
(2015). http://dx.doi.org/10.1111/bph.12832 Google Scholar
E. M. Hetrick and M. H. Schoenfisch,
“Analytical chemistry of nitric oxide,”
Annu. Rev. Anal. Chem., 2 409
–433
(2009). http://dx.doi.org/10.1146/annurev-anchem-060908-155146 Google Scholar
S. Murphy et al.,
“Synthesis of nitric oxide in CNS glial cells,”
Trends Neurosci., 16 323
–328
(1993). http://dx.doi.org/10.1016/0166-2236(93)90109-Y TNSCDR 0166-2236 Google Scholar
K. J. Reeves, M. W. Reed and N. J. Brown,
“Is nitric oxide important in photodynamic therapy?,”
J. Photochem. Photobiol., 95 141
–147
(2009). http://dx.doi.org/10.1016/j.jphotobiol.2009.02.005 Google Scholar
R. Bhowmick and A. W. Girotti,
“Cytoprotective induction of nitric oxide synthase in a cellular model of 5-aminolevulinic acid-based photodynamic therapy,”
Free Rad. Biol. Med., 48 1296
–1301
(2010). http://dx.doi.org/10.1016/j.freeradbiomed.2010.01.040 FRBMEH 0891-5849 Google Scholar
P. V. Finocchietto et al.,
“Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death,”
Exp. Biol. Med., 234
(9), 1020
–1028
(2009). http://dx.doi.org/10.3181/0902-MR-81 EXBMAA 0071-3384 Google Scholar
J. O. Lundberg, E. Weitzberg and M. T. Gladwin,
“The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics,”
Nat. Rev. Drug Discov., 7
(2), 156
–167
(2008). http://dx.doi.org/10.1038/nrd2466 NRDDAG 1474-1776 Google Scholar
A. P. Castano, T. N. Demidova and M. R. Hamblin,
“Mechanisms in photodynamic therapy: part two–cellular signaling, cell metabolism and modes of cell death,”
Photodiagn. Photodyn. Ther., 2 1
–23
(2005). http://dx.doi.org/10.1016/S1572-1000(05)00030-X Google Scholar
A. B. Uzdensky,
“Signal transduction and photodynamic therapy,”
Curr. Sign. Transd. Ther., 3 55
–74
(2008). http://dx.doi.org/10.2174/157436208783334277 Google Scholar
W. H. Chan,
“Photodynamic treatment induces an apoptotic pathway involving calcium, nitric oxide, p53, p21-activated kinase 2, and c-Jun N-terminal kinase and inactivates survival signal in human umbilical vein endothelial cells,”
Int. J. Mol. Sci., 12 1041
–1059
(2011). http://dx.doi.org/10.3390/ijms12021041 1422-0067 Google Scholar
L. Zhou and D. Y. Zhu,
“Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications,”
Nitric Oxide, 20 223
–230
(2009). http://dx.doi.org/10.1016/j.niox.2009.03.001 Google Scholar
BiographyVera D. Kovaleva received her PhD in biophysics in 2016. She is a researcher at the Laboratory of Molecular Neurobiology, Southern Federal University, Rostov-on-Don, Russia. She is the author of 15 journal papers. Anatoly B. Uzdensky received his PhD and Dr Sci degrees in biophysics in 1980 and 2005, respectively. He is a professor in biophysics and head of the Laboratory of Molecular Neurobiology, Southern Federal University, Rostov-on-Don, Russia. He is the author of more than 115 journal papers and 3 books. His current research interests include photodynamic therapy, neuroscience, cell biology, and proteomics. He is a member of SPIE, and European, Russian, and American Societies for Photobiology. |

