|
|
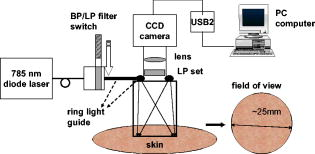
1.IntroductionNIR fluorescence imaging can be a potentially useful technology for clinical dermatologic diagnosis due to the deeper penetration and minimal biological effects on skin of NIR radiation versus ultraviolet (UV) and short wavelength visible light. Although some in-vitro studies have been done for tissue samples with NIR autofluorescence,1, 2, 3, 4 not much progress has been made on in-vivo studies prior to our work,5, 6, 7 especially for human subjects in clinical settings. One reason is that native tissue molecules usually emit very weak fluorescence under NIR light excitation.8 Although exogenous fluorophores can be applied to enhance signal levels, this adds complications to the imaging process, not the least of which is the fact that such fluorophores must be considered as drugs in the clinical and regulatory context. Another reason is the difficulty of eliminating background fluorescence in the NIR range, which comes from various optical components within the imaging system. Those two factors in combination may yield poor in-vivo NIR fluorescence images due to low signal-to-background ratio (SBR). Important tissue fluorophores, such as elastin, collagen, and NADH, mostly fluoresce only under UV or short wavelength visible light excitation, and will appear dark under NIR excitation. Recently, a spectroscopic study of skin by our group6 and an ophthalmologic imaging study by Keilhauer and Delori9 have surprisingly found that melanin in human and animal tissue emits strong fluorescence under NIR excitation. This finding provides a rationale for practical in-vivo NIR fluorescence imaging of human tissue based on melanin as an endogenous fluorophore. In dermatology, melanin is a major tissue chromophore that plays a role in many benign and malignant skin disorders, including melanoma. Under UV and shorter wavelength visible light excitation, melanin has been found to have a much lower fluorescence signal than other tissue components.10, 11, 12, 13, 14, 15 Prior to our observation, the conventional approach to identifying cutaneous melanin essentially relied on measuring the amount of UV and shorter wavelength visible light that has been absorbed. To quantify the concentration of melanin in human skin, empirically based algorithms were developed to extract melanin’s information from its reflectance spectra.16 Nevertheless, it has not yet been possible to use a positive optical method to directly observe and potentially quantify cutaneous melanin. With NIR fluorescence, the optical signal that is directly emitted by melanin should provide more meaningful and complementary information on its distribution and biological activity within tissue. Thus, based on our previous spectroscopic study,6 and with special consideration given to optimizing an imaging system for NIR wavelengths, we have designed and constructed a prototype in-vivo NIR fluorescence imaging system for clinical skin evaluation. The specific goals of this work have been: 1. to visually confirm the melanin autofluorescence that was discovered through point spectroscopic measurements, and 2. to develop a clinically feasible method for exploiting this effect in the diagnosis and evaluation of pigmented skin disorders based on melanin NIR fluorescence imaging. 2.Materials and Methods2.1.System SetupFigure 1 shows the block diagram of the imaging system. Light from a solid-state diode laser (BRM-785-0.35-100-0.22-SMA B&W TEK Incorporated, Newark, Delaware; central wavelength: , FWHM: , power output: ) is coupled through a optical fiber (P200-2-VIS/NIR, Ocean Optics Incorporated, Dunedin, Florida) to a ring light guide (Moritex USA Incorporated, San Jose, California; transmission at ), which provides relatively uniform illumination at the end of the attached probe, with a field of view (FOV) of in diameter. Reflectance and fluorescence photons remitted by the skin are then collected by a lens (Xenoplan , Schneider Optics Incorporated, Hauppauge, New York), optimized for the wavelength range of , toward an NIR-sensitive camera (Alta U1, Apogee Instruments Incorporated, Roseville, California) for image acquisition. The camera incorporates a Kodak 0402-ME charge-coupled device (CCD) sensor with square pixels in a array, and operates at by a software-controlled on-board thermoelectric cooler and fan system. Data acquired by the camera are transferred to a PC system via a USB2 cable ( data rate). The system is designed to perform both NIR reflectance and fluorescence imaging within a clinically practical measurement time (in the order of a few seconds) with fast switching between the two imaging modes by placing appropriate optical filters at the illumination side (i.e., between the laser output and the ring light guide) and the light collection side (i.e., between the ring light guide and the lens) of the system. For reflectance imaging mode, a colored glass long-pass (LP) filter (RG-830, Edmund Optics Incorporated, Barrington, New Jersey) is used to select the NIR spectral range for illumination, and, at the light collection side, an LP filter combination consisting of two colored glass LP filters (RG-830, Edmund Optics Incorporated, Barrington, New Jersey) and an interference filter (RazorEdge Long Wave Pass Edge Filter LP02-785RU-25, Semrock, Rochester, New York) is used to collect the diffusely reflected NIR photons. In such a configuration, the main peak of the laser output at is blocked; the diffusely reflected photons in the broad, spontaneous emission tail (roughly ) of the laser spectrum form the reflectance image. To switch from NIR reflectance imaging mode to the NIR fluorescence imaging mode, a bandpass (BP) filter (MaxLine™ Laser-line Filter LL01-785-25, Semrock, Rochester, New York) is inserted (within ) at the illumination side to replace the LP filter, which eliminates the spontaneous emission tail in the laser output, while the LP filters at the detection side are not changed. In the system, the filter switch is achieved by a computer-controlled actuator (PLB-50, Pacific Laser Equipment, Santa Ana, California), such that minimum perturbation to the system and the imaging procedure is involved. This design of using the same (laser) light source for both fluorescence excitation and reflectance imaging illumination made the system more compact and efficient in switching between the two imaging modes. 2.2.Clinical MeasurementsThe study was approved by the Clinical Research Ethics Board of the University of British Columbia (protocol C05-0569). Informed consent was obtained from every patient or volunteer who participated in the study. The illumination power density at the skin surface was measured to be , an order of magnitude below the ANSI maximum permissible skin exposure limit of for a cw laser beam.17 The NIR autofluorescence images included in this study were acquired with an exposure time of , while the NIR reflectance images were acquired within . Before each measurement, the lesion and its surrounding skin were cleaned with an isopropyl alcohol wipe. The NIR fluorescence and reflectance images are taken sequentially by holding the camera probe in gentle and steady contact with the skin surface for a few seconds. A standard clinical color image is also acquired using a Nikon digital camera (model D100 with Micro-Nikkor f/2.8 lens). The entire imaging procedure takes about . We are currently conducting a systematic clinical study using this prototype imaging system. Example images are discussed next. 3.Results and DiscussionUsing the previous NIR autofluorescence imaging system, we have successfully acquired in-vivo images of various skin lesions. Figure 2 shows a benign junctional melanocytic nevus, a cherry angioma, and a superficial spreading melanoma. The left column shows standard color photographs of the three lesions. In the middle column are NIR reflectance images, while the right column shows the NIR fluorescence images. The junctional melanocytic nevus and the melanoma, both of which contain excessive melanin, showed significantly brighter NIR fluorescence emission than their surrounding normal skin, while their NIR reflectance images are darker than the surrounding normal skin due to melanin absorption of the incident light. Melanin absorption also accounts for their dark appearance in the color photographs. For comparison, images for a cherry angioma are shown in the second row; this type of skin lesion is a common benign neoplasm consisting of increased numbers of blood vessels in the skin. The lesion looks red on the clinical visible color image and dark on the NIR reflectance image due to hemoglobin absorption of the incident light from within the blood vessels of the lesion. In contrast, the NIR fluorescence image of a cherry angioma appears as a darker area compared to its surrounding normal skin. To quantitatively evaluate the results, we adopt the method used by Demos in their study3 and define the relative intensity difference as where is the image intensity averaged over the lesion and is the image intensity averaged over a normal region. For the junctional melanocytic nevus, was estimated from a area at the lesion, and was estimated from four such square areas in the surrounding normal region. The NIR reflectance image has and the NIR autofluorescence image has . For the melanoma, and are both estimated from four areas, sampled from the lesion and the surrounding normal region, respectively. The NIR reflectance image has and the NIR autofluorescence image has .Fig. 2Images from a benign junctional melanocytic nevus, a cherry angioma, and a superficial spreading melanoma. The left column is for clinical photographs, the middle column for NIR reflectance images, and the right column for NIR fluorescence images. 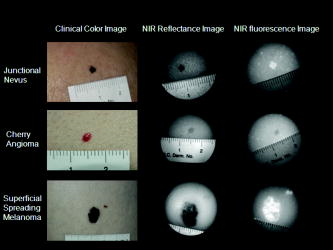 These results confirm our previous point spectroscopy study results,6 where cutaneous melanin fluoresces brightly under NIR excitation, and provide a new platform for imaging the distributions of melanins in skin tissue in vivo. These results are also consistent with bright NIR fluorescence observed by Keilhauer 9 for melanins in the ocular fundus. Our study results provide a new method for imaging the distribution of skin melanins in vivo. While both reflectance imaging and NIR autofluorescence imaging are potentially useful for detecting melanins in tissue, the autofluorescence technique is perhaps especially favorable since it represents a “direct” method that also exhibits higher detection sensitivity. In other words, with the fluorescence method, an increase in signal is measured over a zero background signal, whereas with reflectance, the analogous signal (i.e., absorbed or scattered light) is derived “indirectly” as the difference between the incident and the reflected light. This small decrease in the intensity of a very large signal as measured in the reflectance technique leads to a correspondingly large loss in sensitivity.18 Furthermore, for in-vivo skin tissue, the blood (hemoglobin) absorption is often of a comparable magnitude to melanin absorption, making it difficult to discern the true melanin distribution from single band reflectance imaging. Although there are more sophisticated ways, such as multispectral reflectance imaging, to differentiate these two chromophores, they require more complicated imaging hardware (in both optical components and imaging electronics), lengthened measurement time, complicated light transport modeling, and lengthy data processing time to derive the spatial distribution of these chromophores. In contrast, this issue is naturally solved by our system working in NIR autofluorescence mode, where melanin is a native fluorophore, while hemoglobin within blood vessels behaves as an absorbing chromophore. Figure 3 shows an incidental prominent blood vessel running beneath a melanin-rich seborrheic keratosis from upper left to middle right in the pictures. While the blood vessel is easily visible outside of the seborrheic keratosis on both the clinical visible color image and the NIR reflectance image, it is almost invisible within the lesion due to the confounding melanin absorption effect. On the other hand, the hemoglobin absorption also confounds the assessment of the melanin distribution from the reflectance images. Nevertheless, the NIR autofluorescence image in Fig. 3 clearly identified a segment of the blood vessel within the lesion as a dark spot (yellow arrow), while areas covered with melanins are bright. In Fig. 4, the profiles representing image intensities averaged from five adjacent vertical lines and five adjacent horizontal lines across the same lesion in Fig. 3 are plotted. Again, the NIR autofluorescence signal from the lesion clearly stands out above the normal skin background, while the blood vessel is recognized as decreased autofluorescence signals, as indicated by the arrows. This example demonstrated the great advantage of NIR autofluorescence imaging as a direct method for assessing cutaneous melanin distributions in vivo over the indirect reflectance imaging method. Fig. 3Seborrheic keratosis overlapping a blood vessel: note that the yellow arrows point to where a blood vessel segment is relatively invisible in the clinical photographs and the NIR reflectance image. However, this blood segment is readily visible in the NIR fluorescence image. 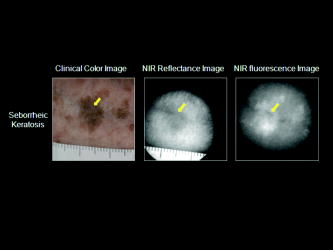 Fig. 4Intensity profiles at each pixel along the horizontal and vertical lines as indicated on the NIR fluorescence image for the same seborrheic keratosis lesion shown in Fig. 3. When the lines are across the blood vessel locations, the image intensities are decreased. 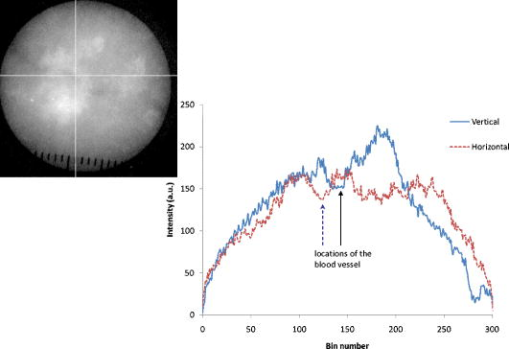 4.ConclusionsWe successfully develop a NIR fluorescence imaging system that can acquire good quality NIR autofluorescence images of the skin in vivo within a short, two-second exposure time that is suitable for clinical applications. The system can also acquire NIR reflectance images by utilizing spontaneous emission from the same laser within the same overall optical configuration. Preliminary results show that the distribution of NIR fluorescence due to cutaneous melanin within abnormally pigmented skin can be captured as an image showing a bright signal that corresponds directly to the presence of melanin. This has confirmed our previous findings from a NIR fluorescence point spectroscopy study of cutaneous melanins, and provides a new method for directly imaging the distributions of cutaneous melanins in skin tissue. The acquired in-vivo NIR autofluorescence images may be useful for clinical evaluation and diagnosis of pigmented skin lesions, including melanoma. A systematic and detailed clinical study is underway in our laboratory to fully explore the clinical utility and limitations of this new imaging technique. AcknowledgmentsThis work is supported by the Canadian Institutes of Health Research (grant number ITM-66116), the National Cancer Institute of Canada with funds from the Canadian Cancer Society, the Canadian Dermatology Foundation, the VGH and UBC Hospital Foundation In It for Life Fund, and the BC Hydro Employees Community Services Fund. We thank Jianhua Zhao and Wei Zhang for their assistance with the experiments. ReferencesE. B. Hanlon, I. Itzkan, R. R. Dasari, M. S. Feld, R. J. Ferrante, A. C. McKee, D. Lathi, and N. W. Kowall,
“Near-infrared fluorescence spectroscopy detects Alzheimer’s disease in vitro,”
Photochem. Photobiol., 70 236
–242
(1999). https://doi.org/10.1111/j.1751-1097.1999.tb07994.x 0031-8655 Google Scholar
G. Zhang, S. G. Demos, and R. R. Alfano,
“Far-red and NIR Spectral wing emission from tissues under 532 and photo-excitation,”
Lasers Life Sci., 9 1
–16
(1999). 0886-0467 Google Scholar
S. G. Demos, R. Gandour-Edwards, R. Ramsamooj, and R. White,
“Near-infrared autofluorescence imaging for detection of cancer,”
J. Biomed. Opt., 9
(4), 587
–592
(2004). https://doi.org/10.1117/1.1688812 1083-3668 Google Scholar
S. G. Demos, R. Gandour-Edwards, R. Ramsamooj, and R. White,
“Spectroscopic detection of bladder cancer using near-infrared imaging techniques,”
J. Biomed. Opt., 9
(4), 767
–771
(2004). https://doi.org/10.1117/1.1753587 1083-3668 Google Scholar
Z. Huang, H. Lui, D. I. McLean, M. Korbelik, and H. Zeng,
“Raman spectroscopy in combination with background near-infrared autofluorescence enhances the in vivo assessment of malignant tissues,”
Photochem. Photobiol., 81 1219
–1226
(2005). https://doi.org/10.1562/2005-02-24-RA-449 0031-8655 Google Scholar
Z. Huang, H. Zeng, I. Hamzavi, A. Alajlan, E. Tan, D. I. McLean, and H. Lui,
“Cutaneous melanin exhibiting fluorescence emission under near-infrared light excitation,”
J. Biomed. Opt., 11
(3), 034010
(2006). https://doi.org/10.1117/1.2204007 1083-3668 Google Scholar
X. Han, H. Lui, D. I. McLean, and H. Zeng,
“A technique for near-infrared autofluorescence imaging of skin: preliminary results,”
Proc. SPIE, 6078 60780S
(2006). https://doi.org/10.1117/12.646416 0277-786X Google Scholar
E. M. Sevick-Muraca, E. Kuwana, A. Godavarty, J. P. Houston, A. B. Thompson, and R. Roy,
“Near-infrared fluorescence imaging and spectroscopy in random media and tissues,”
Biomedical Photonics Handbook, 33-1
–33-66 CRC Press, Boca Raton, FL
(2003). Google Scholar
C. N. Keilhauer and F. C. Delori,
“Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin,”
Invest. Ophthalmol. Visual Sci., 47 3556
–3564
(2006). https://doi.org/10.1167/iovs.06-0122 0146-0404 Google Scholar
S. D. Kozikowski, L. J. Wolfram, and R. R. Alfano,
“Fluorescence spectroscopy of eumelanins,”
IEEE J. Quantum Electron., QE-20 1379
–1382
(1984). https://doi.org/10.1109/JQE.1984.1072333 0018-9197 Google Scholar
J. M. Gallas and M. Eisner,
“Fluorescence of melanin-dependence upon excitation wavelength and concentration,”
Photochem. Photobiol., 45 595
–600
(1987). 0031-8655 Google Scholar
M. Elleder and J. Borovansky,
“Autofluorescence of melanins induced by ultraviolet radiation and near ultraviolet light. A histochemical and biochemical study,”
Histochem. J., 33 273
–281
(2001). https://doi.org/10.1023/A:1017925023408 0018-2214 Google Scholar
S. P. Nighswander-Rempel, J. Riesz, J. Gilmore, J. Bothma, and P. Meredith,
“Quantitative fluorescence excitation spectra of synthetic eumelanin,”
J. Phys. Chem. B, 109 20629
–20635
(2005). https://doi.org/10.1021/jp053704+ 1089-5647 Google Scholar
S. P. Nighswander-Rempel, J. Riesz, J. Gilmore, and P. Meredith,
“A Quantum Yield Map for Synthetic Eumelanin,”
J. Chem. Phys., 123 194901
(2005). https://doi.org/10.1063/1.2075147 0021-9606 Google Scholar
P. Meredith, B. J. Powell, J. Riesz, S. P. Nighswander-Rempel, M. R. Pederson, and E. Moore,
“Towards structure-property-function relationships for eumelanin,”
Soft Matter, 2 37
–44
(2006). https://doi.org/10.1039/b511922g 1744-683X Google Scholar
G. N. Stamatas, B. Z. Zmudzka, N. Kollias, and J. Z. Beer,
“Non-invasive measurements of skin pigmentation in situ,”
Pigment Cell Res., 17 618
–626
(2004). 0893-5785 Google Scholar
, ANSI Standard Z136.1-2007, American National Standards Institute, Washington, D.C. ( American National Standard for the Safe Use of Lasers,
(2007) Google Scholar
H. Zeng,
“Human skin optical properties and autofluorescence decay dynamics,”
109 University of British Columbia,
(1993). Google Scholar
|