|
|
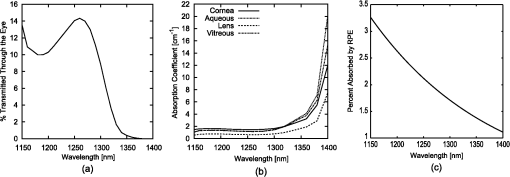
1.IntroductionMilitary and law enforcement agencies in the United States and elsewhere are devoting considerable resources to the area of “nonlethal weapons.” Of particular interest are devices that disrupt normal vision with bright visible light. These devices are often referred to as ocular interruption (OI) devices, as they are designed to saturate retinal photoreceptors and produce glare or transient flash blindness.1–4 One specific class of OI devices, the laser dazzler, employs visible lasers that project a beam toward a distant target. While dazzlers have proven useful in the field, the effectiveness of these devices depends upon the amount of light entering the eye, ambient lighting conditions, and the laser wavelength(s) used.3,4 For example, a green light device provides warning even during daylight hours, whereas either a bright green or red source can temporally disrupt vision in dimmer conditions. The concept of thermal lensing was first reported by Gordon et al.5 The basic principle arises from a phenomena which occurs when a weakly absorbing medium is sufficiently heated to induce a change in the index of refraction and form a virtual lens. This physical response can occur within any transparent material where the absorption properties allow for heating, such as water, air, fused silica, and ethanol. Since the principle is based on the local absorption of energy into the medium (and subsequent temperature rise), it becomes strongly dependent on both time and space. In general, the induced changes in the index of refraction, , can be modeled via Eq. (1): where is the refractive index, () is the first order temperature dependence of the refractive index (or thermo-optic coefficient) of the material, and is the laser-induced change in temperature as a function of space and time.6 The temperature change can be calculated using the heat-transport equation [Eq. (2) shown in cylindrical coordinates]:where is the thermal conductivity of the tissue in , is the specific heat of the tissue in , is the density of the tissue in , is the temperature rise at the coordinate , and is the source term in units of .7Previous research using “translational wavelengths” from 1050 to 1350 nm has led to the hypothesis that laser-induced thermal lensing in the human eye could cause transient visual disruption.8–13 As the light rays are focused toward the retina by the curvature of the cornea and the lens, local areas of absorption within the eye produce both transverse and longitudinal temperature gradients, leading to the formation of a thermal lens. The overall affect results in the creation of a dynamic nonlinear negative focal length-like lens which shifts the focal point of the eye beyond the retina. This shift in focal length causes the focused beam diameter at the retina of a collimated beam incident on the cornea to be enlarged once the thermal lens forms, see Fig. 1. Fig. 1Visible focal region spot of HeNe laser coaligned with a 150 mW 1318 nm laser at (a) initial state () and (b) the end of the IR-laser exposure (). Measurements were made in an artificial eye.10 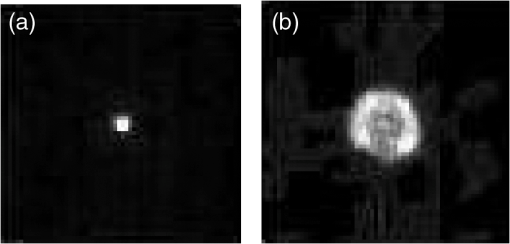 Tests using only 1318 nm wavelength exposures in artificial eyes also clearly demonstrate blurring of the image as a result of this thermal lens formation, see Fig. 1. To determine the viability of using thermal lensing for OI, three critical questions must be answered. First, can safe tests be conducted in the human eye? Second, is there a demonstrable effect on vision from ocular thermal lensing? Finally, once safety is assured and the concept is proven in humans, is there sufficient power within the safety limits to significantly distort vision? 1.1.Safety Considerations at 1319 nmOcular tissue absorption coefficients between 1150 and 1400 nm are known to be low enough to penetrate the cornea and yet high enough that less than 15% of the light within this wavelength range reaches the retina, see Fig. 2(a).14 The value of wavelength-dependent absorption coefficients for the cornea, aqueous, lens, and vitreous for the human eye are illustrated in Fig. 2(b) from 1150 to 1400 nm,14 and the amount of energy absorbed in the retina as a function of wavelength is presented in Fig. 2(c). From the data presented in Fig. 2, approximately 3.6% of the 1319 nm light entering the cornea reaches the retina, 1.6% of that light is then absorbed by the retinal pigmented epithelium (RPE). In other words, less than 2% of the IR energy entering the cornea actually contributes to potential damage on the retina. Fig. 2(a) Initial state image (); (b) steady-state image () from when the IR laser was exposed; and (c) image appearance 0.0667 s after the IR laser source is turned off. The system returns to its initial state appearances about 0.20 s after the IR laser source is turned off. 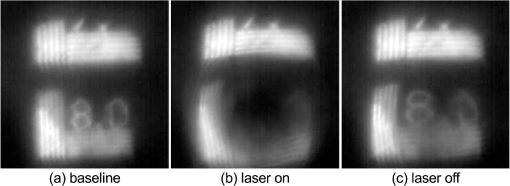 It is hypothesized that as 1319 nm power is increased, heating of 1°C to 3°C is sufficient to induce index of refraction changes which result in the formation of a thermal lens within the eye. This thermal lensing effect then causes an even further increase in safety as the beam diameter at the retina is increased, see Fig. 1. As a result, it was hypothesized that thermal lensing would significantly influence ocular damage thresholds induced by these lasers.12,16–20 Damage threshold experiments at 1319 nm using rhesus models indicate that the 24 h ED50 threshold for a 80 ms exposure is 14.5 W of measured power for a 5 mm beam entering the eye,12 where ED50 is the amount of energy required to create a minimal visible lesion in 50% of exposures. Based on this series of investigations, it was determined that the current ANSI Z136.1-2007 standard (, exposure duration of 1 s for wavelengths between 1200 and 1400 nm) was extremely conservative (approximately 10× lower) than necessary in order to meet the universally accepted margin of safety (10% of the ED50).21,22 In an ideal situation, the subject’s pupil will be much larger than the diameter of the IR laser; however, in a practical setting, the beam size of an OI device will be much larger than the subject’s pupil. Therefore, it becomes important to establish the risk of potential harm of the IR beam to the iris. Absorption of IR energy in the iris is strongly dependent on the amount of pigmentation, or melanin, present in the eye. Similar to skin, a lighter-color (lower melanin) iris will absorb less of the IR energy than a darker-color (high melanin) iris. For this safety analysis, only a worst case damage scenario for a dark (high melanin) iris will be considered. Very little data currently exists on the absorption properties of the iris; however, since the absorption of melanin will dominate for dark pupils the damage threshold of the iris is known to be similar to the threshold for skin. ANSI lists the maximum permissible exposure (MPE) for 1319 nm, 0.25 s exposures to be . This value is considerably lower than the retinal threshold; however, it should be noted that most of the energy from a Gaussian beam will be transmitted through the pupil, making this a conservative estimate of the damage threshold. The third, and final, point of damage for a 1319 nm laser entering the eye is the cornea. Damage threshold studies have again shown that corneal thresholds are several orders of magnitude higher in this wavelength range than the current ANSI standard.21,22 Experiments from Chen et al. using various animal models also further confirmed this hypothesis by reporting the ED50 threshold for the cornea to be approximately 9.04 W for a 5 mm beam incident on the cornea.20 These thresholds are much lower than the retinal hazards for long exposures (0.1 to 10 s); however, a proposed threshold of even is still very conservative in protecting against cornea, iris, and retina lesions. These damage threshold measurements from animal and computational modeling have provided sufficient evidence to suggest that laser thresholds in ocular media could be significantly increased without increasing risk of lesions within the eye. As a result, changes in the threshold limit values (TLVs) have been adopted by the American Conference of Governmental Industrial Hygienists.23 Some changes in the current (2007) ANSI standard have been made; however, the increase to has not yet been fully incorporated due to ANSI’s more extensive review process. However, similar increases in the exposure limits are anticipated in the next ANSI Z136.1 standard (2013). In both standards the MPE and TLV for 1319 nm laser light and a 1 s exposure time will be capped at . With these new exposure limits, it is now possible to test whether thermal lensing in the human eye can cause significant changes in visual function. Previous experimental results using an artificial eye have already shown that the thermal lens influences the focus of visible wavelengths, Figs. 1 and 3; however, it is still unclear if the natural human eye will be able to compensate for these changes in the visible wavefront with accommodation or if the effect will impact a large enough field of view to be detected by the observer. Therefore, this study seeks to determine if a noticeable difference in visual function can be observed in the presence of a thermal lens induced in ocular media using different power levels and exposure durations. 2.Methods2.1.Subject TaskEight healthy subjects between 20 and 65 years of age with uncorrected distance visual acuity of at least participated in the experiment. Each subject was examined by an eye care professional prior to beginning the experiments to rule out any preexisting ocular conditions which may influence the safety of the study. The study was conducted under the tenets of the Declaration of Helsinki,24 and informed consent was obtained from all subjects. Each subject was exposed to eleven different power/duration combinations, see Table 1. During the experiment, exposures were randomized so that subjects were unaware of the current power or exposure duration being used. After each exposure, subjects were asked to indicate (with “Yes” or “No”) whether a visual distortion occurred during the exposure. For each “Yes” response, subjects were then asked to describe the observed changes in the visual stimulus. These comments were recorded either by the subject or by an investigator present in the room. In order to minimize the number of exposures for each subject, each set of exposure conditions (A to K) were administered three to five times, depending on the consistency of the subject’s responses (i.e., if the first three responses were consistently “Yes” or “No” then no further trials were needed). Table 1Eleven exposure combinations used for each subject.
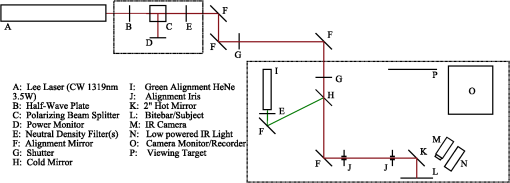
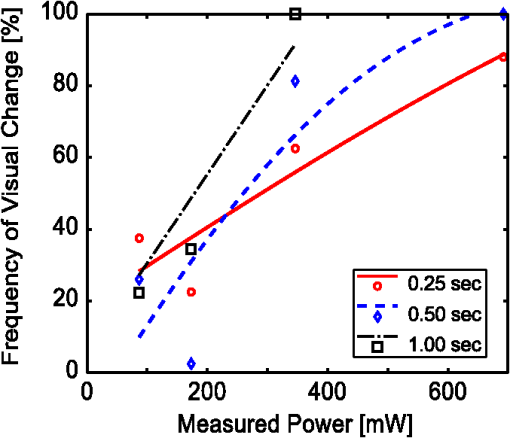
2.2.InstrumentationThe experimental setup, Fig. 4, utilized an open-view (Newtonian) optical system to provide a method of visual stimulus viewing. A 2-inch hot mirror was used to align and deliver the IR beam into the subject’s pupil. A low power () visible laser beam (543 nm) coaligned with the IR laser was used to help align the subject with the IR beam and the center of the visual stimulus. A Nd:YAG Lee Laser (Lee Laser Inc., Orlando, Florida) was configured for a 1319 nm output, and a beam expander (BE02M-C, Thorlabs Inc, Newton, New Jersey) was used to control beam size and collimate the IR laser to a minimum divergence. The IR beam diameter was measured to be 4.44 mm at the subject’s eye position using an IR camera (EPM 2000, Molectron Dectector Inc, Portland, Oregon). A bite bar and forehead rest were also utilized to aid in subject positioning. Once the subject was brought into position, the visible beam was turned off, and the unexposed (left) eye was covered using an eye patch. Prior to the experiment, a dual detector power meter (EPM 2000, Molectron Dectector Inc, Portland, Oregon) was used to calibrate the IR laser power at the subject with the power emitted at one exit of a beam splitter placed just after the laser. The power of the IR laser at the beam splitter was then continuously monitored during data collection, and a shutter system was employed to ensure proper power levels were used. This system was also programmed to automatically cut off the beam if the power level drifted above the desired level. An IR camera (Electrophysics Micron Viewer, Fairfield, New Jersey) was used to view the corneal reflection of the IR beam and allow for video recordings of each exposure which were used to measure the pupil diameter of each subject and confirm each exposure was successfully delivered. To help increase the likelihood of a successful exposure, pupils were enlarged by placing the subjects in a dark room with the only the visual stimulus acting as a light source. The visual stimulus was an Amsler grid subtending 7 deg of visual angle with an average luminance of . The grid was presented on a LCD display positioned 83 cm from the subject’s corneal plane, see Fig. 5(a). Fig. 5Simulations of the distorted Amsler grid blurring for (a) normal conditions (no IR exposure), (b) full distortion/blur (c) 1-D horizontal shift, and (d) 2-D shift. Images were generated using Inkscape Vector Graphics Editor (Inkscape, www.inkscape.org). 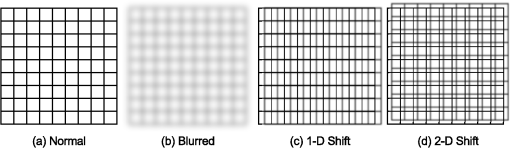 2.3.Data AnalysisThe percentage of “Yes” responses (i.e., the frequency of visual change) was recorded for each exposure condition. These data were analyzed using MATLAB (MathWorks, Natick, Massachusetts) and SPSS (IBM Corp., Armonk, New York). The goal was to determine the effect of IR power, duration, and higher order predictors (such as subject age and pupil diameter) on the frequency of visual change. Video images acquired during data collection were used to estimate alignment accuracy by measuring the position of the IR beam on the cornea. 3.Results3.1.Effects on Visual PerformanceBefore analyzing the data, video footage of each subject’s experiment was used to confirm the laser beam position relative to the pupil for each exposure condition. Little variation in the vertical positioning of the beam was observed; only the horizontal position potentially affected the outcome of the exposure. For most subjects, the horizontal beam position was maintained within the pupil. The only exceptions were for subjects 2 and 4, where occasionally (one or two exposures) the beams did not fully enter the eye. These exposures were removed from the data set for subsequent analysis. The frequency of visual change reported was analyzed for all power and duration combinations for all eight subjects, see Fig. 6. A large amount of variability existed between and within each subject, but this was expected since a threshold level effect was being investigated. However, our first subject reported a visual change for every exposure level. Sham exposures revealed that this individual was experiencing visual changes in the absence of any laser exposure. Therefore, while results for subject 1 are shown in Fig. 6, the results for this subject were excluded from the final determination of the thermal lensing effects on visual function. Fig. 6Interpolated curves of frequency of visual change (i.e., percent of time effect was seen) plotted against power level for the various durations for each subject. 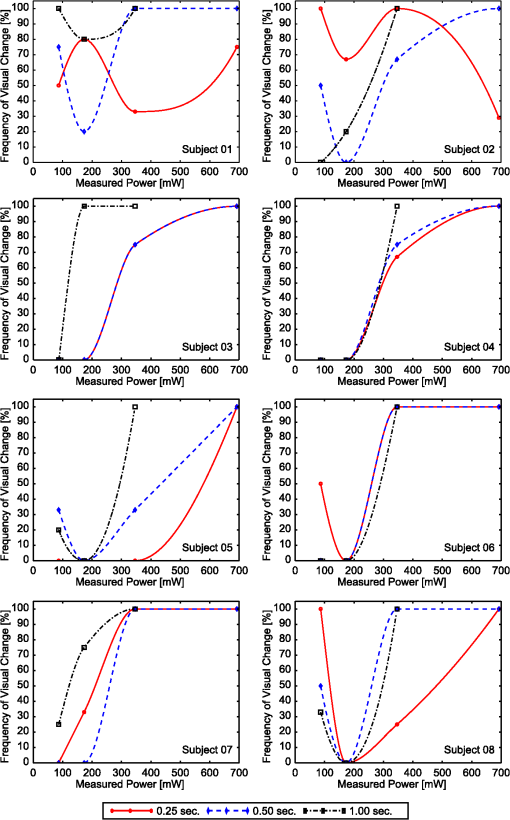 After results were compiled for each individual subject, the responses were averaged to determine an approximate threshold across all subjects. This interpolation was expected to result in a low frequency of visual change for lower energy levels and a high frequency of visual change as the energy was increased. However, several subjects reported visual changes at low exposure levels and the cubic interpolation analysis did not mimic a cumulative probability function. Therefore, to characterize the threshold effect as accurately as possible, the subsequent analysis was based on a Gaussian error function regression, as shown in Fig. 7. Results showed that the power level used strongly correlated with the likelihood that the exposure would be associated with a visual change. Some variation in the frequency of visual change was also observed for different exposure durations; however, given the limited number of subjects allowed for this pilot study, an in-depth statistical analysis to determine the significance of exposure duration would not be appropriate. Therefore, it was concluded that the relationship is most likely related to the total amount of power required to create a sufficiently strong thermal lens which can significantly impact vision. 3.2.Observed Visual DistortionIn addition to the binary “Yes” or “No” response from each subject, a description of the effect was also recorded for each “Yes” response. Descriptions of the observed visual changes varied greatly between subjects. Almost all subjects described a “full blurring” of the visual target during exposures at the highest power level (692 mW). For mid-level exposures (173 to 346 mW), subjects reported some or all of the following: localized blurring in the center, blurring on the edges, a “doubling” of the vertical and/or horizontal lines (i.e., two-dimensional shift), and a directional (or one-dimensional) shift of the target left or right within the field, see Fig. 5. 4.DiscussionThis experiment has demonstrated that thermal lensing can induce safe significant visual distortions of human vision. These effects were possible with IR energy levels below the current standards set by the ACGIH. Having examined the effect at various power levels and exposure durations, it was determined that total IR power delivered to the cornea played a more significant role in predicting a visual effect than the exposure duration. Minor discrepancies between trials with different exposure durations suggest that there might be a minimum thermal lens effect which is needed to overcome the eyes’ ability to compensate for the wavefront distortion through accommodation; however, once this minimum energy level is reached a visual change will be observed in 100% of exposures. Based on the limited number of exposure durations used in this study, it is difficult to determine an accurate radiant energy threshold; however, the effect was more consistent for levels above 346 mW with almost 100% visual disruption for powers of 692 mW. Future studies using a larger number of subjects and a wider range of power levels and exposure durations should be completed in order to more accurately define a threshold of visual effect. Despite being dark-adapted, the average pupil diameter achieved from subjects 1 through 8 was only . This meant that a portion of the IR energy would be absorbed and scattered from the subject’s iris. As previously discussed, this would influence the total amount of IR energy being delivered to the posterior sections of the eye, and may have contributed to the between-subject variations observed in the data. To analyze this, the amount of power needed to induce a visual change in 75% of exposures was plotted against the individual’s pupil diameter, see Fig. 8. Fig. 8Power needed to induce a visual change in 75% of exposures compared to the subject’s measured pupil diameter for subjects 2 through 8. 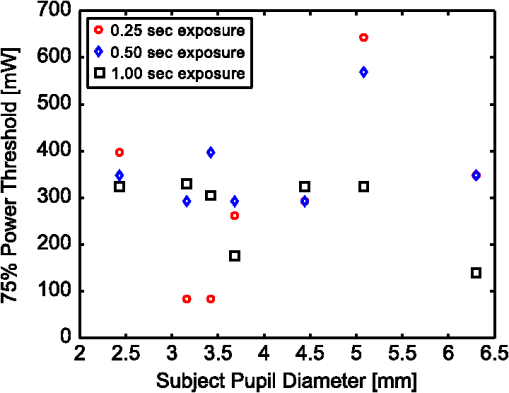 Figure 8 shows very little correlation between the subject’s pupil size and the IR power needed to induce a visual change in 75% of exposures. Subjects in this experiment also came from a range of ethnic backgrounds, and therefore, this data represents a range of melanin distributions within the iris which would have varying influences on the propagation of the 1319 nm laser. There are several reasons why the subject’s pupil size was found to be independent of the threshold for visual function. First, the Guassian beam profile means that most of the energy delivered to the eye will be located at the center of the subject’s pupil. Therefore, any energy which was absorbed in the iris may not significantly contribute to the thermal gradient needed to generate visual distortions. This minimal influence of pupil size could also occur if the effects of a thermal lens generated in the cornea (rather than the retina) contributed more significantly to a change in visual performance. In other words, if the thermal lens generated from the energy absorbed in the cornea layers dominates, then the influence of energy absorbed in layers posterior to the cornea (such as the iris) would be more difficult to distinguish from the overall effect. The within-subject variability may have occurred, in part, due to variability in the alignment of the subject and the IR laser, resulting in the formation of a thermal lens off-axis with respect to the visual axis. Despite the use of a bite-bar and forehead rest, some changes in subject positioning occurred. This was especially evident when a subject came out of the apparatus to verbally describe the visual changes they experienced. More experienced subjects were able to return quickly to the same position for each exposure, but less experienced subjects took significantly longer to align and showed slightly more variation in their results. Future experiments should incorporate a more robust and repeatable method of aligning the subject with the optical delivery system and minimize the number of instances where a subject needs to verbally respond to a stimulus. Small variations between subject responses could have been a result of age and aberration differences between subjects. As the eye matures it begins to lose its ability to accommodate which could decrease the threshold required to induce a thermal lens in these subjects. Higher order aberrations could also impact the natural focus of the infrared (IR) light, causing the thermal lens to form in a slightly different axial location, and thus alter the effect. While all of these variations are important to consider in future experiments, the overall similarity between the subjects, however, suggests the effect of these higher order aberrations to be minimal on a threshold power. In order to more accurately correlate visual effects with the generation of the thermal lens, future experiments should also incorporate the use of intrinsic optical imaging techniques that are capable of measuring the power of the thermal lens created within the eye during each exposure. The measured changes can then be correlated with the visual changes experienced, and determination of a more accurate threshold for visual disruption should be possible. 5.ConclusionStudies have been conducted on eight human subjects, which have successfully demonstrated a safe distortion to the visual field when an IR laser is used to induce a thermal lens within the ocular media. This distortion is possible with power levels under the updated ACGIH injury thresholds for near-IR light entering the eye. These experiments have only begun to scratch the surface for the potential of thermal lensing to influence visual performance. Future studies will incorporate a more robust method of measuring distortion as well as improved features for subject alignment so that a more thorough experiment of the influence laser-induced thermal lensing on visual function (contrast sensitivity, visual acuity, etc.) can be conducted. AcknowledgmentsThe authors would like to acknowledge the Consortium Research Fellowship program for student financial support. We would also like to acknowledge Dr. Thomas Milner (University of Texas at Austin) and Rebecca Vincelette (TASC Inc) for contributing their knowledge and advice in preparing for this project. ReferencesL. L. Holladay,
“The fundamentals of glare and visibility,”
J. Opt. Soc. Am., 12
(4), 271
–319
(1926). http://dx.doi.org/10.1364/JOSA.12.000271 JOSAAH 0030-3941 Google Scholar
J. Brown,
“Flash blindness,”
Am. J. Ophthalmol., 60
(3), 505
–520
(1965). AJOPAA 0002-9394 Google Scholar
J. Hecht,
“Diode-pumped solid-state lasers: laser dazzlers are deployed,”
Laser Focus World, 48
(3),
(2012). LFWOE8 1043-8092 Google Scholar
L. Doswald-Beck,
“New protocol on blinding laser weapons,”
Int. Review Red Cross., 36
(312), 272
–299
(1996). Google Scholar
J. Gordonet al.,
“Long-transient effects in lasers with inserted liquid samples,”
J. Appl. Phys., 36
(1), 3
–8
(1965). http://dx.doi.org/10.1063/1.1713919 JAPIAU 0021-8979 Google Scholar
R. Boyd, Nonlinear Optics, Academic Press, New York
(2008). Google Scholar
L. Irvinet al.,
“BTEC thermal model,”
(2008). Google Scholar
R. Vincelette,
“Thermal lensing in ocular media,”
The University of Texas, Austin
(2009). Google Scholar
C. D. Clark III,
“Modeling laser damage to the retina,”
University of Texas at San Antonio,
(2011). Google Scholar
R. Vinceletteet al.,
“Thermal lensing in ocular media exposed to continuous-wave near infrared radiation: 1150–1350 nm region,”
J. Biomed. Opt., 13
(5), 054005
(2008). http://dx.doi.org/10.1117/1.2978066 JBOPFO 1083-3668 Google Scholar
R. Vinceletteet al.,
“Confocal imaging of thermal lensing induced by near-infrared radiation in an artificial eye,”
IEEE J. Sel. Topics Quantum Electron., 16
(4), 740
–747
(2010). http://dx.doi.org/10.1109/JSTQE.2009.2037441 IJSQEN 1077-260X Google Scholar
R. Vinceletteet al.,
“Trends in retinal damage thresholds from 100 millisecond near-infrared radiation: a study at 1100, 1130, 1150 and 1319 nm,”
Laser. Surg. Med., 41
(5), 382
–390
(2009). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
R. Vinceletteet al.,
“Method for measuring ocular aberrations induced by thermal lensing in vivo,”
Proc. SPIE, 7562 75620T
(2010). http://dx.doi.org/10.1117/12.846070 PSISDG 0277-786X Google Scholar
E. Maher,
“Transmission and absorption coefficients for the ocular media of the Rhesus monkey,”
(1978). Google Scholar
R. Birngruberet al.,
“Theoretical investigations of laser thermal retinal injury,”
Health Phys., 48
(6), 781
–796
(1985). http://dx.doi.org/10.1097/00004032-198506000-00006 HLTPAO 0017-9078 Google Scholar
J. Zuclichet al.,
“Ocular effects of penetrating IR laser wavelengths,”
Proc. SPIE, 2391 112
–125
(1995). http://dx.doi.org/10.1117/12.209874 Google Scholar
J. Zuclichet al.,
“A comparison of laser-induced retinal damage from infrared wavelengths to that from visible wavelenghts,”
Laser. Light Ophthalmol., 8
(1), 15
–29
(1997). LLOPED 0922-5307 Google Scholar
J. Zuclichet al.,
“Ophthalmoscopic and pathologic description of ocular damage induced by infrared laser radiation,”
J. Laser Appl., 10
(3), 114
–120
(1998). http://dx.doi.org/10.2351/1.521836 JLAPEN 1042-346X Google Scholar
J. Zuclichet al.,
“Ocular effects and safety standards implications for high-power lasers in the 1.3–1.4 μm wavelength range,”
(2004). Google Scholar
H. Chenet al.,
“A comparative study on ocular damage induced by 1319 nm laser radiation,”
Laser. Surg. Med., 43
(4), 306
–312
(2011). http://dx.doi.org/10.1002/lsm.21052 LSMEDI 0196-8092 Google Scholar
J. ZuclichD. LundB. Stuck,
“Wavelength dependence of ocular damage thresholds in the near-IR to far-IR transition region: proposed revisions to MPEs,”
Health Phys., 92
(1), 15
–23
(2007). http://dx.doi.org/10.1097/01.HP.0000232188.69779.69 HLTPAO 0017-9078 Google Scholar
ANSI (American National Standards Institute),
(2007). Google Scholar
(2010). Google Scholar
“Declaration of Helsinki,”
Br. Med. J., 313
(7070), 1448
–1449
(1996). BMJOAE 0007-1447 Google Scholar
|
||||||||||||||||||||||||||||||