|
|
1.IntroductionThe process of wound healing is dependent on the coordination of numerous cellular processes. It consists of an inflammatory phase, a proliferative phase, and a remodeling phase. The remodeling phase includes the reorientation and reorganization of the granulation tissue into a scar.1 The current interest in the effects of low-intensity laser therapy (LILT) on wound healing arose from the studies of Mester 2 The advantageous use of the laser radiation is mostly observed in the treatment of persistent wounds. In particular, the irradiation of wounds with helium-neon laser (HeNe, radiation at ) has demonstrated an acceleration of healing in animals and humans.3 The main reason for using the light sources radiating in the red and near-IR spectral region is that water and hemoglobin have a weak absorption in the range of . In addition, photons are preferably scattered in the forward direction, promoting a high transdermal penetration of light.4 Trends in LILT research have looked for grounding the effects of the laser radiation on wound healing in vivo. More particularly, an augmented knowledge of the physical characteristics of the radiation is desirable for the effective application of LILT. For example, in biological tissue, laser random speckles are less pronounced than the speckles caused by laser radiation with a preferential direction and orientation, thus, this phenomenon could enhance the effects of radiation coherence when the tissue is irradiated.5 Furthermore, a recent morphological semiquantitative study concerning LILT effects on wound healing showed that a specific direction of the polarized laser incidence could improve the healing process.6 The polarization state of light can be detected and quantified.7, 8, 9, 10, 11, 12 This fact is particularly significant when birefringent tissues are evaluated for diagnostic purposes, because birefringent tissues present remarkable modifications due to the transition from normal to a pathologic state. The collagen content of the skin is particularly important, since combined with elastin in the dermis it provides the extracellular matrix on which the impermeable epidermal layer is grown. The loss of the collagen structure and integrity is frequently associated with abnormalities of the skin, including skin cancer and thermal injury. Thus, birefringence measurements may be seen as a valuable diagnostic indicator of the skin condition.13 Polarized light microscopy is an example of a tool to analyze birefringent material such as collagen, providing information about the orientation and distribution of the collagen.14 On the illumination of the sample on the polarized microscopy the ratio between incident/emergent light is changed by the retardation or by the optical path difference (OPD) of the object.15 When light contacts a birefringent material, the light wave is decomposed into two perpendicularly polarized beams; one beam is polarized along the slowest direction and another along the fastest direction. Polarized light along any random orientation will become elliptically polarized due to the phase difference . If the incoming polarization is in the same direction as either the slowest or the fastest direction, the light will remain linearly polarized when exiting the sample, giving a brighter image usually called birefringence brightness. is proportional to the thickness of the sample and the difference in refractive indices and it is inversely proportional to the wavelength of the light : , where is the optical retardation or OPD. Although the OPD technique constitutes a powerful instrument to perform quantitative analysis during the healing process, to the best of our knowledge collagen birefringence measurements following LILT have never been reported. In this paper, we quantify the organization of collagen fibers in burned skin during the healing process following LILT applied in two different directions of incidence of the electric field vector of the polarized laser radiation through the OPD technique. 2.Materials and MethodsTwenty male adult Wistar rats weighing each were studied. The animals were anesthetized with Avertin ( body mass) and had their backs shaved. Due to the individual variability in the duration and quality of regeneration, each experimental animal acted as its own control. Thus, irradiated and nonirradiated (control) deep third-degree burns were created in the same rat. Three round burns measuring about in diameter were cryogenerated at the end of the spinal column of each animal using a cylindrical brass rod cooled to . The brass rod was kept in contact with the skin of the animal in the areas that received the injury. The contact was made in two sequences of each with an interval of . This procedure was repeated for . After the last application, lesion was irradiated using the electric field vector of the incident polarized laser radiation aligned in parallel with the rat’s occipital-caudal direction, lesion was irradiated using the electric field vector of the incident polarized laser radiation aligned perpendicularly to the aforementioned orientation, and lesion was not irradiated (control). The dose was per irradiation, corresponding to an exposition of approximately . The laser dose was chosen according to previous studies, which are of relevance for procedures on soft tissues.6, 16 Four animals were irradiated and sacrificed at days 3, 7, 10, and 14 after the burn creation. The last animals were killed at day 17 postwounding (p.w.). During the experiment, the rats were singly caged in a light/dark schedule at , with access to food and water ad libitum. National and international principles of laboratory animal care were followed. The light source was a HeNe laser at , with of output power (Uniphase, USA) mounted in a convenient setup. A lens system and an optical filter were used to ensure an uniform exposure at the wound position, obtaining an expanded beam with at . A Glan-Thompson prism was inserted in the beam path to obtain a plane-polarized beam. The polarizer was held on a precision disk, which enabled us to rotate it , and thus, change the direction of the incident polarization. The output power of the laser was confirmed prior to irradiations, using a power meter (LaserCheck®—Coherent, USA). After sacrifice, the areas of the skin under study were collected and fixed routinely by immersion in Bouin’s solution at for and thereafter dehydrated and embedded in paraffin to provide transversal sections of the skin. After cutting, the samples were deparaffined. A healthy skin area labeled “ ” was also processed and analyzed. Three slides containing two sections from each group “ ,” “ ,” “ ,” and “ ” were analyzed. Within each section were carried out six measurements, called center 1, center 2, side 1, side 2, and edge 1, edge 2 (Fig. 1 ). The same measures were performed in the healthy skin sections. Fig. 1Areas analyzed with polarization microscope: center, sides, and edges of lesion; E (epidermis), D (dermis), and L (lesion). 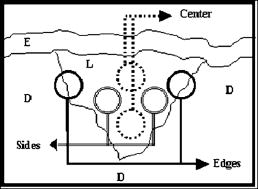 To evaluate the total birefringence (intrinsic and textural) of the samples, sections were mounted with distilled water, which was placed between the slides. This procedure was performed to reduce the refractive index mismatch, which leads to considerable alteration of OPD between slides. The measurements were made with a Zeiss polarized light microscope equipped with a objective and a mica compensator. The same compensator was used to monitor the stability of the system during the experiments: the compensator, without the fibers, was used to obtain standard measurements after each register to guarantee and to test the system stability. Light of wavelength equal to was obtained with an interference filter and was used in all of the analyses. There is a phase difference between the intensity of the incident light and the intensity of the emergent light, which is altered by the retardation of the object and by the polarization system. The angle obtained through the measurements was converted to the OPD (in nanometers) of the birefringent material and was calculated using the Brace-Kohler method as follows:17 where is the retardation of the compensator, derived from the ratio , where is the incident wavelength, in this particular case ; and 11.65 is the constant of the compensator resolution; is , where is the position of the long axis of the collagen bundles relative to the electric vector of the plane of the polarization system.The mean and the standard deviations of the OPD values were computed. Statistical analyses were accomplished using Student’s test, to compare different values obtained among groups , , , and . Significance was accepted at . 3.ResultsDuring the first p.w., there were no statistical significant differences in OPD values among , , and in each area analyzed. Significant differences were observed between and the other specimens in this period. On the 17th day p.w., the overall finding was that the OPD depends on the electric field vector of the incident polarized laser radiation and on the area analyzed within each lesion. Note that the OPD showed a minimum value in the center in comparison to the sides and the edges of the lesion. The OPD values were lower in the center than at the sides and edges, and they progressively increased approaching the lesion edges. Analyzing the center of the lesion, group presented an OPD mean value of ; , , , ; and presented (Fig. 2 ). No statistically significant differences were observed between and , and and . There was significant statistical difference between and , and , and , and and . Fig. 2For OPDs measured at the lesion center, no significant differences were observed between and and and ; there was significant difference statistically between and , and , and , and and . For OPDs measured at the lesion sides, significant differences were observed between and and between and ; no statistically significant differences between and , and , and , and and were observed . For OPDs measured at the lesion edges, there were no significant differences among the samples . 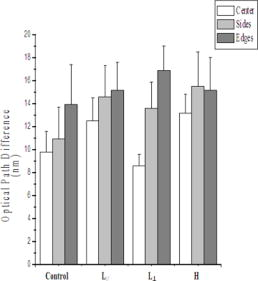 The OPDs measured in the lesion sides for was ; , ; , ; and , (Fig. 2). Significant differences were observed between and and between and ; however, no statistically significant differences were detected between and , and , and , and and . At the lesion edges, the groups , , , and exhibited OPDs of , , , and , respectively (Fig. 2). No significant differences among the samples were observed. Noteworthy was that and exhibited a similar OPD mean value at the lesion center, sides, and edges. 4.DiscussionIn this study, we observed the ability of the components of the skin to alter the polarization state of light. This finding can be attributed to the birefringent nature of collagen fibers, which along with elastin fibers, provides the dermal layer with mechanical strength and organization. There are two types of birefringence, intrinsic birefringence and textural birefringence. The first type is determined by the orientation, oscillation, and the strengths of all electronic transitions of the molecules that constitute the filaments. The second is dependent on partial volume (concentration), aggregational state, and orientation of the bundle components.18 Birefringence measurement remains a powerful technique to study molecular order and the degree of ordered aggregation (packing conditions) in collagen bundles. Changes in either the collagen fibrils or in the extrafibrillar matrix can both, therefore, alter birefringence. A direct illustration of this is provided in situations where the normal structure has been disrupted. For example, the thermal denaturation temperature of collagen range from , and in this range, the individual fibrils that makeup the fibers unravel and there is a reduction in the birefringence.19 Our results agree with those of Tang, 20 who demonstrated that thermally denatured collagen does not exhibit birefringence since the collagen fibers might become merged into an amorphous random state. In fact, birefringence loss is due to the induced necrosis and to the collagen denaturation that corresponds with the separation of the -chain from the triple helix. In addition, the importance of the action of the metalloproteinase matrix in the course of inflammation must be taken in account regarding the collagen fibers breaking down and birefringence loss.21 During the first p.w. no significant differences were observed between irradiated and control lesions, but both exhibited a lower OPD value than for each area analyzed. It is well known that a high fibroblast density, thin randomly oriented collagen fibers, and a rich capillary bed characterize early granulation tissue development, which contributes to a low of OPD value. These characteristics explain our results in the first . On the 17th day, no significant differences were observed between and , which presented the highest birefringence in the center of the lesion. Also, no significant differences were observed between and , which presented the lowest birefringence in the mentioned area. These results suggest that lesions are in a more advanced repair stage because the birefringence in this group was similar to that from healthy skin. The birefringence measured outside of the burn center tended to increase with the distance and reached the normal level at the edges of the lesion. This finding is probably caused by increased healthy tissue content, decreased repaired tissue content, and better fibril organization, which is consistent with a secondary intention repair and in conformity with the results obtained through birefringence measurements in the cornea.19 Also on the 17th day, the groups and showed no significant differences when analyzed at the lesion center, sides, and edges. This finding suggests that the electric field vector of the polarized laser radiation that was perpendicularly aligned to rat’s occipital-caudal direction led to analogous effects to untreated wounds. Consequently, the birefringence is low. This fact is according to Berry, 22 who showed that the wound granulation tissue of untreated rats has minimal birefringence at the third week of wound healing. To summarize, the birefringence analysis showed that the orientation of the collagen bundles represented by OPDs seems to be dependent on the relative orientation between the electric field vector of the polarized laser radiation and a reference direction on the tissue (rat’s occipital-caudal direction). The electric field vector of the polarized laser radiation has a competent effect on the collagen organization in the dermis as observed by the fact that both and exhibited higher birefringence p.w., while and exhibited smaller birefringence during the same period. Some possible factors that may contribute to this variation of OPD during wound healing include the fact that the collagen fibers are randomly distributed in the dermis at the beginning of the healing process, and the thickness of the collagen fibers varies: fibers are thinner in the beginning of the healing process (newly synthesized collagen or younger collagen fibrils) and thicker in the final process. The propagation of polarized light through a biological tissue is an intricate process because it leads to a change in the polarization status of photons owing to tissue birefringence and tissue scattering.12 Since biological tissues are not homogeneous samples, parameters such as the size, shape, and density of the scatters in the medium as well as the polarization state of the incident light are relevant factors.23 It is well known that light polarization remains unchanged through a thin layer of cells; however, in highly scattering mediums, such as living tissue, the light is depolarized after a penetration of a millimeter or so. However, the epidermis and the initial papillary dermis apparently allow the penetration of linearly polarized light with modest depolarization.7 The light can travel a distance of in normal human skin without the complete loss of the linear polarization.23 In fact, Sankaran demonstrated the different patterns of depolarization for linear and circular incident polarization for different tissues. Their results indicate that for dense tissues, linearly polarized light is better preserved than circularly polarized light.11 Note that in this study linear polarized light was used to irradiate the tissue during the first of healing process. The extracellular matrix (ECM) is the scaffold of the skin that supports cells in either unwounded or wounded states. The ECM is dynamic during healing process; it is constantly undergoing remodeling.24 Thus, one can expect that light propagation into a live tissue during the healing process will change due to the dynamic changes inside the tissue. After the initial inflammatory phase, the early wound matrix is gradually replaced by granulation tissue. Indeed, the transmittance of an He–Ne laser and a semiconductor laser in granular tissue is about 2.5 times higher than in normal skin.25 In addition to transmittance, the degree of linear polarization was investigated in healthy and burned skin.26 The results indicated that linearly polarized light could survive in the superficial layers of skin; furthermore, the preservation was even higher in burned skin. Moreover, a more recent work investigated the depolarization properties of gamma-irradiated pig skin at different wavelengths. The authors observed that for the red wavelength, an increase of gamma irradiation dose, which results in skin erythema, decreased the depolarization. As a result, healthy skin promotes more depolarization than a -irradiated skin sample.27 A dense population of blood vessels, macrophages, and fibroblasts embedded within a loose provisional matrix of fibronectin, hyaluronic acid, and collagen characterize the granulation tissue that covers the wound at the beginning of the healing process. The high cellular content and the increased cellular activity of the granulation tissue should contribute to the optical changes during healing process, since Mourant showed that changes in light scattering from both isolated cellular nuclei and cells can be correlated with the DNA content.28 Our findings suggest that in the center of the burn on the 17th p.w., differences were observed between and , indicating that the irradiation might have anticipated the remodeling phase of the healing process. In fact, our results, as well as those reported previously, demonstrated the importance of the direction of incidence of the electric field vector of the polarized laser radiation, since on day 17 p.w. it was reported6 that in , both cellular and extracellular components, as collagen fibrils, appeared to be more organized and thick than in . Linearly polarized light presented better therapeutic results; in addition, a preferential direction of the light incidence was also considered a relevant parameter. According to Nickell, scattering in the skin is anisotropic, with a factor of up to 2 between orthogonal directions.29 The observed scattering anisotropy can be described by assuming a preferential orientation of the collagen fibers in the dermis. As well as the Nickell results, in our study, the awareness of the anisotropy in burned skin could help in the interpretation of the results. If the occipital-caudal direction of incidence is running parallel with the tension lines, which comprise an anatomical equivalent consisting of a preferential parallel orientation and a straightening of collagen bundles,30 it is possible to hypothesize that the polarization memory is better preserved in this direction than perpendicularly with the tension lines, and thus, it could assist in cutaneous wound healing acceleration. Although some reports have suggested that treatment with polarized light may accelerate wound healing,5, 31, 32, 33 literature is still scarce concerning the influence of laser-induced biomodulation on skin repair regarding polarization. For example, it would be interesting to compare the directions of minimum light scattering to those of the skin tension lines to discover the component that makes the difference between the studied incidence directions. 5.ConclusionOur results demonstrated that the electric field vector of the polarized laser radiation affects the collagen organization in the dermis. Lesions irradiated using the parallel polarization aligned with the rat’s occipital-caudal direction showed higher birefringence, indicating that collagen bundles in these lesions are more organized than lesions irradiated using the perpendicular relative direction. AcknowledgmentsThe authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. ReferencesK. E. Moyer,
A. Davis,
G. C. Saggers,
D. R. Mackay, and
H. P. Ehrlich,
“Wound healing: the role of gap junctional communication in rat granulation tissue maturation,”
Exp. Mol. Pathol., 72
(1), 10
–16
(2002). 0014-4800 Google Scholar
E. Mester,
T. Spiry,
B. Szende, and
J. G. Tota,
“Effect of laser rays on wound healing,”
Am. J. Surg., 122
(4), 532
–535
(1971). 0002-9610 Google Scholar
Y. A. Vladimirov,
A. N. Osipov, and
G. I. Klebanov,
“Photobiological principles of therapeutic applications of laser radiation,”
Biochemistry (Mosc.), 69
(1), 81
–90
(2004). 0006-2979 Google Scholar
M. H. Niemz, Laser-Tissue Interactions. Fundamentals and Applications, Springer-Verlag, Berlin Heidelberg (1996). Google Scholar
T. I. Karu,
“Low-power laser therapy,”
Biomedical Photonics Handbook, 48-1
–48-25 CRC Press, Boca Raton, FL
(2003). Google Scholar
M. S. Ribeiro,
D. F. T. Silva,
C. E. N. Araujo,
S. F. Oliveira,
C. M. R. Pelegrini,
T. M. T. Zorn, and
D. M. Zezell,
“Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study,”
J. Clin. Laser Med. Surg., 22
(1), 59
–66
(2004). 1044-5471 Google Scholar
S. L. Jacques,
J. R. Roman, and
K. Lee,
“Imaging superficial tissues with polarized light,”
Lasers Surg. Med., 26 119
–129
(2000). https://doi.org/10.1002/(SICI)1096-9101(2000)26:2<119::AID-LSM3>3.0.CO;2-Y 0196-8092 Google Scholar
I. A. Vitkin and
R. C. N. Studinski,
“Polarization preservation in diffusive scattering from in vivo turbid biological media: effects of tissue optical absorption in the exact backscattering direction,”
Opt. Commun., 190 37
–43
(2001). https://doi.org/10.1016/S0030-4018(01)01080-X 0030-4018 Google Scholar
X. Wang and
L. V. Wang,
“Propagation of polarized light in birefringent turbid media: a Monte Carlo study,”
J. Biomed. Opt., 7
(3), 279
–290
(2002). https://doi.org/10.1117/1.1483315 1083-3668 Google Scholar
K. C. Hadley and
I. A. Vitkin,
“Optical rotation and linear and circular depolarization rates in diffusively scattered light from chiral, racemic, and achiral turbid media,”
J. Biomed. Opt., 7
(3), 291
–299
(2002). https://doi.org/10.1117/1.1483880 1083-3668 Google Scholar
V. Sankaran, J. T. Walsh Jr, D. J. Maitland,
“Comparative study of polarized light propagation in biologic tissues,”
J. Biomed. Opt., 7
(3), 300
–306
(2002). https://doi.org/10.1117/1.1483318 1083-3668 Google Scholar
S. L. Jacques,
J. C. Ramella-Roman, and
K. Lee,
“Imaging skin pathology with polarized light,”
J. Biomed. Opt., 7
(3), 329
–340
(2002). https://doi.org/10.1117/1.1484498 1083-3668 Google Scholar
M. C. Pierce,
J. Strasswimmer,
B. H. Park,
B. Cense, and
J. F. de Boer,
“Birefringence measurements in human skin using polarization-sensitive optical coherence tomography,”
J. Biomed. Opt., 9
(2), 287
–291
(2004). https://doi.org/10.1117/1.1645797 1083-3668 Google Scholar
M. A. Geday,
W. Kaminsky,
J. G. Lewis, and
A. M. Glazer,
“Images of absolute retardance L center dot Delta n, using the rotating polariser method,”
J. Microsc., 198 1
–9
(2000). https://doi.org/10.1046/j.1365-2818.2000.00687.x 0022-2720 Google Scholar
B. C. Vidal,
“Image analysis of tendon helical superstructure using interference and polarized light microscopy,”
Micron, 34
(8), 423
–432
(2003). https://doi.org/10.1016/S0968-4328(03)00039-8 0968-4328 Google Scholar
S. C. Nunez,
G. E. Nogueira,
M. S. Ribeiro,
A. S. Garcez, and
J. L. Lage-Marques,
“He-Ne laser effects on blood microcirculation during wound healing: a method of in vivo study through laser Doppler flowmetry,”
Lasers Surg. Med., 35
(5), 363
–368
(2004). 0196-8092 Google Scholar
C. C. Montarou and
T. K. Gaylord,
“Two-wave-plate compensator method for single-point retardation measurements,”
Appl. Opt., 43
(36), 6580
–6595
(2004). 0003-6935 Google Scholar
B. C. Vidal,
M. L. Mello, and
E. R. Pimentel,
“Polarization microscopy and microspectrophotometry of sirius red, picrosirius and chlorantine fast red aggregates and of their complexes with collagen,”
Histochem. J., 14
(6), 857
–878
(1982). 0018-2214 Google Scholar
Y. F. Huang,
K. M. Meek,
M. W. Ho, and
C. A. Paterson,
“Analysis of birefringence during wound healing and remodeling following alkali burns in rabbit cornea,”
Exp. Eye Res., 73
(4), 521
–532
(2001). 0014-4835 Google Scholar
J. Tang,
F. Zeng,
H. Savage,
P. P. Ho, and
R. R. Alfano,
“Laser irradiative tissue probed in situ by collagen fluorescence imaging,”
Lasers Surg. Med., 27
(2), 158
–164
(2000). https://doi.org/10.1002/1096-9101(2000)27:2<158::AID-LSM7>3.0.CO;2-I 0196-8092 Google Scholar
B. C. Vidal,
“Evaluation of the carbohydrate role in the molecular order of collagen bundles: microphotometric measurements of textural birefringence,”
Cell Mol. Biol. (Oxford), 32
(5), 527
–535
(1986). 0145-5680 Google Scholar
D. P. Berry,
K. G. Harding,
M. R. Stanton,
B. Jasani, and
H. P. Ehrlich,
“Human wound contraction: collagen organization, fibroblasts, and myofibroblasts,”
Plast. Reconstr. Surg., 102
(1), 124
–131
(1998). 0032-1052 Google Scholar
V. Tuchin,
“Optical properties of tissues with strong (multiple) scattering,”
Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 353 SPIE Press, Bellingham, WA
(2000). Google Scholar
P. Martin,
“Wound healing—aiming for perfect skin regeneration,”
Science, 276 75
–81
(1997). 0036-8075 Google Scholar
H. Kolarova,
D. Ditrichova, and
J. Wagner,
“Penetration of the laser light into the skin in vitro,”
Lasers Surg. Med., 24
(3), 231
–235
(1999). 0196-8092 Google Scholar
M. S. Ribeiro,
C. M. R. Pellegrini,
D. F. T. Silva,
D. M. Zezell,
T. M. T. Zorn,
A. Z. Freitas, and
F. G. Costa,
“Comparison of linear polarization degree in healthy and wounded rat skin,”
Proc. SPIE, 4453 45
–48
(2001). 0277-786X Google Scholar
F. Boulvert,
B. Boulbry,
G. L. Brun,
B. L. Jeune,
S. Rivet, and
J. Cariou,
“Analysis of the depolarizing properties of irradiated pig skin,”
J. Opt. A, Pure Appl. Opt., 7 21
–28
(2005). 1464-4258 Google Scholar
J. R. Mourant,
M. Canpolat,
C. Brocker,
O. Esponda-Ramos,
T. M. Johnson,
A. Matanock,
K. Stetter, and
J. P. Freyer,
“Light scattering from cells: the contribution of the nucleus and the effects of proliferative status,”
J. Biomed. Opt., 5
(2), 131
–137
(2000). https://doi.org/10.1117/1.429979 1083-3668 Google Scholar
S. Nickell,
M. Hermann,
M. Essenpreis,
T. J. Farrell,
U. Kramer, and
M. S. Patterson,
“Anisotropy of light propagation in human skin,”
Phys. Med. Biol., 45
(10), 2873
–2886
(2000). https://doi.org/10.1088/0031-9155/45/10/310 0031-9155 Google Scholar
G. E. Pierard and
C. M. Lapiere,
“Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines,”
Am. J. Dermatopathol., 9
(3), 219
–224
(1987). 0193-1091 Google Scholar
M. M. Colic,
N. Vidojkovic,
M. Jovanovic, and
G. Lazovic,
“The use of polarized light in aesthetic surgery,”
Aesthetic Plast. Surg., 28
(5), 324
–327
(2004). 0364-216X Google Scholar
P. Iordanou,
G. Baltopoulos,
M. Giannakopoulou,
P. Bellou, and
E. Ktenas,
“Effect of polarized light in the healing process of pressure ulcers,”
Int. J. Nurs. Pract., 8
(1), 49
–55
(2002). 1322-7114 Google Scholar
J. Kymplova,
L. Navratil, and
J. Knizek,
“Contribution of phototherapy to the treatment of episiotomies,”
J. Clin. Laser Med. Surg., 21
(1), 35
–39
(2003). 1044-5471 Google Scholar
|


