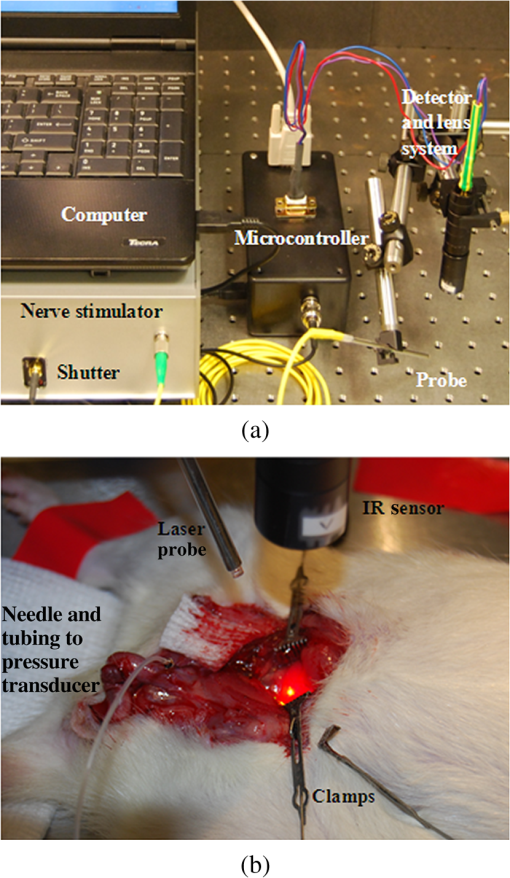
|
|
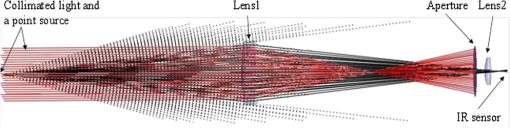
1.IntroductionIdentification of the cavernous nerves (CNs) during prostate cancer surgery is critical in preserving a man’s sexual function following surgery. Because of the close proximity of the nerves to the prostate surface, they are at risk of injury during dissection and removal of a cancerous prostate gland. The exact location and path of these microscopic nerves also varies significantly among patients. These observations may explain the wide variability in sexual potency rates (9 to 86%) following surgery.1 Development of improved methods for intraoperative identification of the CNs may prove valuable for preserving the nerves and improving postoperative sexual function and patient quality of life. Numerous experimental nerve mapping devices and imaging modalities have been tested recently as potential intraoperative tools for identification of the CNs during prostate cancer surgery; however, they all have significant limitations.2,3 Some of these technologies, including electrical nerve mapping devices, have been tested in the clinic.4 However, electrical nerve mapping has proven inconsistent and unreliable in identifying the CNs and evaluating nerve function. Lack of specificity, high false positive responses, and the influence of multiple conflicting factors in recording an electrical response during surgery have been cited.5–7 Furthermore, electrical nerve stimulation (ENS) suffers from several inherent limitations, including need for physical contact between electrode and tissue, limited spatial precision due to electrical current spreading in tissue, and electrical artifacts that may interfere with measurement. Optical nerve stimulation (ONS) using infrared (IR) laser energy provides several advantages, including noncontact stimulation, improved spatial selectivity, and elimination of stimulation artifacts.8 Our laboratory is developing ONS9–14 as a potential alternative to ENS using a standard in vivo rat prostate model.15 The ONS mechanism has recently been identified primarily as a photothermal process.16,17 The nerve temperature during ONS is therefore the most critical parameter. The temperature range necessary for successful ONS is relatively narrow, from to 47°C. Lower temperatures do not result in nerve activation18 and higher temperatures result in undesirable thermal damage to the nerve.19 Significant temperature fluctuations currently occur during both pulsed and continuous-wave (CW) delivery of laser radiation during ONS. Rapid, safe, and reproducible ONS may therefore require application of a constant temperature during laser irradiation. Closed-loop feedback systems utilizing an IR sensor for real-time control of tissue surface temperatures during laser therapy have previously been reported for laser tissue welding and soldering applications.20–23 The therapeutic window for successful laser tissue welding is relatively large (e.g., 50 to 100°C), allowing for significant error in temperature control without adverse thermal effects in the tissue. However, for the specific application of ONS, the therapeutic window for successfully activating the nerves is much narrower, thus representing a greater challenge. The ultimate goal of our laboratory is to develop a safe and effective ONS system as an intraoperative diagnostic tool for identification and preservation of the CNs during surgical excision and removal of a cancerous prostate gland. In this preliminary study, we describe the construction, characterization, and initial testing of a closed-loop, automated, temperature-controlled ONS (TC-ONS) system in an established in vivo rat prostate model for maintaining a precise, preset nerve temperature (). This TC-ONS system may prevent nerve damage due to an uncontrolled escalation in nerve temperature that may otherwise occur during conventional pulsed and CW methods of ONS. 2.Materials and Methods2.1.Surgical PreparationAll studies were performed under an animal protocol approved by the Animal Care and Use Committee at Johns Hopkins Hospital (Baltimore, Maryland). A total of 12 Sprague Dawley rats (400 to 600 g) were anesthetized by intraperitoneal injection with sodium pentobarbital. The rats were secured in the supine position and prepped for surgery. An adjustable heating pad was placed under the rats to maintain their body temperature at or near 37°C during surgery. The CN arising from the ipsilateral major pelvic ganglion situated dorsolateral to the prostate was exposed via a midline suprapubic incision and anterior pelvic dissection. To assess intracavernous pressure (ICP), the shaft of the penis was denuded of skin and the left crural region was cannulated with a 23 gauge needle connected via polyethylene tubing to a pressure transducer (Harvard Apparatus, Holliston, Massachusetts). An increase in ICP after ONS was detected by a data acquisition system (DI-190, Dataq Instruments, Akron, Ohio). ICP response (mmHg) as a function of time (s) was plotted using MATLAB 6.0 software (Mathworks, Natick, Massachusetts) with a data acquisition rate of 8 Hz and an accuracy of 0.5 mmHg. This method is based on a standard technique for measuring ICP during physical nerve stimulation and ENS in rats.24 After completion of the study, the rats were euthanized by intracardiac injection of potassium chloride while under anesthesia, consistent with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association. 2.2.Optical Nerve Stimulation SystemA near-IR, single-mode, pigtailed, diode laser (QFLD-1450, Qphotonics, Ann Arbor, Michigan) emitting up to 150 mW of power at a wavelength of 1455 nm was operated in CW mode. Laser radiation at 1455 nm has an () optical penetration depth of in water (the dominant soft tissue absorber in the near-IR spectrum),25 intended to approximate the CN diameter, for more efficient absorption of the laser energy in the nerve. The 1455 nm laser was integrated into a compact, custom-built ONS system, consisting of the following components. A low-power, red diode laser aiming beam (QFLD-660, QPhotonics) with a wavelength of 660 nm was coupled into a single-mode fiber (SMF). An SMF coupler ( Dual Window Coupler, Fiber Instrument Sales, Oriskany, New York) with a coupling ratio combined both visible and IR laser beams into a single SMF. The fiber coupler output was connected to a custom-built 10-Fr [3.4-mm-outer diameter (OD)] laparoscopic probe consisting of -cladding SMF (SMF-28, Corning, New York) with an aspheric lens (354430C, 2-mm-OD, 5-mm-focal-length, Thorlabs, Newton, New Jersey) attached to the distal tip for beam collimation. The custom 3.4-mm-OD laparoscopic probe provided a collimated 1.0-mm-diameter () laser spot with Gaussian spatial beam profile on the nerve surface at a working distance of . An in-line optical shutter (SH-200-1480-9/125, Oz Optics, Ottawa, Canada) in the IR laser SMF arm provided rapid on/off switching of the laser beam during ONS. Coupling into the SMF and the fusion splices attenuated the maximum power output of the probe to . All ONS experiments were performed with an irradiation time of either 15 or 30 s and a fixed laser spot diameter of 1 mm. For the control studies using CW laser irradiation without temperature control, incident laser powers providing consistent and robust ICP responses were chosen. For the TC-ONS system, different temperature set points (42, 44, 46, and 48°C) were explored. A minimum of five stimulations were performed for each set of parameters. A successful ICP response was judged by the ability to differentiate the peak ICP during ONS from the normal physiological baseline ICP observed before and after ONS. 2.3.Radiometry TheoryRadiometry is based on the principle that any tissue with a temperature above absolute zero emits IR radiation. According to the Stephan–Boltzmann law, the intensity, , of thermal radiation emitted from a surface tissue area, , is given by , where is emissivity, is the Stefan–Boltzmann constant, , and is tissue temperature. The emissivity, , is determined by the tissue’s surface properties. For soft tissue, is due to large water content. In addition, thermal radiation energy is homogenous in all directions. From Wien’s law, tissues at different body temperatures emit radiation at different peak wavelengths: , where is the wavelength of peak blackbody emission at an absolute temperature of and is called Wien’s displacement constant, equal to 2898 μm K. For the relevant temperature range of 305 to 323 K (32 to 50°C) for ONS studies, thermal radiation is emitted primarily in the spectral range of 8.9 to 9.5 μm. 2.4.Temperature Feedback SystemThe TC-ONS system consisted of three main components: an all-SMF laser system with mechanical shutter, a custom laparoscopic laser probe, and a radiometer with a temperature feedback control system. The radiometer system was composed of a thermopile digital sensor module (Heimann HID L14 FL5.5 T50, Boston Electronics, Bookline, Massachusetts), a zinc selenide (ZnSe) plano-convex IR lens (LA7733-F, Thorlabs) with transmission in the spectral range of 600 nm to 16 μm, and a microprocessor (QSK62P, Renesas Technology, Santa Clara, California). A photograph of the real-time, temperature-controlled all-SMF ONS system is shown in Fig. 1 along with the experimental setup. The thermopile sensor used in this system had an absorbing area of ; it was sensitive to the 5.5 to 13.5 μm spectral range and generated a pulse-width modulation signal that was proportional to the temperature. The response time of the detector was 5 ms with a refresh rate between 100 and 250 ms. The mid-IR radiation emitted from the heated nerve surface was collected and transmitted to the IR sensor, generating a signal by use of a focusing lens with a 40 mm working distance. The output signal from the IR sensor was processed using a microprocessor for data acquisition and control. The actual control of laser power over the nerve surface was accomplished by providing rapid on/off switching with an optical shutter, based on an external transistor–transistor logic signal provided from a laptop PC. 2.5.Computer Ray-Tracing SimulationsComputer simulations using basic and complex ray-tracing methods (FRED Optical Engineering Software version 10.41.0, Photon Engineering, Tucson, Arizona) were also performed to provide a better understanding of propagation of the IR radiation through the two-lens optical system. These simulation studies were helpful not only to determine the optimal position of the IR lenses but also to optimize the aperture size and optimal position, which was critical to having a correct focusing power onto the CN and spatial resolution for accurate temperature measurements. Figure 2 shows the computer simulations in two-dimensional format. Light beams from a point source (black) and collimated light beams (red) were used to symbolize the IR radiation emitted from the heated spot on the nerve surface and the IR radiation emitted from surrounding tissue, respectively. These beams were collected with the use of an IR lens (20 mm focal length) as an objective lens (Lens1). Using a 1-mm-diameter aperture, undesired radiation (collimated light) was successfully minimized. The filtered radiation was transmitted through the IR sensor by a focusing IR lens (Lens2) with a focal length of 5.5 mm. 2.6.Calibration of TC-ONS SystemFor calibration of the IR sensor, a black anodized aluminum plate with an emissivity of was used as a blackbody object. Control of the surface temperature of this blackbody was achieved with a hot plate (Cole-Parmer, Vernon Hills, Illinois). Both a thermal camera (A20M, FLIR, Billerica, Massachusetts) and a thermocouple (Type T, Omega Engineering, Stamford, Connecticut) attached to the surface of the metal plate were used to measure the surface temperature with a resolution of . The radiometer system, ZnSe lens, was placed at a distance of about 40 mm horizontal to the blackbody object’s surface and the pulse width from the IR sensor was recorded for a range of temperatures between 30 and 50°C. The pulse width and resulting absolute measured temperature were plotted and a linear trend line equation was used to calibrate the IR sensor for the microcontroller. An “IF” statement on the microcontroller compared the measured temperature from the IR sensor to a temperature threshold value set by the user. While the input remained below the set point, the shutter received a “LOW” signal. If the input exceeded the set point, a “HIGH” signal was transmitted to the shutter, closing it and pausing laser irradiation of the nerve. Once the input signal fell below the set point again, a “LOW” signal reopened the shutter. This negative feedback loop was operating over the entire stimulation period. In summary, for each experiment, a set-point temperature was first chosen, typically within the safe range of nerve temperatures (42 to 47°C), and entered into the PC software. The IR laser was then turned on with an incident laser power sufficient to reach this set-point temperature and preferably with a short ramp-up time, for potential diagnostic applications. During stimulation, the mechanical shutter would then rapidly open and close, maintaining this set-point temperature to within a small error () in response to feedback from the IR sensor, which was in turn monitoring the nerve temperature and in communication with the PC, in a closed feedback loop. 3.ResultsThe CN temperature and ICP response were measured as a function of laser irradiation time for CW mode at different power levels and for TC-ONS mode at different temperature set points. The overall objectives were (1) to produce a rapid ramp-up in nerve temperature to the desired set-point temperature and (2) to maintain this constant set-point temperature during the stimulation period. Figure 3 shows plots of the CN temperature and ICP response as a function of laser power and time. A 15 s baseline temperature is shown ( to 15 s), followed by 15 s stimulation ( to 30 s), and then 30 s postirradiation nerve cooling phase ( to 60 s). Three different ONS modes were plotted under these conditions: CW at a power of 62 mW, TC-ONS at 62 mW with a set point of , and CW at a lower power of 52 mW which resulted in the same final nerve temperature of at the end of stimulation (). This graph shows two major differences. First, by comparing CW stimulation (62 mW) without temperature control to CW stimulation (62 mW) with TC-ONS, it was observed that the temperature set point prevented a continuous rise in the nerve temperature and maintained the nerve temperature at . Second, by comparing CW stimulation (62 mW) with TC-ONS and CW stimulation (52 mW) without temperature control, we observed that at , both graphs showed a final temperature of . However, the TC-ONS provided a faster ramp-up to the final temperature, thus translating into a faster ICP response time (7 versus 12 s) as well. A rapid ICP response is critical if ONS is to be adopted as an intraoperative diagnostic technique in the clinic. Fig. 3Cavernous nerve temperature and ICP response as a function of time for a 15 s stimulation ( to 30 s). Use of higher laser power (62 mW) in CW mode allowed the nerve to reach the necessary temperature range for nerve activation more rapidly, generating a faster ICP response time (). However, high power CW operation produces continuously escalating temperatures, eventually resulting in thermal damage to the nerve. Conservative use of lower laser power (52 mW) resulted in an unnecessary delay in reaching the nerve activation temperatures necessary for a robust ICP response (). The TC-ONS system allowed both use of higher laser power for a shorter and faster initial ramp-up time in temperature and ICP, while also maintaining the nerve at a constant, preset temperature for safety. 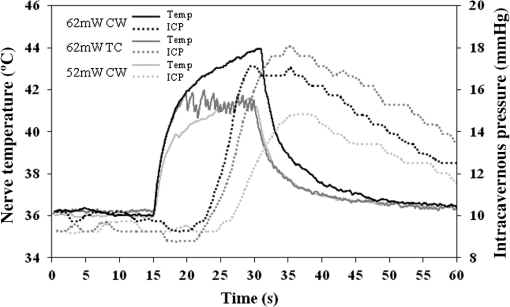 In Fig. 4, the nerve temperature and ICP are plotted as a function of laser power or set-point temperature and time, with a 15 s baseline and a 30 s stimulation time. Five different conditions are shown: CW stimulation (94 mW) without temperature control and stimulation with TC-ONS at set points of 42, 44, 46, and 48°C corresponding to laser powers of 79, 84, 94, and 94 mW, respectively. Similar ICP response times were observed for the similar nerve temperature ramp-up times. However, ICP responses with higher peak values due to the higher final nerve temperatures were also observed. Fig. 4Cavernous nerve temperature and ICP response as a function of time for a 30 s stimulation ( to 45 s). CW stimulation (94 mW) was compared to TC-ONS with set-point temperatures of 42, 44, 46, and 48°C corresponding to laser powers of 79, 84, 94, and 94 mW, respectively. Similar ICP response times were observed for similar nerve temperature ramp-up times. However, higher set-point temperatures resulted in more robust ICP responses. 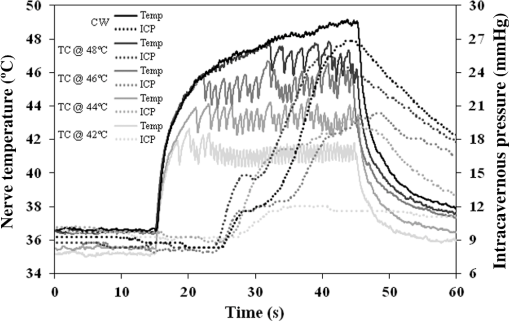 4.DiscussionPreservation of the CNs during prostate cancer surgery has proven challenging due to the close proximity of the CNs to the prostate surface, their microscopic nature, and their variation in location and path among patients. New technologies capable of identifying the CNs would aid in their preservation and result in improved postoperative sexual function and patient quality-of-life. Temperature-controlled laser systems have been previously tested for laser tissue welding and soldering applications. This preliminary study demonstrates the feasibility of TC-ONS of the rat CNs. In summary, application of higher CW laser power allows the nerve to reach the necessary temperature range for nerve activation more rapidly, thus potentially generating both a faster and stronger ICP response. Both a fast and robust ICP response is necessary if ONS is to be suitable as an intraoperative diagnostic technique in the clinic. However, use of higher CW laser power also results in a continuously escalating nerve temperature and, if left uncontrolled, can eventually produce temperatures that cause thermal damage to the nerve (). On the contrary, conservative use of lower CW laser power will result in an unnecessary delay in the temperature rise in the nerve, a potentially slower ICP response time, and a weaker ICP signal, making the method impractical in the clinic. The prototype TC-ONS system tested in these preliminary studies allowed use of higher laser power for a shorter and faster initial ramp-up time in nerve temperature and ICP response, respectively, while also maintaining the nerve at a constant (), preset temperature for safety. Furthermore, it should be noted that, in general, there is a relatively large matrix of laser parameters that need to be studied during CW and, especially, pulsed modes for ONS (Table 1), making optimization of those techniques unnecessarily complex. Another advantage of TC-ONS is that this technique effectively decreases the number of relevant parameters to a single parameter, the nerve activation temperature, which is critical for successful, reliable, and safe ONS. Since ONS is believed to operate based on a photothermal response in the nerve, it is logical that the primary focus of parameter optimization should be the nerve temperature. Table 1Laser parameters that need to be optimized for pulsed, continuous-wave (CW), and temperature-controlled (TC) nerve stimulation for a given laser wavelength.
One limitation of our preliminary TC-ONS design was the difficulty in precisely calibrating the temperature sensor. While the sensor was chosen due to its small size (for future integration into a probe) and low cost, the sensor had limited temperature resolution and stability. The sensor had a resolution of ~1°C compared to ~0.1°C for the thermal camera. Subtle environmental temperature variances around the sensor casing were also believed to cause a drift of up to a few degrees Celsius in the calibrated temperature reading of the sensor. Improved thermal isolation of the sensor will therefore be necessary to maintain stability before this system is ready for clinical testing. Another limitation is the background radiation seen by the IR sensor. IR radiation emitted by the two-lens optical system (Fig. 2), including IR lenses and an aperture, and the walls of the handle must be accounted for during calibration studies of the radiometer. Use of a single broadband IR sensor also results in temperature measurements which are sensitive to the tissue emissivity (). Because of high water content in soft tissue, it is assumed that . However, the nerve structure is also expected to introduce a nonuniform emissivity distribution. A higher spatial resolution can also be achieved by decreasing the aperture diameter in the two-lens optical system if needed. Finally, future work will also include integration of the temperature sensor and the SMF probe into a single, compact, handheld instrument. 5.ConclusionsThis preliminary study has demonstrated that a prototype TC-ONS system is capable of maintaining a constant and relatively precise nerve temperature, critical for safe and reliable stimulation of the prostate CNs. Further development of this system is warranted to provide improved sensor temperature resolution and stability, given the relatively narrow therapeutic window of temperatures for successful ONS. AcknowledgmentsThis research was supported in part by the U.S. Department of Defense Prostate Cancer Research Program, Grant No. PC073709 and the U.S. Department of Energy, Grant No. DE-FG02-06CH11460. Serhat Tozburun was supported in part by an Optics and Photonics Scholarship from SPIE. The authors would like to thank Dr. James Conrad and Dr. Richard Blackmon for their further assistance with programming the microcontroller used in this study. ReferencesA. L. Burnettet al.,
“Erectile function outcome reporting after clinically localized prostate cancer treatment,”
J. Urol., 178
(2), 597
–601
(2007). http://dx.doi.org/10.1016/j.juro.2007.03.140 JOURDD 0248-0018 Google Scholar
K. PonnusamyJ. M. SorgerC. Mohr,
“Nerve mapping for prostatectomies: novel technologies under development,”
J. Endourol., 26
(7), 769
–777
(2012). http://dx.doi.org/10.1089/end.2011.0355 JENDE3 0892-7790 Google Scholar
S. Raiet al.,
“Advances in imaging the neurovascular bundle,”
Curr. Opin. Urol., 22
(2), 88
–96
(2012). http://dx.doi.org/10.1097/MOU.0b013e3283501826 CUOUEQ 0963-0643 Google Scholar
L. Klotz,
“Neurostimulation during radical prostatectomy: improving nerve-sparing techniques,”
Semin. Urol. Oncol., 18
(1), 46
–50
(2000). Google Scholar
H. L. Kimet al.,
“A positive caver map response poorly predicts recovery of potency after radical prostatectomy,”
Urology, 56
(4), 561
–564
(2000). http://dx.doi.org/10.1016/S0090-4295(00)00748-2 0090-4295 Google Scholar
J. HolzbeierleinM. PetersonJ. A. Smith Jr.,
“Variability of results of cavernous nerve stimulation during radical prostatectomy,”
J. Urol., 165
(1), 108
–110
(2001). http://dx.doi.org/10.1097/00005392-200101000-00027 JOURDD 0248-0018 Google Scholar
P. C. Walshet al.,
“Efficacy of first-generation cavermap to verify location and function of cavernous nerves during radical prostatectomy: a multi-institutional study by experienced surgeons,”
Urol., 57
(3), 491
–494
(2001). http://dx.doi.org/10.1016/S0090-4295(00)01067-0 0090-4295 Google Scholar
J. Wellset al.,
“Application of infrared light for in vivo neural stimulation,”
J. Biomed. Opt., 10
(6), 064003
(2005). http://dx.doi.org/10.1117/1.2121772 JBOPFO 1083-3668 Google Scholar
N. M. Friedet al.,
“Identification and imaging of the nerves responsible for erectile function in rat prostate, in vivo, using optical nerve stimulation and optical coherence tomography,”
IEEE J. Sel. Top. Quant. Electron., 13
(6), 1641
–1645
(2007). http://dx.doi.org/10.1109/JSTQE.2007.910119 IJSQEN 1077-260X Google Scholar
N. M. Friedet al.,
“Non-contact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser,”
J. Endourol., 22
(3), 409
–413
(2008). http://dx.doi.org/10.1089/end.2008.9996 JENDE3 0892-7790 Google Scholar
S. Tozburunet al.,
“A compact laparoscopic probe for optical stimulation of the prostate nerves,”
IEEE J. Sel. Top. Quant. Electron., 16
(4), 941
–945
(2010). http://dx.doi.org/10.1109/JSTQE.2009.2035432 IJSQEN 1077-260X Google Scholar
S. Tozburunet al.,
“Continuous-wave infrared optical nerve stimulation for potential diagnostic applications,”
J. Biomed. Opt., 15
(5), 055012
(2010). http://dx.doi.org/10.1117/1.3500656 JBOPFO 1083-3668 Google Scholar
S. Tozburunet al.,
“Continuous-wave laser stimulation of the rat prostate cavernous nerves using a compact and inexpensive all single mode optical fiber system,”
J. Endourol., 25
(11), 1727
–1731
(2011). http://dx.doi.org/10.1089/end.2011.0172 JENDE3 0892-7790 Google Scholar
S. Tozburunet al.,
“Subsurface near-infrared laser stimulation of the periprostatic cavernous nerves,”
J. Biophoton., 5
(10), 793
–800
(2012). http://dx.doi.org/10.1002/jbio.201100134 JBOIBX 1864-063X Google Scholar
D. M. Quinlanet al.,
“The rat as a model for the study of penile erection,”
J. Urol., 141
(3), 656
–661
(1989). JOURDD 0248-0018 Google Scholar
J. Wellset al.,
“Biophysical mechanisms of transient optical stimulation of peripheral nerve,”
Biophys. J., 93
(7), 2567
–2580
(2007). http://dx.doi.org/10.1529/biophysj.107.104786 BIOJAU 0006-3495 Google Scholar
M. G. Shapiroet al.,
“Infrared light excites cells by changing their electrical capacitance,”
Nat. Commun., 3
(736), 1
–10
(2012). http://dx.doi.org/10.1038/ncomms1742 NCAOBW 2041-1723 Google Scholar
P. Cesareet al.,
“Ion channels gated by heat,”
Proc. Natl. Acad. Sci. U. S. A., 96
(14), 7658
–7663
(1999). http://dx.doi.org/10.1073/pnas.96.14.7658 PNASA6 0027-8424 Google Scholar
J. D. Wellset al.,
“Optical mediated nerve stimulation: identification of injury thresholds,”
Lasers Surg. Med., 39
(6), 513
–526
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
R. B. Stewartet al.,
“Laser assisted vascular welding with real time temperature control,”
Lasers Surg. Med., 19
(1), 9
–16
(1996). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
I. Cilesiz,
“Controlled temperature photothermal tissue welding,”
J. Biomed. Opt., 4
(3), 327
–336
(1999). http://dx.doi.org/10.1117/1.429934 JBOPFO 1083-3668 Google Scholar
B. Foreret al.,
“Repair of pig dura in vivo using temperature controlled CO2 laser soldering,”
Lasers Surg. Med., 37
(4), 286
–292
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
G. Normanet al.,
“In vitro conjunctival incision repair by temperature-controlled laser soldering,”
J. Biomed. Opt., 14
(6), 064016
(2009). http://dx.doi.org/10.1117/1.3262610 JBOPFO 1083-3668 Google Scholar
J. Rehmanet al.,
“Intracavernous pressure responses to physical and electrical stimulation of the cavernous nerve in rats,”
Urology, 51
(4), 640
–644
(1998). http://dx.doi.org/10.1016/S0090-4295(97)00693-6 0090-4295 Google Scholar
G. M. HaleM. R. Querry,
“Optical constants of water in the 200 nm to 200 μm wavelength region,”
Appl. Opt., 12
(3), 555
–563
(1973). http://dx.doi.org/10.1364/AO.12.000555 APOPAI 0003-6935 Google Scholar
|