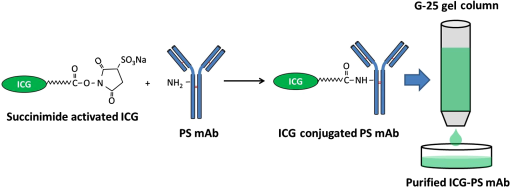
|
|
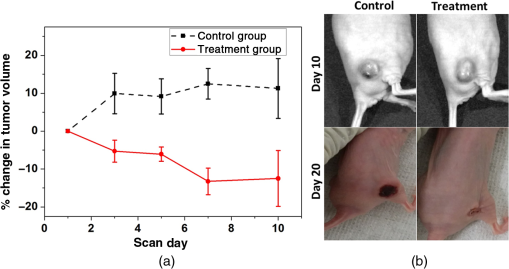
1.IntroductionBreast cancer is one of the most diagnosed and leading causes of cancer deaths in women. Clinically, breast cancers are divided based on its prognostic and therapeutic implications. Triple-negative breast cancers (TNBC) do not express estrogen receptor or progesterone receptor, and do not have HER-2/Neu amplifications. TNBC’s suffer from poor prognosis and have limited response to targeted therapy techniques.1 10% to 15% of all breast cancers are reported to be TNBC.2 While TNBC is treatable by chemotherapy, a noninvasive and nonradioactive imaging modality to track the occurrence of apoptosis is needed for an effective treatment plan. The common targets for specific imaging probes for apoptosis include activated effector caspases3,4 and probes that bind to components of the scrambled plasma membrane such as phosphatidylserine (PS) that are transiently exposed on the outer surface of the cell during apoptosis.4–6 Caspases are cysteine-aspartate proteases that play a pivotal role in the regulation of apoptosis. The intrinsic and extrinsic pathways of apoptosis lead to a transient increase of a small number of intracellular executioner caspases: caspase-3 and caspase-7. To successfully reach the caspase-3 and caspase-7 targets, the apoptosis probes have to penetrate the cell membrane. If there is no apoptosis, the probes do not have the ability to leave the cells resulting in false positives. Despite such drawbacks, there have been several developments on optical and nuclear imaging of apoptosis based on fluorescent probes and caspase inhibitors such as isatins7–9 for in vivo applications. Although these caspase inhibitors have been proposed for imaging of the activated caspases, its rapid and transient increase renders imaging of this apoptosis biomarker particularly challenging.10 In fact, every patient may show a different ideal temporal window for imaging, which occurs between 6 and 24 h after treatment. This requirement to choose the best time to image may limit the clinical application of these agents. On the other hand, PS is a major anionic phospholipid, representing 2% to 10% of total cell phospholipids. Its externalization occurs within a few hours of an apoptotic stimulus by an active transport mechanism that leads to presentation of 50 to 100 million molecules per apoptotic cell on its surface. This makes PS an abundant and accessible target for molecular imaging. During apoptosis, PS is exposed on the cell surface facilitating redistribution of phospholipids across the bilayer, making it an accessible target for molecular imaging.5,6 Currently, annexin V labeled with radioisotope is the most widely used imaging probe for PS-based detection of apoptosis that shows an elevated uptake of the tracer in some lesions that respond to chemotherapy.4,5,11 Annexin V is also one of the few cell death imaging agents that has reached phase II/III clinical trials. However, annexin V has a low PS binding affinity and the binding requires the presence of calcium. In many cases, the endogenous Ca++ level is not high enough to facilitate the binding between annexin V and PS; hence, the localization of radiolabeled annexin in apoptotic cells is suboptimal. Also, this technique is limited by radioisotope half-life, which may require frequent injection and repeated imaging. We propose to address these limitations by labeling, indocyanine green (ICG), a nonradioactive FDA approved near-infrared (NIR) absorbing probe with a long plasma half-life PS monoclonal antibody (mAb) and image the apoptotic tumor site with a relatively new imaging modality known as multispectral optoacoustic tomography (MSOT). Apart from anatomical information, multiwavelength optoacoustic imaging provides functional information of the tissue noninvasively with high contrast and good spatial resolution based on optoacoustic or photoacoustic effect.12–14 The use of exogenous NIR contrast agents such as ICG further improves the capabilities of MSOT to provide additional deep functional information of tissues. 2.Materials and Methods2.1.Conjugation and PurificationIn this work, PS mAb (Cell Signal Solutions, New Jersey) was tagged with succinimide activated ICG (AAT Bioquewst Inc., California). The desired concentration of antibody solution was prepared by diluting the PS mAb stock () in of phosphate buffer solution. For conjugation, 1 mg of ICG-sulfo-EG4-OSu was dissolved in of dimethyl sulfloxide (DMSO) and added to the antibody solution to keep the final concentration of DMSO to . The mixture was incubated at room temperature for 1 h and subjected to gel filtration purification using a G-25 gel filter column. The eluted solution contained the conjugated products while the unbound ICG was trapped in the gel column. The conjugation and purification process is summarized in the schematic as shown in Fig 1. NIR spectrometry and multispectral optoacoustic scans (iThera Medical GmbH, Munich, Germany) were performed on the eluted ICG-PS mAb and chemotherapeutic drug doxorubicin (Sigma-Aldrich, St Louis, Missouri) to determine the maximum absorption peaks in the NIR wavelength range of 680 to 980 nm. 2.2.Mouse Xenograft Model and Animal Study DetailsTriple negative breast cancer cell line, MDA-MB-231, (ATCC, Virginia) was cultured in Dulbecco’s modified eagle’s medium containing 10% fetal bovine serum and 1% penicillin streptomycin at 37°C in an incubator containing 5% . When 80% to 90% confluent, cells were used for subcutaneous innoculation. All animal experiments were performed in compliance with guidelines set by the Institutional Animal Care and Use Committee (IACUC), SingHealth. Breast cancer xenograft models were established by subcutaneous injection of cell suspension (0.2 mL) containing and Matrigel (BD Biosciences, California) in volume ratio on the right flank of immunodeficient nude female mice (NCr) purchased from InVivos Pte Ltd., Singapore. The mice were continuously monitored for tumor growth and were used for further experiments when the tumor volume reached a size of 400 to . During the animal study, the treatment group received a single intraperitoneal injection (IP) injection of doxorubicin at , followed by IP injection of ICG-PS mAb. The control group received a single dose of normal saline, followed by an IP injection of ICG-PS mAb in the peritoneum. The longer plasma circulatory time of ICG-PS mAb was used to capture the temporal window of apoptosis occurrence by periodic scanning. MSOT scans were performed on days 0, 1, 3, 5, 7, and 10 with day 0 being the baseline scan to investigate the localization of the ICG-PS mAb. Tumor volume was measured on all scan days using Vernier caliper. 2.3.Multispectral Optoacoustic Tomography Experimental Parameters and Scan ProtocolThe mouse was placed in a belly down position in a water-repellent transparent polyethylene membrane to prevent direct animal contact with water before placing it in the temperature-controlled imaging water chamber of the 64-channel MSOT scanner for good acoustic coupling. Ultrasound gel was applied on the mouse skin surface to match the speed of sound in tissue and water. During the scan, the mice were anesthetized by continuously maintaining the isoflurane at 1.5% to 2% and oxygen flow rate at 0.8 to . The mouse/phantom was translated through the transducer array by the animal stage along its axis to capture the corresponding axial image slices. For data acquisition, the region of interest (ROI) of multiple axial slices was set to a step size of 0.5 mm with 9 ns excitation pulses from a tunable optical parametric oscillator pumped by Nd-YAG laser at a repetition rate of 10 Hz from 700 to 900 nm wavelength range. The generated ultrasound is detected by 64 cylindrically focused ultrasound transducers with a central frequency of 5 MHz (60% bandwidth), organized in a concave array of 172 deg angular coverage and a radius of curvature of 4 cm. A wavelength interval of 10 nm for each axial slice was selected and the averaged optoacoustic signals from five frames per wavelength at each step were recorded. Signals obtained were then processed to reconstruct the anatomical and functional (multispectral) tomographic images using the integrated ViewMSOT software. 2.4.Multispectral Optoacoustic Tomography Phantom StudyFor the phantom study, the polyurethane MSOT phantom provided by iThera was used. The cylindrical phantom with a diameter of 2 cm is specially designed to mimic the shape, size, and optical properties of a mouse. Additionally, it has two inner cylindrical channels, each with a diameter of 3 mm and capacities, one for holding the control medium and the other for holding the dissolved contrast agent in the same medium. The control medium and ICG-PS mAb/doxorubicin were pipetted into the respective channels and placed in a phantom holder, which in turn was placed in the MSOT imaging chamber. For data acquisition, we set up an ROI of multiple transverse slices with a step size of 1 mm across the channel containing the probe and control, applied excitation wavelengths from 700 to 900 nm in steps of 10 nm for each transverse slice, and recorded the averaged optoacoustic signals from 5 frames per wavelength and position. 3.ResultsThe greenish appearance of the eluted product was an indication of successful conjugation of ICG to PS mAb while the unconjugated ICG remained in the gel column. NIR spectroscopy of the conjugates later confirmed the binding of ICG to antibody. Like ICG-PS mAb, doxorubicin also has a longer plasma half-life that may contribute to the optoacoustic signal generation during MSOT scan resulting in a false-positive signal in the tumor region. To overcome this, NIR absorbance of both doxorubicin and ICG-PS mAb were performed to check for absorption maximum in the NIR region. NIR absorption spectrum [Fig. 2(a)] of the conjugates showed peaks at 715 and 780 nm due to ICG while doxorubicin did not show any absorbance. The results not only confirm the binding of ICG to PS mAb but also showed that the peaks fall within 680 to 980 nm wavelength range of the MSOT system. Prior to injecting ICG-PS mAb and doxorubicin into the animal, a phantom study was conducted to investigate the optoacoustic effect of the compound. The resultant spectra of the compound from the reconstructed MSOT data matched closely with that of NIR absorption spectra. Due to the negligible absorbance of doxorubicin in the NIR region, it did not show any optoacoustic signal generation as compared to ICG as shown in Fig. 2(b). Fig. 2NIR spectrum of ICG-PS mAb and doxorubicin. (a) Absorption spectrum obtained from spectrometer. (b) NIR spectrum obtained from MSOT phantom study. 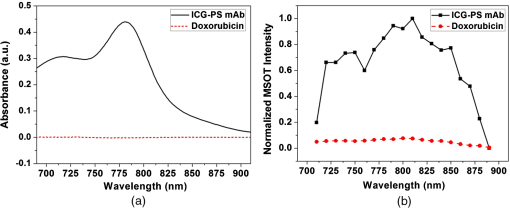 Pre- and posttreatment MSOT scans were conducted on control and treated tumor groups. The axial images showing the cross section of mice along the tumor are as shown in Fig. 3(a). The tumor site in this figure is highlighted with white circles. The first and second columns represent the multispectral optoacoustic images of control and treatment groups obtained on days 0, 1, 3, 5, 7, and 10, respectively. As shown in Fig. 3(a), the control tumor showed no sign of ICG signal in the tumor region on all scan days despite the presence of ICG-PS mAb in circulation as seen in the images. In the treatment group, there is a gradual increase in the accumulation of ICG-PS mAb over time in the tumor site. To estimate the percentage change in MSOT signal intensity due to the uptake of ICG-PS mAb, an ROI was drawn to include the entire volume of the tumor. The percentage change in intensity with respect to the baseline (day 0) was calculated. As shown in Fig. 3(b), the treatment group showed highest increase in intensity on day 5, while the control group showed very minimal change in signal intensity throughout the study. Tomographic slices showing the distribution and accumulation of ICG-PS mAb mostly in the periphery of the tumor on day 5 are presented in Fig. 3(c). Fig. 3(a) MSOT images showing an increased uptake of ICG-PS antibody in the treatment group tumor region indicating the cells have undergone apoptosis. The control group shows no uptake of ICG-PS antibody in the tumor region. (b) The percentage change in the MSOT signal intensity in the tumor region on different scan days as compared to the baseline for both control and treatment groups. (c) Tomographic slices of the scanned tumor area on day 5 (treatment group) with each slice separated by 1 mm. 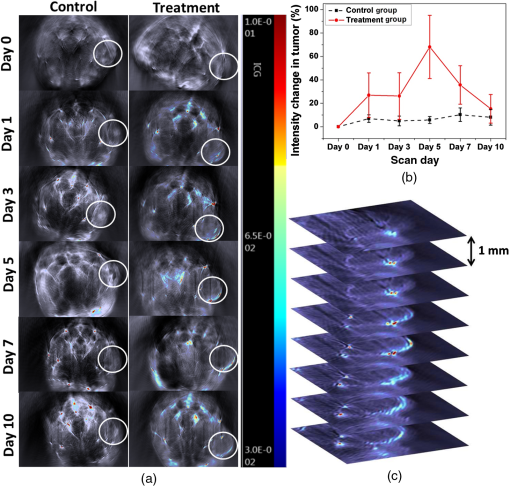 The physical change in the tumor volume on all scan days was measured for both groups to confirm the treatment outcome. Percentage change in tumor volume was then calculated for each tumor using the following equation: where is the volume on the previous scan day and is the volume on scan day.The MSOT scan results showed good correlation with the treatment results as the tumor in the treated mouse gradually reduced in size, while the tumor in the control mouse continued to grow as shown in Fig. 4(a). After 20 days, the tumor in the treated mouse completely regressed but the tumor in the control mouse continued to grow as shown in Fig. 4(b). The maximum reduction in tumor volume also occurred between 5 and 7 days as shown in Fig. 4(a) which correlates well with the MSOT signal. 4.DiscussionThe ICG-PS mAb conjugates were purified by a simple gel filtration technique, which separates the unconjugated ICG from the reaction solution. In this method, the ICG-PS mAb and the unconjugated PS mAb remain in the eluted solution. When injected, the unconjugated PS mAb will compete with the ICG-PS mAb for the PS target on the apoptotic cell surface, reducing specific binding sites for ICG-PS mAb. To overcome this, the compound has to be further purified to remove unconjugated PS mAb. High-pressure gel filtration purification is one such technique that can be used to separate the ICG-PS mAb conjugates from the unconjugated antibody, thus removing the competition from unbound PS mAb. This will increase specific binding of ICG-PS mAb which can in turn increase the optoacoustic signal from the targeted apoptotic tumor site during MSOT scan. The commonly used positron emission tomography (PET) and single photon emission tomography (SPECT) employ short half-lived radioisotopes and ligands of short plasma half-life. Thus, it is not possible to capture occurrence of apoptosis that may occur 1 to 10 days postchemotherapy. The present antibody-based study takes advantage of long circulatory half-life of PS antibodies to localize in the apoptotic site over a long period of time. As shown in Fig. 4(b), the highest localization of ICG-PS mAb was observed on the fifth day and started to decrease gradually thereafter. The spectral stability of ICG varies with different aqueous conditions. In tissues and cells, the NIR absorption peak is red shifted due to binding of ICG to cell/blood proteins,15,16 which may lead to missing the absorption maximum of ICG if a single-wavelength photoacoustic tomography is used. Multiwavelength optoacoustic tomography can overcome this issue by covering the entire wavelength range in a single scan for imaging ICG that are bound to tissues and thus eliminating the need for multiple scans from a single-wavelength photoacoustic tomography. Noninvasive in vivo imaging of apoptosis employing multiwavelength optoacoustic tomography was successfully used to capture the occurrence of apoptosis in TNBC tumors. This technology can be a valuable tool in monitoring therapy response and strategizing for an efficient treatment of TNBC. As demonstrated in this work, the conjugation of antibody to ICG is relatively easy and can be extended to other apoptosis indicating antibodies and proteins, such as phospatidylethanolamine17 and lactadherin.18 This technique has the potential to be used in clinical applications since ICG is the only optical contrast agent approved by the FDA and has been used clinically in many areas that include the assessment of lymphatic vascular structure and function,19 vascular repair,20 measurement of liver function,21,22 and retinal angiography.23,24 5.ConclusionPS mAb conjugated with ICG is nonradioactive and has a longer plasma half-life. Hence, imaging of apoptosis can be performed over a period of time without the limitation of radioisotope half-life, while taking advantage of the high sensitivity and high resolution of MSOT. We have demonstrated that MSOT images show the occurrence of apoptosis in the treatment group with a steady increase in signal intensity in the tumor region as compared to the control group. Combined with suitable probes, MSOT is of potential in imaging of apoptosis and can be a good tool for evaluating the therapeutic effect of various chemotherapy drugs. AcknowledgmentsThe authors would like to acknowledge financial support received from Singhealth Foundation Research (Grant No. SHF/FG549P/2013). ReferencesJr. W. J. Irvin and L. A. Carey,
“What is triple-negative breast cancer?,”
Eur. J. Cancer, 44 2799
–2805
(2008). http://dx.doi.org/10.1016/j.ejca.2008.09.034 Google Scholar
C. K. Anders and L. A. Carey,
“Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer,”
Clin. Breast Cancer, 9 S73
–S81
(2009). http://dx.doi.org/10.3816/CBC.2009.s.008 Google Scholar
M. Scabini et al.,
“In vivo imaging of early stage apoptosis by measuring real-time caspase-3/7 activation,”
Apoptosis, 16 198
–207
(2011). http://dx.doi.org/10.1007/s10495-010-0553-1 Google Scholar
F. G. Blankenberg,
“In vivo detection of apoptosis,”
J. Nucl. Med., 49
(Suppl 2), 81s
–95s
(2008). http://dx.doi.org/10.2967/jnumed.107.045898 Google Scholar
J. Toretsky et al.,
“Preparation of F-18 labeled annexin V: a potential PET radiopharmaceutical for imaging cell death,”
Nucl. Med. Biol., 31 747
–752
(2004). http://dx.doi.org/10.1016/j.nucmedbio.2004.02.007 Google Scholar
K. Ogawa and M. Aoki,
“Radiolabeled apoptosis imaging agents for early detection of response to therapy,”
Sci. World J., 2014 11
(2014). http://dx.doi.org/10.1155/2014/732603 Google Scholar
G. Smith et al.,
“Design, synthesis, and biological characterization of a caspase 3/7 selective isatin labeled with 2-18F]fluoroethylazide,”
J. Med. Chem., 51 8057
–8067
(2008). http://dx.doi.org/10.1021/jm801107u JMCMAR 0022-2623 Google Scholar
Q.-D. Nguyen et al.,
“Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific (18)F]-labeled isatin sulfonamide,”
Proc. Natl. Acad. Sci. U. S. A., 106 16375
–16380
(2009). http://dx.doi.org/10.1073/pnas.0901310106 Google Scholar
L. E. Edgington et al.,
“Non-invasive optical imaging of apoptosis using caspase-targeted activity based probes,”
Nat. Med., 15 967
–973
(2009). http://dx.doi.org/10.1038/nm.1938 1078-8956 Google Scholar
B. A. Smith and B. D. Smith,
“Biomarkers and molecular probes for cell death imaging and targeted therapeutics,”
Bioconjugate Chem., 23 1989
–2006
(2012). http://dx.doi.org/10.1021/bc3003309 BCCHES 1043-1802 Google Scholar
S. Rottey et al.,
“Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy,”
J. Nucl. Med., 47 1813
–1818
(2006). JNMEAQ 0161-5505 Google Scholar
J. Xia, J. Yao and L. V. Wang,
“Photoacoustic tomography: principles and advances,”
Electromagn. Waves, 147 1
–22
(2014). http://dx.doi.org/10.2528/PIER14032303 Google Scholar
A. Taruttis and V. Ntziachristos,
“Advances in real-time multispectral optoacoustic imaging and its applications,”
Nat. Photonics, 9 219
–227
(2015). http://dx.doi.org/10.1038/nphoton.2015.29 Google Scholar
P. J. van den Berg, K. Daoudi and W. Steenbergen,
“Review of photoacoustic flow imaging: its current state and its promises,”
Photoacoustics, 3 89
–99
(2015). http://dx.doi.org/10.1016/j.pacs.2015.08.001 Google Scholar
S. Fickweiler et al.,
“Indocyanine green: intracellular uptake and phototherapeutic effects in vitro,”
J. Photochem. Photobiol., B, 38 178
–183
(1997). http://dx.doi.org/10.1016/S1011-1344(96)07453-2 Google Scholar
C. Ciamberlini et al.,
“Indocyanine green videoangiography using cooled charge-coupled devices in central serous choroidopathy,”
J. Biomed. Opt., 2 218
–225
(1997). http://dx.doi.org/10.1117/12.268939 Google Scholar
K. Emoto et al.,
“Exposure of phosphatidylethanolamine on the surface of apoptotic cells,”
Exp. Cell. Res., 232 430
–434
(1997). http://dx.doi.org/10.1006/excr.1997.3521 ECREAL 0014-4827 Google Scholar
S. K. Dasgupta, P. Guchhait and P. Thiagarajan,
“Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5,”
Transl. Res., 148 19
–25
(2006). http://dx.doi.org/10.1016/j.lab.2006.03.006 Google Scholar
N. Unno et al.,
“A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography,”
J. Vasc. Surg., 52 946
–952
(2010). http://dx.doi.org/10.1016/j.jvs.2010.04.067 Google Scholar
N. Unno et al.,
“Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema,”
J. Vasc. Surg., 45 1016
–1021
(2007). http://dx.doi.org/10.1016/j.jvs.2007.01.023 Google Scholar
T. Ishizawa et al.,
“Real-time identification of liver cancers by using indocyanine green fluorescent imaging,”
Cancer, 115 2491
–2504
(2009). http://dx.doi.org/10.1002/cncr.v115:11 CANCAR 0008-543X Google Scholar
K. Gotoh et al.,
“A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation,”
J. Surg. Oncol., 100 75
–79
(2009). http://dx.doi.org/10.1002/jso.v100:1 JSONAU 0022-4790 Google Scholar
S. L. Owens,
“Indocyanine green angiography,”
Br. J. Ophthalmol., 80 263
–266
(1996). http://dx.doi.org/10.1136/bjo.80.3.263 Google Scholar
L. A. Yannuzzi,
“Indocyanine green angiography: a perspective on use in the clinical setting,”
Am. J. Ophthalmol., 151 745
–751
(2011). http://dx.doi.org/10.1016/j.ajo.2011.01.043 AJOPAA 0002-9394 Google Scholar
BiographyRavi Kumar Kannadorai received his PhD from Nanyang Technological University, Singapore. He is a research fellow with the Department of Nuclear Medicine, Singapore General Hospital. He is currently working on tumor imaging with various imaging modalities such as MSOT, PET, and SPECT. His other research interests include tumor therapy using SIRT and plasmonic photothermal effect, creating tumor models in mice, In Vivo Imaging System, and fluorescence spectroscopy. Sunil Kumar Udumala received his MSc degree in biochemistry during which he worked on end-stage renal disease (ESRD) from Uppsala University and Karolinska Institute. He is a senior research associate at the Radiological Sciences ACP, Singapore General Hospital. In 2009, he joined Eli Lily and Astar, Singapore and established new functional and screening cell-based assays to validate drug targets. At Roche, he set up the imaging lab with a focus on preclinical imaging and radiotracers development. Yu Wing Kwong Sidney is a senior principal research scientist with the Department of Nuclear Medicine, Singapore General Hospital. His research interest is in molecular tumor imaging with various imaging modalities such as PET, MRI, CT, and more recently, MSOT. He and his team have vast experiences in conjugation and radiolabeling of large molecules such as peptides and antibodies, creating various tumor models in mice, and imaging of the animals. |