|
|
1.IntroductionThe articular cartilage benefits from a specialized extracellular matrix architecture that is optimized for weight-bearing and resisting the stress induced in articulation. The collagen fibers in cartilage are organized in a unique “arcade” like formation.1 The fibers are oriented perpendicularly to the interface between calcified cartilage and noncalcified cartilage in the deep radial zone (or the “deep zone”), then bend in a transitional zone (or the “intermediate zone”) to be eventually parallel to the surface in the superficial zone (or the “tangential zone”). The superficial zone is thin and only takes up 10% to 20% of the total cartilage thickness. However, it plays a critical role in protecting the inner zones and maintaining proper mechanical responses for cartilage to function normally. The collagen fibers in the superficial zone are organized in a preferential orientation2 to deal with the directional mechanical demand in cartilage.3 The alteration of fiber orientation in the superficial zone has been observed in early osteoarthritis.4,5 A careful consideration of fiber orientation may also be important in clinical treatments such as osteochondral transplantation.2 Because of the importance of fiber orientation in the superficial zone of the cartilage, a technology that can directly visualize the collagen organization would be valuable to both basic research and clinical practice. The “split-line” method has been used to reveal fiber orientation on the cartilage surface.6,7 Split-lines are the cracks created on the articular cartilage surface when pricking the cartilage using a fine needle. These cracks are caused by the collagen fibers splitting along the lines of tensile stress, which are aligned with the collagen fibers in the superficial layer as revealed in scanning electron microscopy (SEM) studies.8 The collagen fibrils resisted strains better along the split-line directions than perpendicular to the split-lines.9 Although the “split-line” method can reveal the global fiber orientation in the superficial zone of the cartilage, it is destructive and impractical for diagnosis purpose. Clinical imaging technologies such as magnetic resonance imaging (MRI) are nondestructive, but their current resolution is insufficient to resolve the detailed structural features in the thin cartilage.10 High-resolution microscopic MRI (μMRI)11 is promising for differentiating histological zones in cartilage. However, current hardware is only limited to imaging prepared small samples. Small angle x-ray scattering (SAXS)12 was also explored to obtain collagen orientation in cartilage from the side of the sample; but special x-ray facility such as synchrotron radiation12 is often needed in SAXS. Currently, polarization light microscopy (PLM) is recognized as the primary tool to detect fiber orientation in cartilage.13,14 The highly organized collagen structure in cartilage produces a strong optical birefringence, which can be detected using polarized light. Unfortunately, PLM has limited imaging depth and can only image prepared thin cartilage sections from the cross sectional side. PLM cannot image collagen fiber orientation in intact cartilage from the synovial surface. Optical coherence tomography (OCT) is an emerging high-resolution nondestructive three-dimensional (3-D) optical imaging technology.15 OCT achieved micrometer scale resolution and can detect structural and morphological changes in cartilage as well as surface roughness associated with osteoarthritis.16–19 Because of the presence of optical birefringence in cartilage, polarization-sensitive OCT (PSOCT) has also been widely applied in cartilage imaging.20,21 A common finding in many PSOCT studies was the “banding” pattern in images of normal cartilage due to birefringence, which disappeared in cartilage with progressive degeneration.22 Therefore, the loss of birefringence can be used as a biomarker for detecting early cartilage degeneration.23 Despite its great potential for nondestructive cartilage imaging, very few PSOCT studies investigated the possibility of imaging the fiber orientation in cartilage. It is known that the optic axis measured in PSOCT is related to the fiber orientation. Unfortunately, conventional PSOCT only measures the “cumulative” optic axis that represents the integrated signal from the tissue surface to a particular imaging depth. Such integrated optic axes do not represent the actual fiber orientation.24 It has recently been shown that the 3-D fiber orientation in cartilage can be obtained by analyzing the depth profiles of tissue birefringence25 or optic axis26 when evaluated at multiple different incident angles. However, only single point measurement was achieved in these early studies26,27 and thus it is challenging to apply these approaches to obtain the spatial- and depth-resolved orientation in the whole cartilage. Optical polarization tractography (OPT) was a newly developed method for imaging fiber orientation in tissue. It utilized a Jones calculus based method to extract the true “local” optic axis from PSOCT measurements.28,29 Validation studies have shown that OPT achieved histology-like accuracy in imaging fiber orientation in tissues.30 As an optical polarization based method, OPT can image the fiber orientation and birefringence at the sample surface as in conventional PLM. However, OPT can nondestructively image fiber orientation and birefringence at different depths from the tissue surface, which is not feasible in PLM. Recent studies have demonstrated that OPT can be potentially applied to image fiber structures in skeletal muscle,31 heart muscle,32 and blood vessels.33 In this study, we showed that OPT can image collagen fiber orientation in the superficial layer of the articular cartilage. The fiber orientation measured in OPT agreed very well with those obtained using the standard split-line procedure. In addition, the superficial zone thickness can also be estimated using the depth profile of the local birefringence obtained in OPT. 2.Method2.1.Cartilage Samples and Split-Line CreationArticular cartilage samples were obtained at the first phalanges of fresh pig feet acquired from a local supermarket. A total of 112 split-line patterns were produced in five cartilage samples using a fine-tip conical shaped needle of in diameter (Dritz® long ball point pins, #16950, Prym Consumer USA, Spartanburg, South Carolina). The needle was perpendicularly inserted into the cartilage surface until reaching the subchondral bone. This insertion caused the collagen fibers in the superficial zone to crack along the direction of tensile stress. The needle was dipped in a commercial grade India ink before insertion, which stained the exposed cartilaginous matrix in black and made the split-lines clearly visible.6 After split-lines were created, the cartilage sample was imaged from the whole cartilage surface using the OPT system as described in Sec. 2.2. After the OPT scan, the cartilage samples were fixed in 10% formalin solutions, decalcified, embedded in paraffin, and cut into thick sections. Microscopic images of the histology sections stained using hematoxylin and eosin (H&E) were acquired using a Nikon Eclipse E800 microscope equipped with a color camera (RETIGA 1300, QImaging, Canada). 2.2.Optical Polarization Tractography ImagingThe OPT system is based on a wavelength, spectral-domain full-range Jones matrix optical coherence tomography (JMOCT) system that has been described in detail previously.29,34 JMOCT measures the Jones matrix that can characterize tissue polarization properties including retardance, diattenuation, and optic axis. A two-axis galvanometer scanner (GVS212, Thorlabs, Newton, New Jersey) was used to scan the incident light on the cartilage surface along the B- and C-scan directions. At each scanning position, an A-scan of the pixel-wise Jones matrix was measured by imaging the sample using incident light of alternating left- and right-circular polarization. The modulation of the light polarization was achieved by using an electro-optical modulator (EO-AM-NR-C1, Thorlabs, Newton, New Jersey). For each incident polarization, the horizontal and vertical linearly polarized components of the interference spectra were simultaneously imaged by using a single line-scan camera (AVIIVA SM2, e2v, France). The depth-resolved “cumulative” Jones matrix was then constructed from these four interference signals as described in detail previously.34 Such a pixel-wise Jones matrix characterized the round-trip “cumulative” polarization properties from the surface to a measurement depth. In order to derive the true fiber orientation, the “local” polarization properties (retardance, diattenuation, and optic axis) were then computed from the single-trip Jones matrices using a Jones calculus based methodology.29,32 Briefly, this algorithm calculated the local optical retardance using Jones matrices measured from two consecutive depths based on similar matrix transformation.24,28 An iterative algorithm was then used to calculate the optic axis at each depth starting from the sample surface.24,29 The local retardance was applied to calculate the sample birefringence , where is the A-scan pixel size and is the optical wavelength. The fiber orientation was estimated based on the extraordinary optic axis. It was mapped between [, 90 deg] with the zero degree aligned with the C-scan direction. The resolution of the current OPT imaging system was measured as (in air) and in the axial and lateral direction, respectively. The cartilage sample was imaged at a speed of to construct an image volume of . The corresponding raw pixel size in the image volume was 3, 6, and along the B-, C-, and A-scan direction, respectively. During the image processing, the images were resized to pixel-size along all dimensions using cubic spline interpolation. To facilitate the comparison with the large split-lines, a median filter was applied to improve the signal-to-noise of the OPT images in the en face () plane at all depths. The OPT method produced three depth-resolved images for each sample in this study. First, the 3-D image of fiber orientation () was obtained using the local optic axis. The corresponding tractography representation was obtained using the streamline functionality in MATLAB (The MathWorks, Inc., Natick, Massachusetts). Second, the 3-D image of the local birefringence () was generated. The birefringence value represents the difference of the refractive indices along the ordinary and extraordinary optic axes. A higher indicated a stronger anisotropic fiber structure; whereas a lower suggested a more isotropic structure along the path of the light. Last, OPT also generated the intensity images of the sample as in conventional OCT.34 The summation of the interference intensities from the horizontal and vertical polarization components was calculated for both the left- and right-circularly polarized incident light and then averaged to calculate the intensity image. Therefore, the resulting intensity image was polarization independent and represented the tissue structure related light reflectance changes. 2.3.Quantification of Split-LinesFrom the OPT intensity images of the cartilage sample, the orientation of each individual split-line was quantified using an image processing procedure. To improve the image contrast of the split-lines, the en face intensity OPT image was averaged from the surface to in depth. We found that the positions and orientations of the split-lines did not change over the depth in the intensity images. First, a small region enclosing each individual split-line was manually selected using the polygonal region-of-interest (ROI) functionality in MATLAB. Because of the ink stain, the split-lines appeared darker than the surrounding tissue inside the ROI. Therefore, a simple threshold based method was applied to segment all image pixels located on this split-line. The and positions of these image pixels were then fitted using a linear line equation intercept, where represents the angle of this split-line. The goodness of fitting was evaluated by using the coefficient of determination () and root-mean-squared-error (RMSE). The above procedure was repeated for each split-line in the intensity image to obtain the corresponding split-line orientation . The RMSE in fitting all 112 split-lines used in this study were consistently and 94% (106/112) of the lines had fitting RMSE. These results indicated that split-lines were reliably quantified. 2.4.Comparison of Split-Line Orientation with Optical Coherence Tomography ResultsThe OPT fiber orientation was obtained from all images pixels located on a split-line. The fiber orientation representing the entire split-line was calculated by averaging the orientation from all pixels using circular averaging33 where is the local fiber orientation obtained in OPT at each pixel on the split line and and were averaged over all pixels on the split-line.Correlation analysis was then carried out to compare the angles obtained in split-line with those obtained in OPT at the same location. In addition to the coefficient of determination , the overall RMSE between the two measurements were also calculated to estimate the overall error between the two measurements where represents the total number of split-lines. These analyses were repeated for the OPT images obtained at all depths to comparatively study the effect of imaging depth in fiber orientation measurements.3.ResultsFigure 1 shows example image data obtained from OPT scanning. Figure 1(a) shows a photograph of split-line patterns created on a cartilage sample. Due to the ink staining, the split-lines are clearly visible on the cartilage surface. The orientation of all split-lines formed a general converging pattern from the side toward the groove. The marked square in Fig. 1(a) (a area) indicates the OPT scanning area. Figure 1(b) shows the 3-D intensity image of the sample obtained in OPT. The A-, B-, and C-scan directions were marked using arrows in Figs. 1(a) and 1(b). The split-lines are also visible in the intensity image. Fig. 1Example OPT images of an articular cartilage sample. (a) A photograph of the split-line pattern at the cartilage surface. The dash-line box indicates the OPT image area. (b) The 3-D intensity image of the sample volume. A sagittal (C-scan) cutting plane is illustrated with marked directions of A-, B-, and C-scan. Also shown are the corresponding cross sectional images of (c) intensity, (d) color-coded local optic axis, and (e) local birefringence at the sagittal cutting plane marked in (b). (f) A histology image (H&E stain) of the same cartilage sample. A zoom-in view of a small section [box in (f)] was shown in (g). SZ, superficial zone; TZ, transitional zone; and RZ, radial zone. 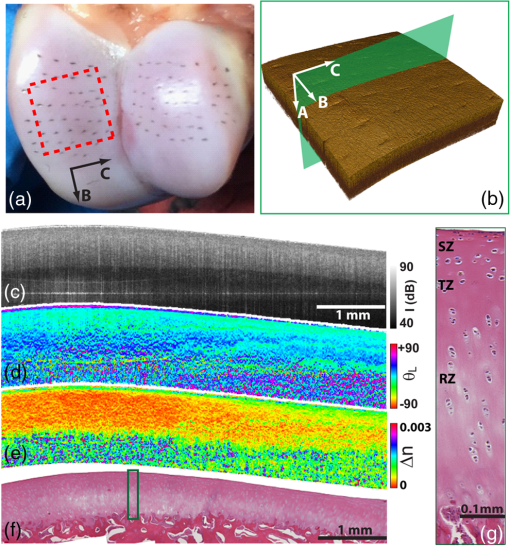 Figures 1(c), 1(d), and 1(e) show representative cross sectional images of the intensity, color-coded local optic axis, and local birefringence. These cross sectional images were constructed from a sagittal cutting plane as shown in Fig. 1(b). The cartilage body had a stronger intensity than the underneath calcified cartilage/bone. The cartilage was thicker at the left end than the right end (toward the groove). The cartilage thickness can be identified from the intensity image as shown previously.16 The fiber orientation was relatively homogeneous close to the surface, but showed a large variation in the calcified cartilage/bone region [Fig. 1(d)]. The birefringence appeared in green color within a very thin layer right underneath the surface. Then the birefringence decreased in value in the radial zone (yellow color), and increased significantly (blue color) in the bone region [Fig. 1(e)]. Similar to the fiber orientation, the birefringence was significantly noisier in the calcified/bone region. The noisy and high birefringence values in the bone region may be caused by the low signal intensity [Fig. 1(c)]. The general cartilage morphology shown in the histology [Fig. 1(f)] agreed well with that obtained in the intensity image [Fig. 1(c)]. From a magnified small histology section [marked on Fig. 1(f)], different cartilage zones can be roughly visualized based on the morphology of the chondrocytes. In the superficial zone (SZ), chondrocytes appeared as flattened ellipsoids that were tangential to the cartilage surface. The spheroidal shaped chondrocytes appeared to be arranged perpendicular toward the cartilage surface in the radial zone (RZ). However, a precise boundary between different zones was elusive from the H&E histology. The fiber orientations can be better visualized in the en face plane at different depths from the cartilage surface [Fig. 2]. As a reference, the corresponding images of OPT intensity and birefringence were also presented. These images were extracted from the 3-D OPT volumes () obtained in the same sample shown in Fig. 1. The split-lines were clearly visible in the en face intensity images. The orientation of the split-lines stayed the same over the entire depth. However, the fiber orientation obtained in the OPT varied over depth. At depth, the streamlines in the top half of the image had negative angles; whereas the bottom half showed positive angles. At depth, most of the streamlines appeared to be horizontal. Then at depth, most of fibers were aligned in the positive direction. The OPT orientation measured at depth appeared to be most similar to the split-line results. Fig. 2The en face tractographic images extracted at different depths from the OPT image volume as shown in Fig. 1(a). The corresponding intensity images showing the split-lines and the birefringence images are shown in the second and third columns, respectively. These images were extracted from the 3-D OPT volumes () obtained in the same sample shown in Fig. 1. 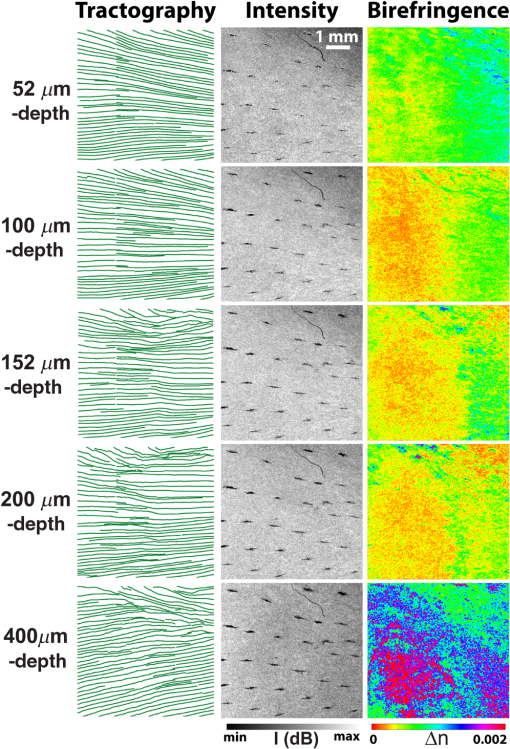 Figure 2 shows that the tissue birefringence varied over the depth in cartilage when examined in the en face planes. This observation is consistent with that from the cross sectional images [Fig. 1]. The color-coded birefringence image appeared in blue-purple color at depth that was inside the calcified cartilage/bone region. Within the region of noncalcified cartilage, the birefringence appeared to decrease initially because it had a smaller value at depth than at depth. It then remained about the same from 100 to . As elaborated in the discussions, such depth-dependent birefringence profiles within the cartilage are likely attributed to “arcade” fiber structure in the noncalcified cartilage as the fibers become more aligned (or “isotropic”) with the incident light in the radial zone. Figure 3 showed a quantitative comparison between the fiber orientation measured in OPT and the split-lines angles in this cartilage sample (Fig. 1). The orientation of each split-line was quantified and represented using a short line segment [Fig. 3(b)]. The fiber orientations obtained in OPT at depth were shown as solid lines in the same figure along with the fitted split-line segments. As shown in Fig. 3(c), the two methods agreed very well with each other and were highly correlated (). Fig. 3(a) The image of split-lines obtained from averaged intensity images from 0 to in depth. (b) The fitted split-lines (short line segments) and OPT results extracted at depth (solid lines in green). (c) The correlation between fiber orientations measured from split-lines and OPT using data shown in (b). (d) The coefficient of determination () obtained from the correlation between split-lines and OPT at different depths. Also shown is the depth profile of the overall error [Eq. (2)] between the angles measured in split-lines and OPT. 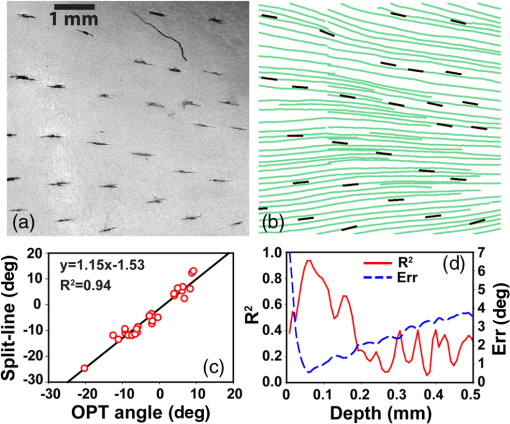 Because the fiber orientation measured in OPT changed with depth (Fig. 2), the correlation between OPT and split-lines was also depth dependent [Fig. 3(d)]. The coefficient of determination of the correlation () increased from 0.4 at surface to 0.94 at depth and then decreased to at depth. The remained more than 0.8 between and in depth. Accordingly, the overall RMSE between the OPT orientation and split-line angles reached a minimum of 0.58 deg at depth. The error was consistently low () between 44 and in depth. Similar results were obtained from split-lines obtained in other cartilage samples. Figure 4 shows the correlation between OPT and split-lines obtained in all five samples with a total of 112 split-lines. Figure 4(a) shows the correlation between split-line and OPT measurements at the depth of . The correlation result had a high of 0.95, a very close to 1.0 slope, and a small intercept of , all indicating an excellent agreement between the two measurements. Further analysis indicated that the difference between the angles obtained in split-line and OPT was less than or equal to 5 deg in more than 89% of the split-lines [Fig. 4(b)]. Figure 4(c) shows the coefficient of determination () and overall error calculated from the correlation between split-lines and OPT at different depths. The stayed above 0.9 using OPT results measured at depths between 40 and ; whereas the corresponding overall RMSE was between the two methods. Fig. 4(a) The correlation between the split-line orientation and OPT in all cartilage samples (a total of 112 split-lines). The OPT results were extracted at the depth of . (b) The distribution of the difference in orientation measured from split-lines and OPT. The line curve shown is a Gaussian fitting of with and . 89.3% of the split-lines had a angle difference from the OPT results. (c) The coefficient of determination () of the correlation between all split-lines and OPT as well as the overall error [Eq. (2)] calculated at different depths. 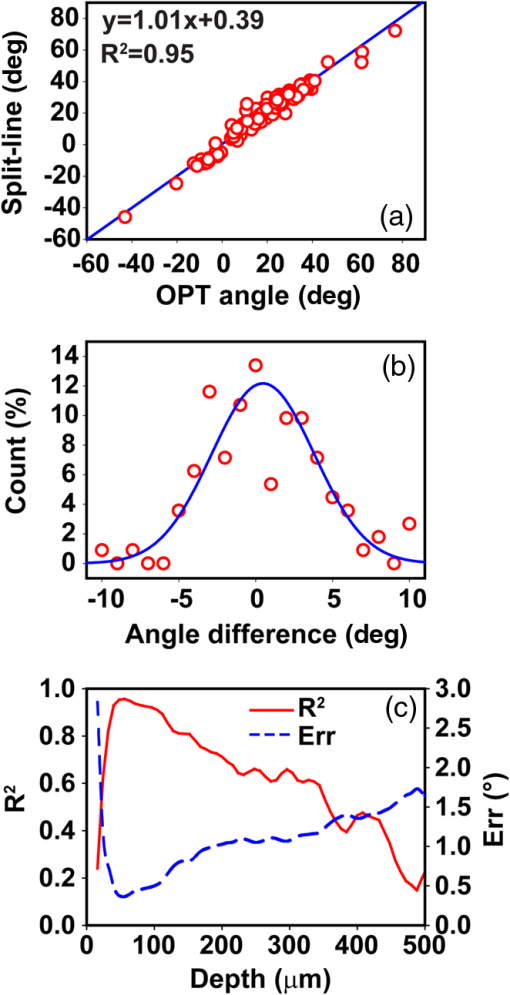 4.DiscussionDespite being a destructive method, the split-line procedure was the only currently available way to map the global fiber orientation on the whole cartilage surface. Our results indicated that the fiber orientation measured in OPT was highly consistent with the split-line results. In addition to being a nondestructive imaging method, OPT also provides a continuous map of the fiber orientation on the entire cartilage surface; whereas the split-lines can be produced only at discrete locations. However, it is important to understand why only the OPT results obtained at depths between and showed excellent agreement with the split-line results. The lower limit of is likely due to special structures in the superficial layer of the cartilage. Studies have shown that additional microscopic structures exist at the “most superficial” layer of the superficial zone.1,35 This special layer may extend deep into the superficial layer and have different tissue composition and morphology. Fiber orientation changes inside the superficial zone were also observed in both PLM36 and SEM studies.37 It is possible that the split-line orientation was less influenced by this “most superficial” layer of the superficial zone. The upper depth limit of obtained in the correlation analyses was likely determined by the thickness of the superficial zone.1,14 The OPT only measures the fiber orientation in an “evaluation plane” that is perpendicular to the incident light.30 When the fibers start to arch in the transitional zone toward the perpendicular orientation in the deep zone, their projections in the evaluation plane also changed. We noticed that the sample birefringence (Figs. 1 and 2) appeared to reach a minimum at , suggesting the fibers may have become perpendicular to the surface at this depth. To investigate this further, the cartilage sample was cut to reveal the internal cross section of the sample and OPT was applied to image it directly from the side. Such a “side-scan” scheme was similar to the conventional PLM imaging of the cross sectional cartilage samples11,14 where the superficial zone can be readily resolved (Fig. 5). In particular, the streamlines [Fig. 5(c)] clearly revealed the “arcade” like architecture in different zones. As a color-coded representation of the same cross sectional axis image, Fig. 5(b) showed a blue layer at small depths that indicated a close to zero-degree orientation in the superficial zone. As a comparison, Fig. 5(d) shows the “top-scan” birefringence images obtained at the same location. The “top-scan” results were obtained by imaging the cartilage from the synovial surface as in the previous figures. Fig. 5The OPT results of intensity (a), optic axis (b), and tractography (c) obtained from the side of the cartilage sample (the “side-scan”). (d) The birefringence image of the same sample obtained from the cartilage surface (the “top-scan”). (e) The quantitative depth profiles of the fiber orientation (from the “side-scan”) and birefringence (from the “top-scan”) obtained from a ROI as marked in (b) and (d). The solid line on the fiber orientation curve is the fitting results using the hyperbolic tangent function. The dashed line labeled as “SZ” marks the boundary of the superficial zone. 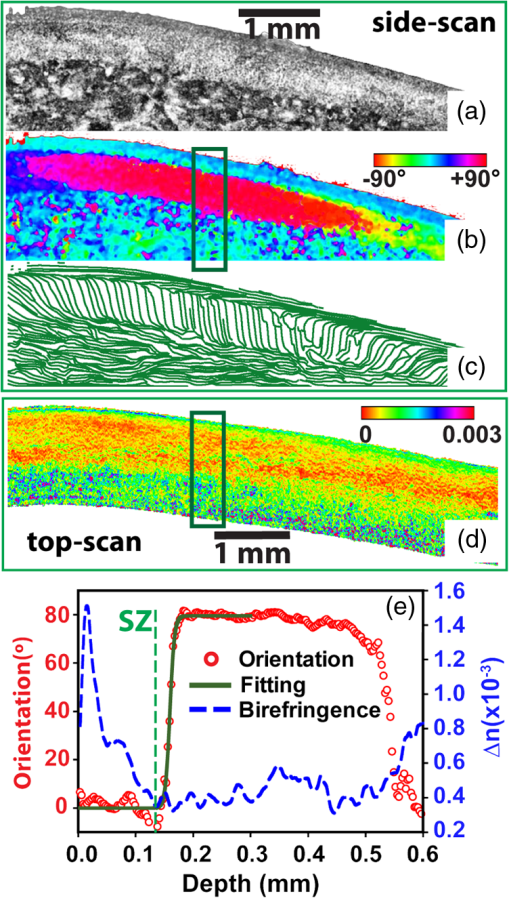 Figure 5(e) shows a comparison between the quantified one-dimensional (1-D) depth profiles of the side-scan fiber orientation and top-scan birefringence. These curves were calculated by averaging all 1-D scans from a ROI ( wide) marked in Figs. 5(b) and 5(d). The depth in Fig. 5(e) was calculated assuming an optical refractive index of 1.5 as reported in a previous study.16 The side-scan orientation curve can be well fitted using the hyperbolic tangent function as described by Xia et al.11 The superficial zone boundary was determined at the depth where the orientation changed more than 1 deg, which resulted in a superficial layer thickness of at this location. The aforementioned superficial zone thickness determined from the “side-scan” coincided with the depth where the “top-scan” birefringence was about to reach the baseline [Fig. 5(e)]. In addition, the birefringence reached the maximum at depth, which coincided with the thickness of the “most superficial” layer reported in a previous study.35 The middle 1/3 of the depth between the maximum and the first minimum of the birefringence was about 50 to , from which the OPT results showed consistent agreement with the split-line results. This observation can serve as an empirical rule to determine the best depth when applying OPT to image fiber orientation in the superficial zone of the cartilage. The birefringence measured in OPT represents the true birefringence only when the collagen fibers are perpendicular to the incident light,25 which is valid in the superficial zone. However, the collagen fibers are almost perpendicular to the cartilage surface in the radial zone, where the fibers appear more “isotropic” to the incident light. Therefore, the measured birefringence appeared to be very small within the radial zone. Similarly, OPT measures the true fiber orientation in the superficial zone where the fibers are tangential to the cartilage surface. The fiber orientations measured in the radial zone only represent the projected fiber orientation within the plane perpendicular to the incident light.30 The birefringence depth profile obtained from the top-scan OPT provides a nondestructive way to estimate the thickness of the superficial zone. As shown in Fig. 5(e), the superficial zone coincided with the depth where the top-scan birefringence started to approach its baseline. Applying this criterion to other locations of the same cartilage, we obtained an average superficial zone thickness of for this cartilage sample. As shown previously,16 the thickness of the whole noncalcified cartilage can be obtained from the intensity image. Therefore, the OPT can potentially measure both the cartilage thickness and its superficial zone thickness in addition to the fiber orientation in the superficial zone. 5.ConclusionWe showed in this study that OPT provides a nondestructive way for visualizing fiber orientation in the superficial zone of fresh cartilage. In addition, the depth-resolved image of the tissue birefringence obtained in OPT can be used to assess the thickness of the superficial zone. Although only porcine phalangeal cartilage was tested in this study, we hypothesize that our results and analyses are still applicable when applying OPT to other cartilage samples. The basic fiber architecture in cartilage has been reported to be similar in several different species.1 Nevertheless, additional studies are necessary to fully understand the capability and limitation of using the OPT technology in cartilage imaging. AcknowledgmentsThe authors thank Rick Disselhorst from the meat science laboratory at University of Missouri for providing cartilage samples and Diane McConnell for providing histology results. The authors Yuanbo Wang, Dongsheng Duan, and Gang Yao have a pending patent application on the optical polarization tractography technology. ReferencesJ. M. Clark,
“The organisation of collagen fibrils in the superficial zones of articular cartilage,”
J. Anat., 171 117
–130
(1990). JOANAY 0021-8782 Google Scholar
S. Below et al.,
“The split-line pattern of the distal femur: a consideration in the orientation of autologous cartilage grafts,”
Arthroscopy, 18
(6), 613
–617
(2002). http://dx.doi.org/10.1053/jars.2002.29877 ARTHE3 0749-8063 Google Scholar
V. B. Shim et al.,
“The influence and biomechanical role of cartilage split line pattern on tibiofemoral cartilage stress distribution during the stance phase of gait,”
Biomech. Model. Mechanobiol., 15
(1), 195
–204
(2015). http://dx.doi.org/10.1007/s10237-015-0668-y BMMICD 1617-7959 Google Scholar
S. Saarakkala et al.,
“Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics,”
Osteoarthritis Cartilage, 18
(1), 73
–81
(2010). http://dx.doi.org/10.1016/j.joca.2009.08.003 OSCAEO 1063-4584 Google Scholar
D. Heinegard and T. Saxne,
“The role of the cartilage matrix in osteoarthritis,”
Nat. Rev. Rheumatol., 7
(1), 50
–56
(2011). http://dx.doi.org/10.1038/nrrheum.2010.198 Google Scholar
G. Meachim et al.,
“Collagen alignments and artificial splits at the surface of human articular cartilage,”
J. Anat., 118
(1), 101
–118
(1974). JOANAY 0021-8782 Google Scholar
P. Boettcher et al.,
“Mapping of split-line pattern and cartilage thickness of selected donor and recipient sites for autologous osteochondral transplantation in the canine stifle joint,”
Vet. Surg., 38
(6), 696
–704
(2009). http://dx.doi.org/10.1111/vsu.2009.38.issue-6 VESUD6 Google Scholar
W. Petersen and B. Tillmann,
“Collagenous fibril texture of the human knee joint menisci,”
Anat. Embryol., 197
(4), 317
–324
(1998). http://dx.doi.org/10.1007/s004290050141 ANEMDG 0340-2061 Google Scholar
M. E. Mononen et al.,
“Effect of superficial collagen patterns and fibrillation of femoral articular cartilage on knee joint mechanics—a 3-D finite element analysis,”
J. Biomech., 45
(3), 579
–587
(2012). http://dx.doi.org/10.1016/j.jbiomech.2011.11.003 JBMCB5 0021-9290 Google Scholar
Y. Xia,
“Resolution ‘scaling law’ in MRI of articular cartilage,”
Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society, 15
(4), 363
–365
(2007). http://dx.doi.org/10.1016/j.joca.2006.12.004 OSCAEO 1063-4584 Google Scholar
Y. Xia et al.,
“Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage,”
Osteoarthritis Cartilage, 9
(5), 393
–406
(2001). http://dx.doi.org/10.1053/joca.2000.0405 OSCAEO 1063-4584 Google Scholar
C. Moger et al.,
“Regional variations of collagen orientation in normal and diseased articular cartilage and subchondral bone determined using small angle X-ray scattering (SAXS),”
Osteoarthritis Cartilage, 15
(6), 682
–687
(2007). http://dx.doi.org/10.1016/j.joca.2006.12.006 OSCAEO 1063-4584 Google Scholar
J. Rieppo et al.,
“Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation,”
Osteoarthritis Cartilage, 17
(4), 448
–455
(2009). http://dx.doi.org/10.1016/j.joca.2008.09.004 OSCAEO 1063-4584 Google Scholar
J. I. H. Lee and Y. Xia,
“Quantitative zonal differentiation of articular cartilage by microscopic magnetic resonance imaging, polarized light microscopy, and Fourier-transform infrared imaging,”
Microsc. Res. Tech., 76
(6), 625
–632
(2013). http://dx.doi.org/10.1002/jemt.v76.6 MRTEEO 1059-910X Google Scholar
H. Jahr, N. Brill and S. Nebelung,
“Detecting early stage osteoarthritis by optical coherence tomography?,”
Biomarkers, 20
(8), 590
–596
(2015). http://dx.doi.org/10.3109/1354750X.2015.1130190 Google Scholar
J. M. Herrmann et al.,
“High resolution imaging of normal and osteoarthritic cartilage with optical coherence tomography,”
J. Rheumatol., 26
(3), 627
–635
(1999). Google Scholar
C. R. Chu et al.,
“Arthroscopic microscopy of articular cartilage using optical coherence tomography,”
Am. J. Sports Med., 32
(3), 699
–709
(2004). http://dx.doi.org/10.1177/0363546503261736 Google Scholar
S. Nebelung et al.,
“Three-dimensional imaging and analysis of human cartilage degeneration using optical coherence tomography,”
J. Orthop. Res., 33
(5), 651
–659
(2015). http://dx.doi.org/10.1002/jor.v33.5 JOREDR 0736-0266 Google Scholar
X. Li et al.,
“High-resolution optical coherence tomographic imaging of osteoarthritic cartilage during open knee surgery,”
Arthritis Res. Ther., 7
(2), R318
–R323
(2005). http://dx.doi.org/10.1186/ar1491 Google Scholar
S. J. Matcher,
“A review of some recent developments in polarization-sensitive optical imaging techniques for the study of articular cartilage,”
J. Appl. Phys., 105
(10), 102041
(2009). http://dx.doi.org/10.1063/1.3116620 JAPIAU 0021-8979 Google Scholar
N. Brill et al.,
“Polarization-sensitive optical coherence tomography-based imaging, parameterization, and quantification of human cartilage degeneration,”
J. Biomed. Opt., 21
(7), 076013
(2016). http://dx.doi.org/10.1117/1.JBO.21.7.076013 JBOPFO 1083-3668 Google Scholar
W. Drexler et al.,
“Correlation of collagen organization with polarization sensitive imaging of in vitro cartilage: implications for osteoarthritis,”
J. Rheumatol., 28
(6), 1311
–1318
(2001). Google Scholar
C. R. Chu et al.,
“Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography,”
J. Biomed. Opt., 12
(5), 051703
(2007). http://dx.doi.org/10.1117/1.2789674 JBOPFO 1083-3668 Google Scholar
C. Fan and G. Yao,
“Mapping local optical axis in birefringent samples using polarization-sensitive optical coherence tomography,”
J. Biomed. Opt., 17
(11), 110501
(2012). http://dx.doi.org/10.1117/1.JBO.17.11.110501 JBOPFO 1083-3668 Google Scholar
N. Ugryumova, S. V. Gangnus and S. J. Matcher,
“Three-dimensional optic axis determination using variable-incidence-angle polarization-optical coherence tomography,”
Opt. Lett., 31
(15), 2305
–2307
(2006). http://dx.doi.org/10.1364/OL.31.002305 OPLEDP 0146-9592 Google Scholar
Z. Lu, D. Kasaragod and S. J. Matcher,
“Conical scan polarization-sensitive optical coherence tomography,”
Biomed. Opt. Express, 5
(3), 752
(2014). http://dx.doi.org/10.1364/BOE.5.000752 BOEICL 2156-7085 Google Scholar
N. Ugryumova et al.,
“Novel optical imaging technique to determine the 3-D orientation of collagen fibers in cartilage: variable-incidence angle polarization-sensitive optical coherence tomography,”
Osteoarthritis Cartilage, 17
(1), 33
–42
(2009). http://dx.doi.org/10.1016/j.joca.2008.05.005 OSCAEO 1063-4584 Google Scholar
C. Fan and G. Yao,
“Mapping local retardance in birefringent samples using polarization sensitive optical coherence tomography,”
Opt. Lett., 37
(9), 1415
(2012). http://dx.doi.org/10.1364/OL.37.001415 OPLEDP 0146-9592 Google Scholar
C. Fan and G. Yao,
“Imaging myocardial fiber orientation using polarization sensitive optical coherence tomography,”
Biomedical Optics Express, 4
(3), 460
(2013). http://dx.doi.org/10.1364/BOE.4.000460 BOEICL 2156-7085 Google Scholar
Y. Wang et al.,
“Histology validation of mapping depth-resolved cardiac fiber orientation in fresh mouse heart using optical polarization tractography,”
Biomed. Opt. Express, 5
(8), 2843
–2855
(2014). http://dx.doi.org/10.1364/BOE.5.002843 BOEICL 2156-7085 Google Scholar
Y. Wang et al.,
“Optical polarization tractography revealed significant fiber disarray in skeletal muscles of a mouse model for Duchenne muscular dystrophy,”
Biomed. Opt. Express, 6
(2), 347
(2015). http://dx.doi.org/10.1364/BOE.6.000347 BOEICL 2156-7085 Google Scholar
Y. Wang and G. Yao,
“Optical tractography of the mouse heart using polarization-sensitive optical coherence tomography,”
Biomed. Opt. Express, 4
(11), 2540
(2013). http://dx.doi.org/10.1364/BOE.4.002540 BOEICL 2156-7085 Google Scholar
L. Azinfar et al.,
“High resolution imaging of the fibrous microstructure in bovine common carotid artery using optical polarization tractography,”
J. Biophotonics,
(2015). http://dx.doi.org/10.1002/jbio.201500229 Google Scholar
C. Fan and G. Yao,
“Full-range spectral domain Jones matrix optical coherence tomography using a single spectral camera,”
Opt. Express, 20
(20), 22360
(2012). http://dx.doi.org/10.1364/OE.20.022360 OPEXFF 1094-4087 Google Scholar
R. Fujioka, T. Aoyama and T. Takakuwa,
“The layered structure of the articular surface,”
Osteoarthritis Cartilage, 21
(8), 1092
–1098
(2013). http://dx.doi.org/10.1016/j.joca.2013.04.021 OSCAEO 1063-4584 Google Scholar
D. Mittelstaedt et al.,
“Quantitative determination of morphological and territorial structures of articular cartilage from both perpendicular and parallel sections by polarized light microscopy,”
Connect. Tissue Res., 52
(6), 512
–522
(2011). http://dx.doi.org/10.3109/03008207.2011.595521 Google Scholar
R. Teshima et al.,
“Structure of the most superficial layer of articular cartilage,”
J Bone Joint Surg. Br., 77
(3), 460
–464
(1995). Google Scholar
|

