|
|
1.IntroductionCytochrome oxidase (CCO) is the terminal electron acceptor of the mitochondrial electron transfer chain and catalyzes over 95% of oxygen metabolism, thereby driving adenosine triphosphate (ATP) synthesis.1 The CCO redox state reflects the balance between electron donation from cytochrome and oxygen reduction to water. Although many factors can influence the CCO redox state,2 the most significant is the availability of molecular oxygen.3 The difference spectrum between oxidized and reduced CCO has a distinct band in the near-IR region, which can be measured using near-IR spectroscopy4, 5 (NIRS). Assuming the total concentration of CCO remains constant during an experiment, changes in the NIRS CCO signal represent changes in the CCO redox state. This signal has the potential to provide a noninvasive marker of changes in mitochondrial oxygen delivery and utilization, and might facilitate detection of ischemic thresholds and guide subsequent clinical interventions. The in vivo use of NIRS was first described by Jobsis5 in 1977, and it has been used in animals and humans to measure change in concentration of oxyhemoglobin , deoxy-hemoglobin , and oxidized cytochrome oxidase .6, 7, 8, 9, 10, 11 NIRS exploits the fact that biological tissue is relatively transparent to near-IR light between 700 and , enabling interrogation of structures beneath the tissue surface.5 Biological tissue is a highly scattering medium, complicating the calculation of chromophore concentration, but if the average path length of light through tissue is known, the modified Beer-Lambert law, which assumes constant scattering losses, enables calculation of absolute changes in chromophore concentration.12 Specific extinction coefficients of the oxidized-reduced CCO difference spectrum in the near-IR (NIR) region are similar in magnitude to those of oxy- and deoxyhemoglobin,2 but the concentration of CCO in the brain is approximately one order of magnitude less than these other two chromophores.13 This complicates its detection and raises the possibility that NIRS measured changes in might be subject to artefacts resulting from measurement algorithms.10, 14 However, mitochondrial inhibitor and perfluorocarbon-blood exchange studies in animals have recently shown15, 16 that measurements are stable during large contemporaneous and . Furthermore, data from human visual stimulation studies suggest that cerebral is not merely crosstalk artifact.17 Importantly, has been validated, in animals, as a marker of cellular energy status against magnetic resonance spectroscopy measured reduction in phosphocreatine and nucleoside triphosphates levels.18, 19 Although cerebral has been measured in humans in clinical situations associated with reduced cerebral oxygen delivery, namely, cardiac surgery8 and obstructive sleep apnea,20 these studies are hard to standardize and controversy remains regarding the relationship between and oxygen delivery. This paper aims to quantify broadband NIRS measured cerebral during hypoxemia in healthy human volunteers and examine its relationship to cerebral oxygen delivery and NIRS hemoglobin measurements. 2.Materials and MethodsThis study was approved by the Joint Research Ethics Committee of the National Hospital for Neurology and Neurosurgery and the Institute of Neurology. We studied eight healthy volunteers (seven male and one female, with a median age , and range of 30 to 36). Broadband spectrometer (BBS) optodes were placed apart in a black plastic holder and fixed to the right side of the forehead in the midpupilary line. Light from a stabilized tungsten halogen light source was filtered with long-pass and heat-absorbing filters, and transmitted to the head via a -diam glass optic fiber bundle. Light incident on the detector optode was focused via an identical fiber bundle onto the entrance slit of a spectrograph ( , Instruments SA, France) with a grating. NIR spectra between 650 and were collected at on a cooled-charge coupled device detector (Wright Instruments, United Kingdom) giving a spectral resolution of . An oximeter probe (Novametrix Medical Systems Inc., USA) measured arterial oxygen saturation , and a Portapres finger cuff (Biomedical Instrumentation, TNO Institute of Applied Physics, Belgium) measured mean blood pressure (MBP) and heart rate (HR). Blood flow velocity in the basal right middle cerebral artery (vMCA) was collected using transcranial Doppler ultrasonography (Nicolet, United Kingdom). A modified anesthetic machine delivered gas to the subject via a mouthpiece. Inspired oxygen concentration and end tidal partial pressure of carbon dioxide were measured using an inline gas analyzer (Hewlett Packard, United Kingdom) and a optical sensor (Novametrix Medical Systems Inc.), respectively. The study commenced with of monitoring at normoxia and normocapnea. Nitrogen was then added to the inspired gases, to induce a gradual fall in to 80%, and immediately after this was achieved, the was returned to normoxia for . This cycle was repeated three times. was continuously fed back to subjects and they adjusted their minute ventilation to maintain normocapnea throughout the study. Absolute , , and were calculated from changes in light attenuation using a multiple regression technique termed the algorithm.21 Correction factors for the wavelength dependence of the optical path length were applied to the chromophore absorption coefficients. Individual baseline optical path length was calculated using second differential analysis of the water feature22 of the initial of spectral data. Change in total hemoglobin concentration was defined as and change in hemoglobin difference concentration as .23 Cerebral oxygen delivery in milliliters is defined as where CBF is cerebral blood flow , 1.39 is the oxygen carrying capacity of hemoglobin (in millileters per gram Hb), Hb is arterial hemoglobin saturation (in grams per deciliter), of oxygen in blood (ml/mmHg ) and is arterial partial pressure of oxygen (in millimeters of Hg).The mean vMCA measured using transcranial Doppler ultrasonography correlates with cerebral blood flow.24 Ignoring the small dissolved oxygen component, we define estimated cerebral oxygen delivery as where is an individual specific constant.Assuming constant arterial haemoglobin concentration during the study, percentage change in is calculated as percentage change from baseline of . The start and end of each hypoxemic period was identified from the data. Individual subjects desaturate at different rates and, to enable description of the group data, each individual hypoxemia was divided into equal time periods, with each time point representing an eighth of the total time course of the hypoxemia. This produced nine time points with point 1 representing the point just prior to the start of hypoxemia and point 9 the nadir of hypoxemia. The same technique was applied separately to the recovery period, producing points 9 (just prior to start of recovery) to 17 (end of recovery period). At each time point, the mean of the preceding seconds of data was calculated. Data from the three experimental cycles were averaged to give a single course of hypoxemia and recovery for each subject. Group median changes from baseline at each time point were produced. Statistical analysis was carried out using SAS software (v8.2, SAS Institute, USA) and values were considered significant. Group changes were compared with baseline using nonparametric analysis of variance (ANOVA) and post hoc pairwise comparisons. Correlations between variables were assessed by applying Spearman rank correlation to data from the 17 time points, with Bonferroni-corrected two-tailed tests of significance. A multiple linear regression model was produced from the hypoxemic period group data (time points 1 to 9) with as the dependent variable, and and as the independent variables. To assess whether the measured was crosstalk artifact, a predicted for the recovery period was derived from the recovery period and using the multiple linear regression model. and for the recovery period were compared using a mixed model analysis. 3.ResultsTable 1 shows baseline systemic data for the subject group. The median time of hypoxia required to achieve arterial oxygen saturation of 80% was (range ). The length of each recovery period was fixed at for all subjects. Table 1Median and interquartile range (IQR) (n=8) for baseline FiO2 , SaO2 , EtCO2 , HR, MBP, and vMCA.
Figure 1 shows data for a single subject, demonstrating the experimental time course. Group changes from baseline during hypoxemia and recovery for , HR, MBP, and vMCA are shown in Fig. 2 and for , , , , , and in Fig. 3 . There were no significant changes in the measured optical pathlength during the study . Table 2 shows changes in variables from baseline to the nadir of hypoxemia and from baseline to the end of the normoxic recovery period. Fig. 2Group median and interquartile range for changes from , , , and ; ⋆, ; †, ; ‡, ; and §, for change from baseline. 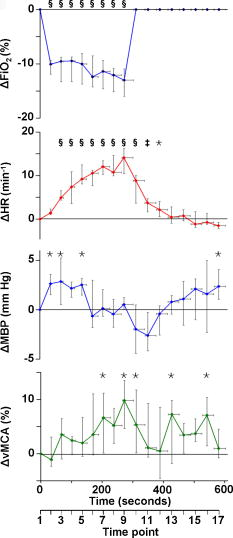 Fig. 3Group median and interquartile range for changes from baseline of , , , , , and ; ⋆, ; †, ; ‡, ; §, and for change from baseline. 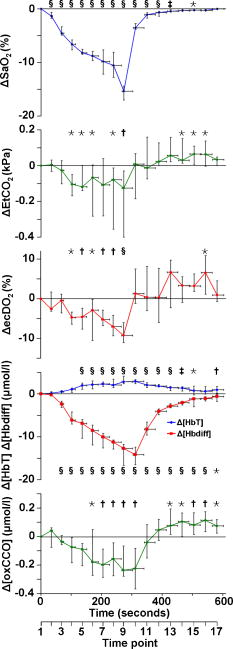 Assessment of the data during both hypoxemia and recovery revealed a significant correlation between and ( , ), but no correlation between and ( , ) or between and ( , ). Multiple linear regression of the group data from the hypoxemic period revealed: To check for crosstalk between the hemoglobin and CCO signals, Eq. 3 was used to derive for the recovery period. and were different (Fig. 4 ).Fig. 4Group median and interquartile range for changes from baseline of (◆), and (∎) during recovery period (time points 10 to 17). Predicted and measured results were different . 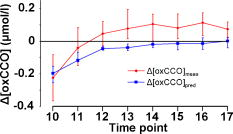 Table 2Median and IQR (n=8) for changes from baseline to nadir of hypoxemia, and end of recovery period for ΔFiO2 , ΔSaO2 , ΔEtCO2 , ΔHR , ΔMBP , ΔvMCA , ΔecDO2 , Δ[Hbdiff] , Δ[HbT] , and Δ[oxCCO] . 4.DiscussionWe described significant cerebral measured using NIRS during hypoxemia to an of 80% in healthy adult humans. We found distinct differences between the measured CCO and hemoglobin signals. Figure 3 shows rising during the hypoxemic challenge before gradually returning toward, but not reaching, baseline values after of normoxic recovery. This infers an increase in cerebral blood volume during hypoxemia, probably as a result of hypoxemic vasodilatation. , which provides an assessment of changes in the balance of and ,23 shows the opposite pattern, decreasing during hypoxemia and returning toward, but not reaching, baseline values after of normoxic recovery. decreases during hypoxemia and returns to baseline before with a subsequent increase above baseline during the normoxic recovery period. Increased cerebral during the recovery period after hypoxemia has been demonstrated in animal models11 and has not been fully explained. Calculation of the correlation between and , , and was performed on the data from both the hypoxemic and recovery phases of the study to assess the ability of the three measures to detect both decreased and increased . Both and did not rise above, or drop below, baseline, respectively, in response to the increase in during recovery and this results in the lack of significant correlations. There was a significant linear correlation between and , inferring that this NIRS measurement has clinical relevance as a measure of changes in cerebral oxygen delivery. We therefore suggest that provides a more reliable assessment of changes in cerebral oxygen delivery than either or . Although NIRS-measured hemoglobin concentrations reflect intravascular oxygenation, the CCO signal indicates changes in mitochondrial oxygen delivery and utilization. In health, there is likely to be a close relationship between intravascular and mitochondrial oxygen delivery. However, in pathological situations, this relationship may be altered by tissue edema, which reduces oxygen diffusion from capillary to mitochondrion. In addition, mitochondrial dysfunction, which reduces the ability to metabolise oxygen, may occur. It is anticipated that in these situations, the mitochondrial CCO signal will yield different information to the intravascular hemoglobin signal, and will provide clinicians with a bedside tool with which to ensure adequate mitochondrial oxygen delivery and thus potentially preserve cell function. NIRS monitoring of cerebral hemoglobin changes is liable to “contamination” of the cerebral signal by hemoglobin in the skin vasculature. The CCO signal is less prone to extracerebral contamination, since CCO is present in low concentrations in skin compared to brain and zero concentration in red blood cells.25 Edwards 7 studied neonates using a commercial six-wavelength NIRO 1000 spectrometer. They found no during alterations in between 85 and 99%. Our previous work in patients with obstructive sleep apnea demonstrated a reduction in during severe desaturation,20 but this clinical paradigm did not allow for controlled manipulation and cellular and cerebrovascular responses in this patient group, who are exposed to repeated severe hypoxic episodes, may not reflect those of healthy individuals. The clinical relevance of cerebral has been demonstrated by NIRS measurements in adult patients undergoing cardiac surgery, where cerebral correlates with neurological outcome.8, 9 Changes in arterial carbon dioxide tension have been shown to effect NIRS measured in both neonatal humans7 (increase in of ) and piglets11, 16 (increase in of ), and to isolate the effect of hypoxemia, we used an feedback loop to minimize changes in . Despite this, we found a small but significant median reduction in of at the nadir of hypoxemia. We do not believe this magnitude of change in will affect the CCO signal, although we are carrying out further studies to test this hypothesis. Controversy exists over how readily CCO becomes reduced following reduced oxygen tension. Several different algorithms exist for the conversion of light attenuation to chromophore concentration changes and the choice of algorithm can affect the results.21 Some animal studies suggest that CCO reduction only occurs during extreme reduction in cerebral oxygen delivery,6, 11, 16 while others have found a gradual reduction in CCO during hypoxemia.26 These variations may relate to the experimental challenges, which comprised graded hypoxia,26 anoxia,11, 16 or induced hypotension.6 Evidence for “late” reduction in CCO in some animal studies following anoxia is a delay between changes in hemoglobin concentrations and CCO redox state,11, 16 and we also show a temporal delay between the first significant drops in and . These animal data have been interpreted as suggesting that CCO reduction does not occur at moderate hypoxemia, but the instigation of anoxia may be too swift a challenge to enable full investigation of the effects of moderate hypoxemia. In addition, these studies6, 11, 16, 26 used animals initially ventilated with supranormal concentrations of oxygen, resulting in baseline arterial oxygen tensions between 14.7 and . Elevated baseline values might further delay the onset of changes in CCO redox during hypoxemia, leading to the conclusion that CCO reduction only occurs after severe reduction in oxygen delivery. These comparisons are further complicated by the fact that some studies have been performed in perfluorocarbon exchanged animals26 with resultant greatly decreased tissue oxygen delivery compared to the blooded animal for a given arterial oxygen tension. We show that in the healthy human brain, gradual CCO reduction takes place during moderate hypoxemia (Fig. 3)—an essential prerequisite for a useful clinical marker of dysoxia. The challenge we utilize in this study is obviously far less severe than that used in many animal studies, and we demonstrate only modest reductions in . We suggest that CCO redox may show a biphasic response to hypoxemia. Our finding of an early modest reduction in may be followed by a threshold (below the extent of our challenge) beyond which a steeper reduction occurs. Our further work investigating CCO redox changes in brain-injured patients who occasionally suffer more severe hypoxemia may address this point. The SNR for the calculation of optical path length using second differential spectroscopy was estimated using Monte Carlo simulation.22 From these data, we would estimate the predicted accuracy of our pathlength calculation to be in the region of 5.2%. We found no significant change in mean optical pathlength during the study. Therefore, if was an artifact of and , then a relationship between , , and derived from the hypoxemia part of the study should also apply during the recovery period. If this were the case, for the recovery period derived using Eq. 3 would not differ from during recovery. and were different (Fig. 4), suggesting that is not merely a crosstalk artifact resulting from the large changes in and . However, additional modelling and experimental studies are required to further investigate the use of the algorithm to detect changes in the CCO signal in a multilayer system, and we are addressing this issue using a combination of continuous wave and phase-resolved spectroscopy together with knowledge of the CCO concentrations in the various cranial layers and their respective optical characteristics. We are currently studying patients with traumatic brain injury to investigate the response of the NIRS CCO signal to periods of intracranial perturbation. NIRS provides the opportunity to make regional measurements of brain metabolism, making probe positioning less critical than in hyperfocal measurements made by invasive techniques, such as cerebral microdialysis, while still retaining the ability to target the tissue at greatest risk of secondary injury: a feature lost when using global measures such as jugular venous oximetry. We aim to show that NIRS measurement of CCO redox changes in patients with brain injury is a useful, noninvasive real-time marker of alterations in mitochondrial oxygen availability. Identification of failing mitochondrial metabolism might then enable NIRS measurement of to guide neuroprotective treatment strategies. The work described in this paper is an essential step toward understanding CCO signal changes in the injured brain. 5.ConclusionWe described, for the first time, the quantification of cerebral during hypoxemia in healthy adults and showed that this measurement provides a marker of reduced cellular oxygen availability in healthy humans. We demonstrated a protocol that produces and provides an ideal paradigm for the in vivo development of NIRS algorithms and instrumentation. AcknowledgmentsMT is a Welcome Research Fellow, under Grant No. 075608 and IT is supported by UCL/UCLH trustees. We thank Dr. Veronica Hollis for technical assistance ReferencesO. M. Richter and
B. Ludwig,
“Cytochrome c oxidase—structure, function, and physiology of a redox-driven molecular machine,”
Rev. Physiol. Biochem. Pharmacol., 147 47
–74
(2003). 0303-4240 Google Scholar
C. E. Cooper,
S. J. Matcher,
J. S. Wyatt,
M. Cope,
G. C. Brown, E. M. Nemoto, and
D. T. Delpy,
“Near-infrared spectroscopy of the brain: relevance to cytochrome oxidase bioenergetics,”
Biochem. Soc. Trans., 22
(4), 974
–980
(1994). 0300-5127 Google Scholar
C. E. Cooper and
R. Springett,
“Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy,”
Philos. Trans. R. Soc. London, Ser. B, 352
(1354), 669
–676
(1997). https://doi.org/10.1098/rstb.1997.0048 0962-8436 Google Scholar
M. Ferrari,
D. F. Hanley,
D. A. Wilson, and
R. J. Traystman,
“Redox changes in cat brain cytochrome-c oxidase after blood-fluorocarbon exchange,”
Am. J. Physiol. Heart Circ. Physiol., 258
(6), 1706
–1713
(1990). 0363-6135 Google Scholar
F. F. Jöbsis,
“Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,”
Science, 198
(4323), 1264
–1267
(1977). https://doi.org/10.1126/science.929199 0036-8075 Google Scholar
C. E. Cooper,
D. T. Delpy, and
E. M. Nemoto,
“The relationship of oxygen delivery to absolute haemoglobin oxygenation and mitochondrial cytochrome oxidase redox state in the adult brain: a near-infrared spectroscopy study,”
Biochem. J., 332
(3), 627
–632
(1998). 0264-6021 Google Scholar
A. D. Edwards,
G. C. Brown,
M. Cope,
J. S. Wyatt, D. C. McCormick, S. C. Roth,
D. T. Delpy, and
E. O. Reynolds,
“Quantification of concentration changes in neonatal human cerebral oxidized cytochrome oxidase,”
J. Appl. Physiol., 71
(5), 1907
–1913
(1991). 8750-7587 Google Scholar
Y. Kakihana,
A. Matsunaga,
K. Tobo,
S. Isowaki,
M. Kawakami,
I. Tsuneyoshi,
Y. Kanmura, and
M. Tamura,
“Redox behavior of cytochrome oxidase and neurological prognosis in 66 patients who underwent thoracic aortic surgery,”
Eur. J. Cardiothorac Surg., 21
(3), 434
–439
(2002). 1010-7940 Google Scholar
G. Nollert,
P. Mohnle,
P. Tassani-Prell,
I. Uttner,
G. D. Borasio,
M. Schmoeckel, and
B. Reichart,
“Postoperative neuropsychological dysfunction and cerebral oxygenation during cardiac surgery,”
Thorac. Cardiovasc. Surg., 43
(5), 260
–264
(1995). 0171-6425 Google Scholar
T. Sakamoto,
R. A. Jonas,
U. A. Stock,
S. Hatsuoka,
M. Cope,
R. J. Springett, and
G. Nollert,
“Utility and limitations of near-infrared spectroscopy during cardiopulmonary bypass in a piglet model,”
Pediatr. Res., 49
(6), 770
–776
(2001). 0031-3998 Google Scholar
R. Springett,
J. Newman,
M. Cope, and
D. T. Delpy,
“Oxygen dependency and precision of cytochrome oxidase signal from full spectral NIRS of the piglet brain,”
Am. J. Physiol. Heart Circ. Physiol., 279
(5), 2202
–2209
(2000). 0363-6135 Google Scholar
D. T. Delpy,
M. Cope,
P. van der Zee,
S. Arridge,
S. Wray, and
J. Wyatt,
“Estimation of optical pathlength through tissue from direct time of flight measurement,”
Phys. Med. Biol., 33
(12), 1433
–1442
(1988). https://doi.org/10.1088/0031-9155/33/12/008 0031-9155 Google Scholar
G. C. Brown,
M. Crompton, and
S. Wray,
“Cytochrome oxidase content of rat brain during development,”
Biochim. Biophys. Acta, 1057
(2), 273
–275
(1991). 0006-3002 Google Scholar
L. Skov and
G. Greisen,
“Apparent cerebral cytochrome aa3 reduction during cardiopulmonary bypass in hypoxaemic children with congenital heart disease. A critical analysis of in vivo near-infrared spectrophotometric data,”
Physiol. Meas, 15
(4), 447
–457
(1994). 0967-3334 Google Scholar
C. E. Cooper,
M. Cope,
R. Springett,
P. N. Amess,
J. Penrice,
L. Tyszczuk,
S. Punwani,
R. Ordidge,
J. Wyatt, and
D. T. Delpy,
“Use of mitochondrial inhibitors to demonstrate that cytochrome oxidase near-infrared spectroscopy can measure mitochondrial dysfunction noninvasively in the brain,”
J. Cereb. Blood Flow Metab., 19
(1), 27
–38
(1999). 0271-678X Google Scholar
V. Quaresima,
R. Springett,
M. Cope,
J. T. Wyatt,
D. T. Delpy,
M. Ferrari, and
C. E. Cooper,
“Oxidation and reduction of cytochrome oxidase in the neonatal brain observed by in vivo near-infrared spectroscopy,”
Biochim. Biophys. Acta, 1366
(3), 291
–300
(1998). 0006-3002 Google Scholar
K. Uludag,
J. Steinbrink,
M. Kohl-Bareis,
R. Wenzel,
A. Villringer, and
H. Obrig,
“Cytochrome-c-oxidase redox changes during visual stimulation measured by near-infrared spectroscopy cannot be explained by a mere cross talk artefact,”
Neuroimage, 22
(1), 109
–119
(2004). 1053-8119 Google Scholar
R. J. Springett,
M. Wylezinska,
E. B. Cady,
V. Hollis,
M. Cope, and
D. T. Delpy,
“The oxygen dependency of cerebral oxidative metabolism in the newborn piglet studied with 31P NMRS and NIRS,”
Adv. Exp. Med. Biol., 530 555
–563
(2003). 0065-2598 Google Scholar
M. Tsuji,
H. Naruse,
J. Volpe, and
D. Holtzman,
“Reduction of cytochrome aa3 measured by near-infrared spectroscopy predicts cerebral energy loss in hypoxic piglets,”
Pediatr. Res., 37
(3), 253
–259
(1995). 0031-3998 Google Scholar
A. D. McGown,
H. Makker,
C. Elwell,
P. G. Al Rawi,
A. Valipour, and
S. G. Spiro,
“Measurement of changes in cytochrome oxidase redox state during obstructive sleep apnoea using near-infrared spectroscopy,”
Sleep, 26
(6), 710
–716
(2003). 0161-8105 Google Scholar
S. J. Matcher,
C. E. Elwell,
C. E. Cooper,
M. Cope, and
D. T. Delpy,
“Performance comparison of several published tissue near-infrared spectroscopy algorithms,”
Anal. Biochem., 227
(1), 54
–68
(1995). https://doi.org/10.1006/abio.1995.1252 0003-2697 Google Scholar
S. J. Matcher,
M. Cope, and
D. T. Delpy,
“Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy,”
Phys. Med. Biol., 39
(1), 177
–196
(1994). https://doi.org/10.1088/0031-9155/39/1/011 0031-9155 Google Scholar
P. J. Kirkpatrick,
J. Lam,
P. Al-Rawi,
P. Smielewski, M. Czosnyka,
“Defining thresholds for critical ischemia by using near-infrared spectroscopy in the adult brain,”
J. Neurosurg., 89
(3), 389
–394
(1998). 0022-3085 Google Scholar
J. M. Valdueza,
J. O. Balzer,
A. Villringer,
T. J. Vogl,
R. Kutter, and
K. M. Einhaupl,
“Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography,”
AJNR Am. J. Neuroradiol., 18
(10), 1929
–1934
(1997). 0195-6108 Google Scholar
D. L. Drabkin,
“Metabolism of the hemin chromoproteins,”
Physiol. Rev., 31
(4), 345
–431
(1951). 0031-9333 Google Scholar
R. Stingele,
B. Wagner,
M. V. Kameneva,
M. A. Williams,
D. A. Wilson,
N. V. Thakor,
R. J. Traystman, and
D. F. Hanley,
“Reduction of cytochrome-c oxidase copper precedes failing cerebral utilization in fluorocarbon-perfused cats,”
Am. J. Physiol., 271
(2), 579
–587
(1996). 0002-9513 Google Scholar
|



