|
|
1.IntroductionThe near-infrared laser (NIRL) therapy has become an effective method in clinics. The curative effect of NIRL therapy was strongly dependent on the selections of the laser parameters, especially on the optimization of the laser exposures1 and wavelengths,2 and so on. In recent years, researchers have made efforts to improve the quality of laser therapy through exploring the mechanism that laser-induced photobiological effects,3,4 such as laser-induced changes in morphology or electrophysiological function, etc. On this way, the NIRLs had been proved to elicit the action potentials (AP) directly5 in sciatic muscle cells of leopard frog and pyramidal neurons in rats, respectively, without any chemical or physical6 and optogenetic7 pretreatments. Both high-intensity mode locked laser8 and low-intensity pulsed laser5,9 were used in these studies. These facts inspired us to study the fundamental mechanism underlying the 980-nm laser interaction with biological tissue in clinics10 through monitoring the electrophysiological function directly. The previous studies had revealed that when the laser wavelength was above , photothermal effect (PE) would play a major role in mediating the laser interaction with biological cells. This could be confirmed by monitoring the laser-induced solution volume changes around the target cell based on a differential-phase optical coherence tomography (DOCT) technique.11 Later, an open pipette method12 was employed to measure the absorption-driven temperature rises (ATR), and a faster response to temperature rises than the DOCT method was obtained. The most significant progress was achieved by Shapiro et al.13 based on the function variations of a living cell. They reported that the membrane capacitance changes played a fundamental mediation role to couple the PE generated in the extracellular solution to the electrophysiological function changes of cells. Therein, the PE generation in cells’ surrounding area was confirmed by replacing water () in the solution with heavy water (), based on the fact that has approximately a fivefold lower optical absorption coefficient at the laser wavelength of 1889 nm than .14 And, a fine analysis of the asymmetrical properties of the capacitive current has been measured and made this capacitance mechanism even more quantitative.15 Thus, a water-mediated PE mechanism was consistent with the optical absorption properties of the extracellular solution. However, another study16 provided an abnormal result. They reported that even the absorption coefficients in water of the 1440 and 1465-nm laser were very close ( at 1440 nm, and at 1465 nm),14 but in order to pace the younger quail hearts ( days) and older mouse hearts ( days), they were required 200 and 400 mW, respectively. Thus, a pure PE mechanism near the cell surface was insufficient to explain the results. And, thereby, a direct observation on the function of the living cell was required to identify the biological roles of the laser parameters. The motivation of this work was to quantitatively evaluate how the electrophysiological functions of nerve to response the different exposure levels of 980-nm laser irradiation. This could be a reference to the dose selection in some clinic applications, such as cosmetic dermatology, at the corresponding wavelength. Here, the near infrared laser at 980-nm wavelength was employed to irradiate the Spraque–Dawley rat hippocampal neurons in vitro. We chose sodium (Na) channel protein of the neuron cell in vitro to carry out the experiments. This is not only because that Na channel function dominated the AP generation, but also its rapid electrophysiological kinetics can be a fast response to the laser interaction, and thereby can be an initiation for the other following regulations. We showed that the Na current kinetics could be accelerated by laser irradiation, this acceleration effect caused by laser stimulation was determined by both the laser powers and the temperature properties of the Na channel protein. 2.Absorption-Driven Temperature Rises Estimation and the Na Channel Function Monitoring2.1.Theoretical Calculation of the Absorption-Driven Temperature RisesThe temperature rises in the surrounding area of the neuron cell in vitro were theoretically calculated to estimate the effect of laser irradiation. The mathematical model of heat conduction equation was employed to describe the laser-induced ATR generation and given in a cylindrical coordinate as:17 where represents the thermal diffusion coefficient, is the thermal conductivity, is the density of water, and is the heat capacity, represents the thermal source from the laser irradiation, and is the optical absorption coefficient, is the irradiation laser power and obeys the Beer–Lambert law with the initial laser power , is the radius of the effective beam waist.The solutions to Eq. (1) can be solved by using a Gaussian approximation, and can be obtained in consideration of the thermal lens effect.17 The ATR in extracellular solution was derived as Eq. (2) of Ref. 17 and copied here: where is the optical absorption coefficient of water at 980-nm wavelength, is the radius of beam waist at axial distance , is the radius of the fiber core, is the characteristic time of thermal lens, , , and . The laser pulse duration was chosen as , represents the 980-nm infrared laser power.This equation predicted a linear relationship between the laser power and , when there were no remarkable changes in the other parameters under different doses of laser exposures. In order to confirm this deduction, we employed an open pipette method to measure the directly and analyzed the thermal properties of the extracellular solution underlying a 980-nm wavelength infrared laser irradiation. 2.2.Open Pipette Method for Absorption-Driven Temperature Rises MeasurementThe open pipette method was a rapid temperature measurement technique in the liquid environment with a spatial resolution at several micrometers scale.12 This method was carried out by using a microampere current amplifier, such as patch clamp etc., to measure the resistance changes of a glass pipette. In Fig. 1(a), the glass pipette was filled with electrolyte solution and formed a several mega-ohm (MOhm) resistance, typically 4–6 MOhm. With a synchronized laser irradiation, the local temperature changes will cause a resistance variation in the pipette. It was this temperature-dependent pipette resistance that was able to respond to the PE at an accuracy of 0.1 ms in time, which was determined by the patch clamp, and micrometer resolution in space, which was given by the diameter of the pipette tip, about . Then, the measured pipette resistance was calibrated as a function of the ATR in solution.12,18,19 And it is typically about 4–6 MOhm at room temperature, which depends on the shape of the pipette tip and if it can be directly measured by a patch clamp system. The patch clamp generated a specific voltage at 100 mV, which was applied to the pipette tip, and the corresponding current was read to calculate the resistance. This resistance will be changed according to the temperature variations of the solution. The temperature was controlled by using a temperature controller (TC-324B, Warner), by which the extracellular solution temperature was controlled. The calibration was carried out with an increase and decrease corresponding to the temperature changes from 25°C to 55°C for several times. And, the variations of the solution temperature were controlled within the accuracy of 0.1°C from the basic room temperature 25°C. The conductance of pipette can be approximately expressed as:20 , where is the solution conductance, is the resistivity determined by the open pipette, is the temperature, is the solution conductance at , is the temperature coefficient of solution conductance. Thus, we can relate the temperature rises to the pipette resistance. In an open pipette method, the cell was absent, but the virtual cell position can be found by moving the pipette tip about away from the optical fiber tip to the cell position. Thus, the cell position can be estimated based on the geometric relationship between the irradiation fiber and the cell as presented in Fig. 1(b). Fig. 1The infrared laser was synchronized with the patch clamp for experiments. (a) The open pipette method, and (b) the geometric relationship between the irradiation optical fiber and the target cell in the mediation extracellular solution indicated by the light pink color. The three blue labels indicate the successive monitoring variables from the infrared laser input to the ATR, which was measured by in the extracellular solution and finally examined by the Na channel function changes. is the radius of the beam waist, which was calculated based on the geometric relationship. Numerical aperture (NA) of the fiber is , the refractive indexes are 1.46 for the fiber core and 1.33 for water, the measured tilt angle of the fiber is , and the diameter of the single mode fiber (SMF-28 ) fiber core is about , respectively. And was corresponding to the axial distance from the end of the SMF-28 fiber to the cell position. Then, the expanded beam diameter can be calculated as: , where the divergence angle is obtained by , and it is . 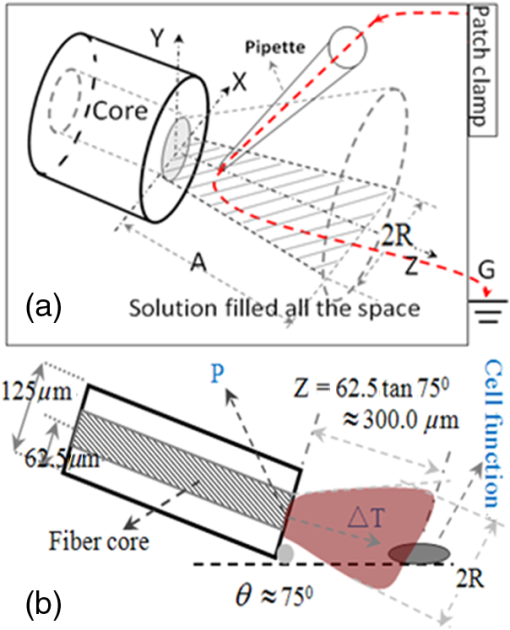 2.3.Monitoring of Infrared Laser Modulation on Na Channel FunctionThe hippocampal neuron cell was acutely dissociated from 7- to 10-day-old Spraque-Dawley rats. The bath solution contained: 150 mM NaCl, 5 mM KCl, 1.1 mM , 2.6 mM , 10 mM HEPES, and 10 mM Glucose (pH 7.4). The pipette solution contained: 65 mM KCl, 80 mM KF, 5 mM KOH, 2 mM , 10 mM EGTA, and 10 mM HEPES (pH 7.4) and 6-mM tetraethylammonium (TEA; pH 7.4) were used to block the voltage-dependent potassium channels. The voltage-dependent Na channel protein in hippocampal neuron was the studied protein.21 As shown in Fig. 1(b), the neuron was irradiated by a 980-nm infrared laser. The laser light was generated by a laser unit (LU0980M180, Lumics, Berlin, Germany) that was synchronized by using a patch-clamp amplifier at an accuracy of 0.1 ms. The output power and duration of 980-nm laser was controlled by a laser diode controller (ITC510, Thorlabs, Newton, New Jersey), as used in Refs. 18 and 19. The system design implemented the transient adjustment of laser power through software programming. The laser light was propagated on to the cell surface through a SMF-28 fiber. The fiber was mounted on an angle-adjustable universal joint, which was controlled by a motorized manipulator (MS314, World Precision Instrument). The Na current of neuron was employed to represent the Na channel function and recorded in whole-cell configuration by a HEKA EPC-10 patch clamp. The three blue labels in Fig. 1(b) represented the three successive monitoring variables, which were the incident 980-nm infrared laser power , the ATR in extracellular solution, and the recorded Na channel function, respectively. 3.ResultsThe protocol for the electrophysiological experiment was presented in Fig. 2(a). The depolarization voltage, which means the intracellular potential when the extracellular solution was grounded in the most cases, was divided into three segments, “control,” “laser,” and “recovery,” respectively. In the control segment, a Na current was elicited corresponding to the normal electrophysiological condition of neurons; in the laser segment, the Na current was recorded right after a 500 ms-duration 980-nm wavelength laser irradiation in order to escape the other laser effect in a large part and in the recovery segment, the Na current was elicited to study the ability of the neuron cell function recovery from laser irradiation. Fig. 2The experimental design for recording the Na channel response to infrared laser stimulation. (a) The protocol for depolarizing voltage and laser irradiation in whole cell patch clamp recording. The top scheme shows the protocol of the depolarizing voltage, laser duration, and the three contrast segments of “control,” “laser,” and “recovery,” respectively. The holding voltage was set as , and the depolarized voltage jumped from to 0 mV with a 5-mV increment. The “500 ms Laser on” is indicated by a light pink color rectangle and the configuration of 500-ms laser irradiation applied on the cell with the control, laser, and recovery segments was presented. (b) The fitting curve of the Na current in the control, laser, and recovery segments. In order to compare the laser effect on the Na currents, the whole cell Na current were drawn in the same time axis. The Na current curves at in the control, laser, and recovery segments, respectively, were fit by the HH model . The inserted table demonstrates that the laser irradiation accelerated the time constant of the sodium channel current from in the control segment to in the laser segment, and in the recovery segment . And the changes in were also found. 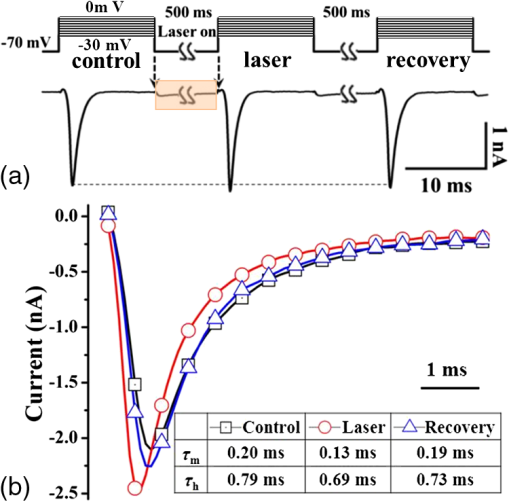 As shown in Fig. 2(b), the Na currents were obtained under depolarization potential in the three segments, respectively, and were plotted in the same coordinate in order to make a comparison. The Na current with a laser irradiation was significantly different from that in control condition. The Na currents were fitted by the HH model18 to extract the time constants, which were used to quantitatively represent the Na current kinetics. The HH model was expressed as: where represented the maximum Na current, and are the time constants of Na current activation and inactivation corresponding to the Na permeability increase and decrease, respectively. And the time constant, , of Na current activation was employed to represent the changes in Na current kinetics under laser irradiation. In order to determine the exposure dependency of infrared laser modulation on neuron cell, the powers of irradiation laser were chosen as 10, 43, 72, and 100 mW, respectively. The applied depolarization voltages were jumped from to 0 mV with a 5 mV-step increment. The time constants of activation were extracted from Na currents in the three segments as the protocol given in the Fig. 2(a) (), and they were plotted in Fig. 3 as a function of both the membrane potentials and the 980-nm infrared laser exposures. The membrane potential was equivalent to the intracellular potential when the extracellular solution was grounded.Fig. 3The time constants of Na current activation underlying control, laser, and recovery conditions were plotted as a function of cell membrane potentials, with to 0 mV with a 5-mV step increments, respectively. The 980-nm laser irradiations were applied in the corresponding laser segments with the laser powers of 10, 43, 72, and 100 mW, respectively. 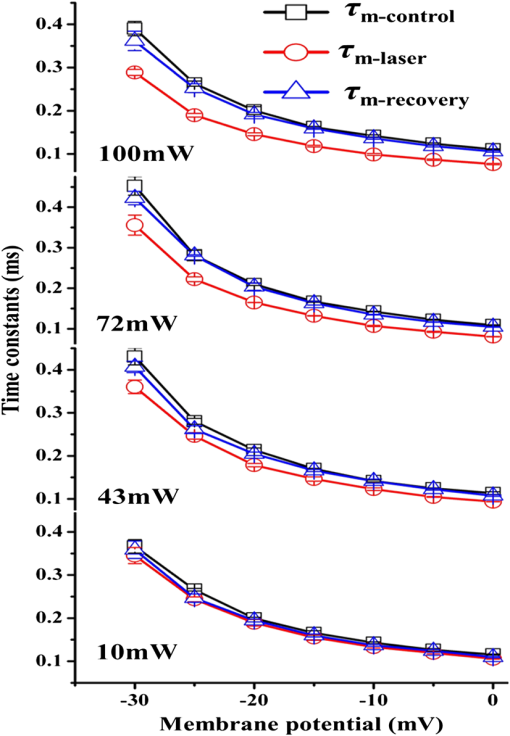 We found that the laser-induced changes in time constants would be accelerated as the 980-nm laser exposures increased from 10 to 100 mW. In order to quantify this exposure-dependent effect, a ratio of time constants between laser and control segments, -laser/-control, was taken to measure the laser modulation effect on Na current kinetics. This ratio was also acted as a function of both the laser exposures and membrane potentials, which ranged from 10 to 100 mW and from to 0 mV with a 5 mV-step increment, respectively, as given in Fig. 4 (). A dependency between the ratio variations and the laser exposures was observed. Based on the experimental results given in Fig. 4, no significant changes were observed in the ratios at the different membrane potentials. Hence, we took an average on the ratios underlying each exposure of 980-nm laser irradiation. For example, the red dashed line in Fig. 4 represented that the averaged ratio of the time constants was 0.85 when the 980-nm laser exposure was at 43 mW. Fig. 4The ratios of time constants, , were obtained under laser irradiation at 10, 43, 72, and 100 mW, and the depolarization membrane voltage ranged from to 0 mV. The ratios of time constants were approximately independent of the membrane potentials, but nearly linear dependent on the 980-nm laser power. The red dashed line indicates that the averaged ratio of the time constants generated by a 43 mW 980-nm laser was about 0.85 (). 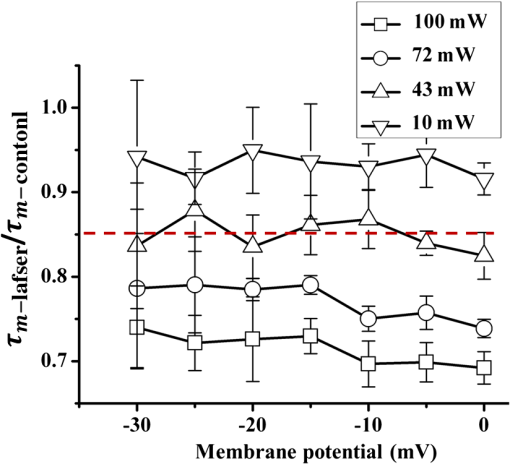 According to the temperature characteristic of Na channel protein, these averaged ratios were able to be related to the surrounding temperature of neuron cell in a quantitative way by choosing the of Na channel protein:22,23 where, and represented the surrounding temperature of neuron cell in the control and laser segment, respectively. And the temperature rise can be a measure of the corresponding ATR.In Fig. 5(a), the dotted line demonstrated the theoretical results of the temperature rise , which was calculated based on the heat conduction equation as given by Eq. (2). In order to obtain the experimental temperature rise based on an open pipette method, the equivalent principle18 was introduced by considering the areas of the light pink rectangles as shown in Fig. 5(b), which was equivalent to their correspondingly overlapped dashed areas determined by the time span of Na current activation, from 0.5 to 1.6 ms. And then, the average ATR in this time span was estimated. As shown in Fig. 5(a), the dash-dotted line showed the temperature rise measured by the open pipette method.12,18 After putting the two s, from Eq. (2) and measured by the open pipette method separately, into Eq. (4), we obtained the averaged ratios corresponding to the different laser exposures. The results were marked as “based on Eq. (2)” and “based on the open pipette,” respectively, in Fig. 5(a). When compared, these two averaged ratios with the ratios obtained from the HH model fitting of the measured Na currents, we found a good match between these three results while the power of 980-nm laser increased from 10 to 100 mW. And an approximation linear relationship existed in the interaction between the 980-nm laser exposures and the ATR generation in the extracellular solution. Fig. 5(a) The averaged ratio of time constants, , obtained from the HH model fitting and calculations based on Eq. (4) are shown and are well linearly dependent on the 980-nm laser power chosen as 10, 43, 72, and 100 mW, respectively. And the ATRs that were measured by the open pipette method12,18 and calculated based on the heat conduction equation, respectively, are presented and matched with each other. (b) The temperature rises, which were caused by 10, 43, 72, and 100 mW laser irradiations at 980-nm wavelength, respectively, were measured by an open pipette method. The averaged ATRs were estimated corresponding to the time span of Na current activation, from 0.5 to 1.6 ms, as used in Ref. 18, based on the equivalent principle. The equivalent means that the areas of the light pink rectangles equal to their overlapped dashed areas, which are determined by the span of the Na current activation. The average temperatures corresponding to the activation read as 3.72°C, 2.54°C, 1.41°C, and 0.32°C, respectively, as the upper edge of the light pink color rectangular indicates, and the results are shown in Fig. 5(a) to compare with those calculated from Eq. (2). The time point “0” was represented as the point of the laser irradiation being turned off and simultaneously initiated a depolarization voltage to elicit Na current. 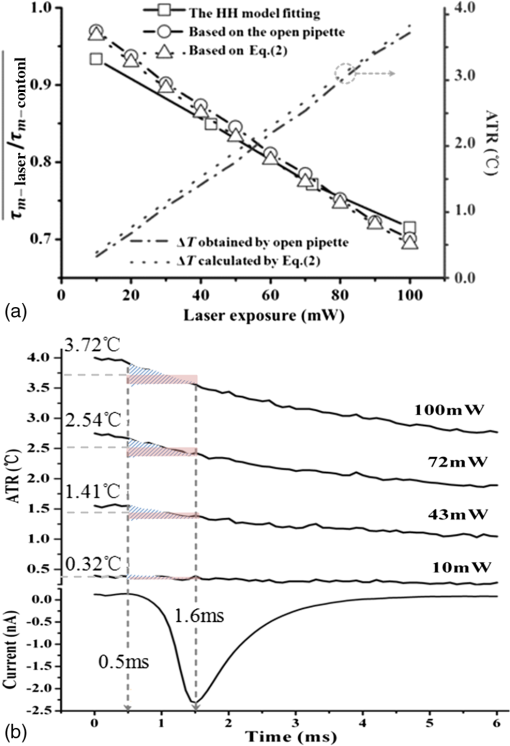 In order to analyze the intrinsic characteristic underlying the interaction between laser irradiation and Na current kinetics, the data underlying the two ATR lines shown in Fig. 5(a) were plotted in Table 1. If we chose the initial input of the infrared laser exposures as , the corresponding output of the ATR was as measured in Fig. 5(b). Then, the second column in Table 1 demonstrated that the laser exposures shown in the first column was partitioned into the product of initial value 10 mW with a scale , which were highlighted in red color in the third column. After making a similar processing for the output of the ATR based on the initial value , the corresponding scale can be achived in the last column. When compared with the two scales, and , we found they were quite close and determined a linearity between the NIRL exposures and the ATRs generation in the extracellular solution. Table 1A linear relationship between the scales of the infrared laser exposures and that of the absorption-driven temperature rises (ATR) generation in the extracellular solution.
Note: The bold numbers demonstrate the linear relationship between the scales of the infrared laser exposures and that of the ATR generation in the extracellular solution. Thus, a two-step model can be successively summarized in Fig. (6) to describe the process that 980-nm infrared laser induced the ATR in the extracellular solution and the neuron cell responded to this ATR. The infrared laser interaction with the neuron cell in vitro can be partitioned into two steps. In the first step, a linear mediation property between the responsive ATR in extracellular solution and the laser exposures was obtained. And second, the ATR modulated the cell function according to the temperature properties of Na channel protein. The linear property could help us to find an optimized exposure of laser irradiation. The mechanism of modulation on the Na channel function by temperature rise could guide the choice of laser parameters in clinic therapy based on the optical absorption properties of the target biological tissue. Fig. 6A two-step schematic underlying the interaction of the infrared laser with the neuron cell in vitro was presented to describe the relationship between the exposures of the infrared laser, the ATR generated in the extracellular solution, and the averaged ratios of the time constant. The schematic demonstrates that the linear relationship between the scales of the infrared laser exposures and that of the ATR generation in the extracellular solution exists. 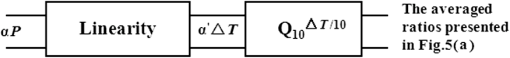 4.DiscussionIn this article, a two-step model was proposed to describe the interaction of the 980-nm laser with a neuron cell in vitro. The laser irradiation generated an ATR in the extracellular solution, and then this ATR affected on the neuron Na channel function. The ATR was both obtained by theoretically solving the heat conduction equation24 and measured by using an open pipette method. The Na current of neuron cell in vitro was recorded by using a patch clamp technique in synchronization with a 980-nm laser irradiation in the accuracy of 0.1 ms. The activation time constants, , were calculated by fitting the Na currents with Hodgkin–Huxley (HH) model.25 Then, the modifications of the Na channel function corresponding to the laser irradiations were quantified. The ratio between the two time constants, that with and without laser irradiations, was calculated. Then this averaged ratio was related to the temperature characteristics of the Na channel protein when chosen its .22,23 This result showed that the Na current activation kinetics was accelerated by 980-nm laser irradiation, and this effect could be ascribed to the average ATR in extracellular solution. The averaged ATR was determined by the optical absorption properties of 980-nm laser in water. The following analysis revealed that the averaged ratio at a specific laser exposure dose could be well related to the temperature properties of the Na channel protein. Finally, these results proved that the 980-nm laser exposure-dependent modulation of Na current kinetics in the neuron cell in vitro was linearly mediated corresponding to the PE in the cell surrounding area. 5.ConclusionsThe significance of this result was experimentally explored how the Na channel protein responded to exposure-dependent 980-nm near-infrared laser irradiation in vitro. A two-step model presented that a linear mediation property existed between the responsive ATR in extracellular solution and the laser exposures in the first step, and the averaged ATR could affect the Na channel functions according to the measured of this membrane protein. This quantitative result could be a good reference for the laser-based clinic treatment, especially for the nerve-based laser therapy, such as relieving short-term pain for rheumatoid arthritis,26 osteoarthritis,27 acute and chronic neck pain,28 and the treatment of diabetic wound healing29 etc., because that pain feeling was conventionally ascribed to the Na channel function.21 Therefore, these experimental results presented that there was a quantitative dependency of the Na current kinetics in neuron cell in vitro on the 980-nm laser exposures from 10 to 100 mW, and this relationship was linearly mediated corresponding to the laser-induced PE. This experimental result could provide a reference to optimize the selection of the NIRL exposures based on the optical absorption properties of the regional tissue, such as water content etc., during the nerve-related therapy. AcknowledgmentsThis study was financially supported by the National Natural Science Foundation of China (No. 31070757, No. 31370835) and Fundamental Research Funds for the National Universities (No. DUT12ZD210). The authors thank the reviewers for their valuable comments. ReferencesL. J. Boulnois,
“Photophysical processes in recent medical laser developments: a review,”
Lasers Med. Sci., 1
(1), 47
–66
(1986). http://dx.doi.org/10.1007/BF02030737 LMSCEZ 1435-604X Google Scholar
B. Wassmeret al.,
“Comparative study of wavelengths for laser lipolysis,”
Photomed. Laser Surg., 28
(2), 185
–188
(2010). http://dx.doi.org/10.1089/pho.2008.2480 PLDHA8 1549-5418 Google Scholar
S. L. Jacques,
“Laser-tissue interactions. Photochemical, photothermal, and photomechanical,”
Surg. Clin. North Am., 72
(3), 531
–558
(1992). SCNAA7 0039-6109 Google Scholar
C. A. Soareset al.,
“Photobiological effect of low-level laser irradiation in bovine embryo production system,”
J. Biomed. Opt., 19
(3), 035006
(2014). http://dx.doi.org/10.1117/1.JBO.19.3.035006 JBOPFO 1083-3668 Google Scholar
J. Wellset al.,
“Optical stimulation of neural tissue in vivo,”
Opt. Lett., 30
(5), 504
–506
(2005). http://dx.doi.org/10.1364/OL.30.000504 OPLEDP 0146-9592 Google Scholar
K. Lugoet al.,
“Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots,”
Biomed. Opt. Express, 3
(3), 447
–454
(2012). http://dx.doi.org/10.1364/BOE.3.000447 BOEICL 2156-7085 Google Scholar
F. Zhanget al.,
“Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures,”
Nat. Protoc., 5
(3), 439
–456
(2010). http://dx.doi.org/10.1038/nprot.2009.226 NPARDW 1754-2189 Google Scholar
H. Hiraseet al.,
“Multiphoton stimulation of neurons,”
J. Neurobiol., 51
(3), 237
–247
(2002). http://dx.doi.org/10.1002/(ISSN)1097-4695 JNEUBZ 0022-3034 Google Scholar
J. Wellset al.,
“Application of infrared light for in vivo neural stimulation,”
J. Biomed. Opt., 10
(6), 064003
(2005). http://dx.doi.org/10.1117/1.2121772 JBOPFO 1083-3668 Google Scholar
H. O. TabakogluN. TopalogluM. Gulsoy,
“The effect of irradiance level in 980-nm diode laser skin welding,”
Photomed. Laser Surg., 28
(4), 453
–458
(2010). http://dx.doi.org/10.1089/pho.2009.2569 PLDHA8 1549-5418 Google Scholar
J. Wellset al.,
“Biophysical mechanisms of transient optical stimulation of peripheral nerve,”
Biophys. J., 93
(7), 2567
–2580
(2007). http://dx.doi.org/10.1529/biophysj.107.104786 BIOJAU 0006-3495 Google Scholar
J. YaoB. LiuF. Qin,
“Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies,”
Biophys. J., 96
(9), 3611
–3619
(2009). http://dx.doi.org/10.1016/j.bpj.2009.02.016 BIOJAU 0006-3495 Google Scholar
M. G. Shapiroet al.,
“Infrared light excites cells by changing their electrical capacitance,”
Nat. Commun., 3
(3), 736
(2012). http://dx.doi.org/10.1038/ncomms1742 NCAOBW 2041-1723 Google Scholar
D. M. WieliczkaS. WengM. R. Querry,
“Wedge shaped cell for highly absorbent liquids: infrared optical constants of water,”
Appl. Opt., 28
(9), 1714
–1719
(1989). http://dx.doi.org/10.1364/AO.28.001714 APOPAI 0003-6935 Google Scholar
Q. Liuet al.,
“Exciting cell membranes with a blustering heat shock,”
Biophys. J., 106
(8), 1570
–1577
(2014). http://dx.doi.org/10.1016/j.bpj.2014.03.008 BIOJAU 0006-3495 Google Scholar
T. Y. Wanget al.,
“Optical stimulation enables paced electrophysiological studies in embryonic hearts,”
Biomed. Opt. Express, 5
(4), 1000
–1013
(2014). http://dx.doi.org/10.1364/BOE.5.001000 BOEICL 2156-7085 Google Scholar
H. Cabreraet al.,
“A thermal lens model including the Soret effect,”
Appl. Phys. Lett., 94
(5), 051103
(2009). http://dx.doi.org/10.1063/1.3078287 APPLAB 0003-6951 Google Scholar
X. Liet al.,
“Temporal modulation of sodium current kinetics in neuron cells by near-infrared laser,”
Cell Biochem. Biophys., 67
(3), 1409
–1419
(2013). http://dx.doi.org/10.1007/s12013-013-9674-9 CBBIFV 1085-9195 Google Scholar
S. Lianget al.,
“Temperature-dependent activation of neurons by continuous near-infrared laser,”
Cell Biochem. Biophys., 53
(1), 33
–42
(2009). http://dx.doi.org/10.1007/s12013-008-9035-2 CBBIFV 1085-9195 Google Scholar
W. K. Pratt,
“Proposed new electrolytic conductivity primary standards for KCl solutions,”
J. Res. Natl. Inst. Stand. Technol., 96 191
–201
(1991). http://dx.doi.org/10.6028/jres.096.008 JRITEF 1044-677X Google Scholar
W. Greffrathet al.,
“Inward currents in primary nociceptive neurons of the rat and pain sensations in humans elicited by infrared diode laser pulses,”
Pain, 99
(1–2), 145
–155
(2002). http://dx.doi.org/10.1016/S0304-3959(02)00071-4 PAINDB 0304-3959 Google Scholar
G. E. KirschJ. S. Sykes,
“Temperature dependence of Na currents in rabbit and frog muscle membranes,”
J. Gen. Physiol., 89
(2), 239
–251
(1987). http://dx.doi.org/10.1085/jgp.89.2.239 JGPLAD 0022-1295 Google Scholar
C. L. Schauf,
“Temperature dependence of the ionic current kinetics of Myxicola giant axons,”
J. Physiol., 235
(1), 197
–205
(1973). JPHYA7 0022-3751 Google Scholar
M. J. van GemertG. W. LucassenA. J. Welch,
“Time constants in thermal laser medicine: II. distributions of time constants and thermal relaxation of tissue,”
Phys. Med. Biol., 41
(8), 1381
–1399
(1996). http://dx.doi.org/10.1088/0031-9155/41/8/009 PHMBA7 0031-9155 Google Scholar
A. L. HodgkinA. F. Huxley,
“A quantitative description of membrane current and its application to conduction and excitation in nerve,”
J. Physiol., 117
(4), 500
–544
(1952). JPHYA7 0022-3751 Google Scholar
S. M. Meireleset al.,
“Assessment of the effectiveness of low-level laser therapy on the hands of patients with rheumatoid arthritis: a randomized double-blind controlled trial,”
Clin. Rheumatol., 29
(5), 501
–509
(2010). http://dx.doi.org/10.1007/s10067-009-1347-0 CLRHD6 1434-9949 Google Scholar
G. Jamtvedtet al.,
“Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews,”
Phys. Ther., 88
(1), 123
–136
(2008). http://dx.doi.org/10.2522/ptj.20070043 0031-9023 Google Scholar
R. T. Chowet al.,
“Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials,”
Lancet, 374
(9705), 1897
–1908
(2009). http://dx.doi.org/10.1016/S0140-6736(09)61522-1 LANCAO 0140-6736 Google Scholar
J. P. Da Silvaet al.,
“Laser therapy in the tissue repair process: a literature review,”
Photomed. Laser Surg., 28
(1), 17
–21
(2010). http://dx.doi.org/10.1089/pho.2008.2372 PLDHA8 1549-5418 Google Scholar
BiographyXinyu Li received his BS degree in applied physics from Dalian University of Technology in 2008. Now, he is a PhD candidate in the Lab of Biomedical Optics, Dalian University of Technology. His research interests are in the areas of laser interaction with biological tissue and low-coherence optical interferometry. Jia Liu received her BS and MS degrees in biology from Liaoning Normal University, China, in 2007 and 2010, respectively. Now, she is a PhD candidate in the Lab of Biomedical Optics, Dalian University of Technology. Her research interest is laser interaction with biological tissue. Shanshan Liang received her BS degree in applied physics in 2007, and received her PhD degree in biomedical optics in 2013 from Dalian University of Technology. Now, she is a postdoctoral researcher at Beckman Laser Institute, University of California, Irvine. Her research interests include optical coherence tomography, fluorescence imaging, photoacoustic imaging systems, and laser interaction with biological tissue. Changsen Sun received his BS and MS degrees in electronic engineering, Jinlin University of Technology, in 1989 and 1992, respectively. His received his PhD degree in optical engineering at the Dalian University of Technology in 1999. Now, he is a professor in the College of Physics and Optoelectronic Engineering, Dalian University of Technology, China. His interests include laser interaction with biological tissue and low-coherence optical information processing. |
||||||||||||||||||||||||||||||||||||

