|
|
1.IntroductionSkin aging is the continuous change of the skin’s structure and functional capacity. In addition to the “normal” course of intrinsic or chronological aging, environmental (e.g., sun light) and life style (e.g., smoking) factors contribute to the so-called extrinsic skin aging. Skin areas that are predominantly exposed to ultra violet radiation (e.g., face, hands, and dorsal aspect of the forearm) are prone to develop various signs of extrinsic aging. These include deep wrinkles, loss of elasticity, and changes in skin pigmentation. Histologically, extrinsically aged skin shows degenerated collagen and elastic fibers in the upper and middermis, which is referred to as solar elastosis.1–3 Facial appearance contributes to the perceived age of individuals and is an indicator of overall health.4,5 Thus, there is growing interest in skin aging research and so-called antiaging strategies. The evaluation of cosmetic products and rejuvenation procedures is often based on histological assessments that provide knowledge of the morphological and molecular characteristics of the skin.6,7 However, the invasive procedure to obtain biopsies contains disadvantages, such as tissue destruction, pain and an increased risk for infections. Optical coherence tomography (OCT) allows a noninvasive and rapid cross-sectional visualization of the skin. It is a widely applied imaging method in many fields of dermatology,8,9 for instance, to follow up wound healing, to diagnose skin cancer, or to assess the effectiveness of skin care products.10–13 To date, only a few studies have used OCT to investigate skin aging. The most often reported parameter in skin aging research measured in OCT images is epidermal thickness (ET). ET has been often observed to decrease with age on intrinsically and extrinsically aged skin areas.14–16 However, OCT images obviously provide much more information than ET only. Therefore, the idea emerged, whether there are other traits or criteria that might be used to characterize signs of skin aging in OCT images. The aim of this study was to develop and to evaluate possible additional parameters to measure skin aging using OCT. 2.Methods2.1.Study Design and ParticipantsAn exploratory validation study was conducted at the Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charité-Universitätsmedizin Berlin, Germany, between January and April 2014. Caucasian women of two age groups with healthy skin were recruited. Written informed consent was obtained from all subjects before participation. They were instructed not to use any cosmetic products on their arms at least 12 h prior to the measurements. 2.2.OCT MeasurementsThe OCT system Telesto (Thorlabs, Lübeck, Germany) was used in this study for in vivo evaluation of skin aging. This measurement method is based on the detection of the optical path length differences of reflected and backscattered light of a sample and a reference beam (interferometry). The OCT system contains a low-coherence near infrared (about 1300 nm) light source and a spectrometer that detects the phase delay of the wavelengths. The hand-held OCT probe was positioned directly onto the skin. Two-dimensional images were acquired with a scan length of around 10 mm, a lateral resolution of up to , and a maximum penetration depth of around 1.5 mm. ET was measured using the software ImageJ and a plug-in developed by Thorlabs. ET measurements were performed on three predefined measurement sites (center of the image and two lateral sites with a fixed distance to the center) by height-width titration, which is the length of a vertical line against the image per se.16 The average ET was calculated from these three measurements. All ET measurements were made by two investigators blinded to the participants’ ages and skin areas. 2.3.OCT Items and Score DevelopmentIn order to develop possible detectable characteristics of skin aging, a literature review was conducted in Medline. Studies that applied different skin imaging techniques, such as sonography,17 histology,10 and laser scanning microscopy,18 as well as studies reporting features of skin morphology,19 wound healing,10,20 and pressure ulcer development17 were reviewed. Based on these reports, seven possible characteristics were developed inductively and iteratively by the authors. The items were discussed with a physician and a researcher, both experienced in OCT imaging, and further adapted. The preliminarily determined items were defined as follows: (1) “stratum corneum (SC) reflectivity,” (2) “epidermal reflectivity,” (3) “upper dermal reflectivity,” and (4) “lower dermal reflectivity.” These four “reflectivity” items describe the intensity of the signal detected by the camera backscattered from the respective tissues. (5) “Dermoepidermal contrast” describes the ability to distinguish between epidermis and dermis. (6) “Vessel density” is the absence or presence of dermal vessels, which were identified as elongated hyporeflective structures in the lower dermis. (7) “Surface unevenness” was defined as the degree to which the skin surface appears to be uneven or folded. In a next step, OCT images taken from the right inner upper arm, right volar forearm, and right dorsal forearm of each participant were evaluated by three raters experienced in OCT imaging (two blinded to the participants’ ages and one unblinded author) independently from each other. The raters were trained by the first author on item scale definitions using example images not included in the current analysis. Each item was graded using a four-point scale (0, absent; 1, low; 2, moderate; 3, high), except “vessel density,” which was graded using a four-point scale ranging from 0 = no vessels to 3 = many vessels. This four category classification was applied to avoid a neutral position and to ensure differentiation.21 2.4.Criterion Validation of the Item “Surface Unevenness”Criterion validity of the item “surface unevenness” was explored by correlating the score with instrumental measures of the skin microtopography of the identical skin areas. The Visioscan® VC 98 camera and the corresponding software SELS 2000 (both Courage & Khazaka Electronic GmbH, Cologne, Germany) were used to measure the DIN/ISO parameters (arithmetic mean roughness from five consecutive sampling lengths) and (maximal roughness). Both parameters were chosen because they exhibited the highest reliability in comparable age groups.22,23 Roughness measurements were conducted in a controlled room temperature of and relative humidity and after the participants’ acclimatization for at least 30 min. 2.5.Statistical AnalysisDemographic characteristics of the sample were described using means, standard deviations (SD), ranges, and frequencies. OCT item scores were analyzed descriptively using medians and interquartile ranges. Interrater agreement () of item scores between the three raters was calculated. This expresses the proportion in percent of which the three raters achieved identical results per OCT image.24 Construct validity was evaluated using Mann–Whitney tests to compare medians of the item scores between the two age groups per skin area. ET and skin surface roughness were presented as means and SDs. Group comparisons were performed using student’s t test for independent samples. Criterion validity was tested using Spearman’s correlation coefficients () between the OCT item score “surface unevenness” and skin roughness measurements and . A -value of (two-sided) was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics, Version 22.0 (IBM Corp., Armonk, New York). 3.Results3.1.Sample CharacteristicsDemographic characteristics of the study sample are summarized in Table 1. Sixteen female subjects were included in the validation study, eight subjects in the young group [mean age 33.5 (SD 2.1) years] and eight subjects in the old group [mean age 76.6 (SD 1.9) years]. Mean body mass indices were and . Equal distributions of Fitzpatrick phototypes II and III were observed in both groups. Table 1Sample characteristics.
Note: BMI, body mass index and SD, standard deviation. 3.2.Epidermal ThicknessMean ET ranged from on the dorsal forearm to on the inner upper arm of the young group (Table 2). Differences between age groups were not statistically significant. Table 2Epidermal thickness (μm) measured in optical coherence tomography (OCT) images.
3.3.OCT Item ScoresAll seven items were detected and could be quantified in our sample. Figure 1 shows the OCT images representing the lowest (left column) and highest (right column) mean score per item. Fig. 1Optical coherence tomography images selected among the 16 participants representing the lowest and highest mean scores per item and image. Mean scores are the averages of the scores of three raters. Scale bar . 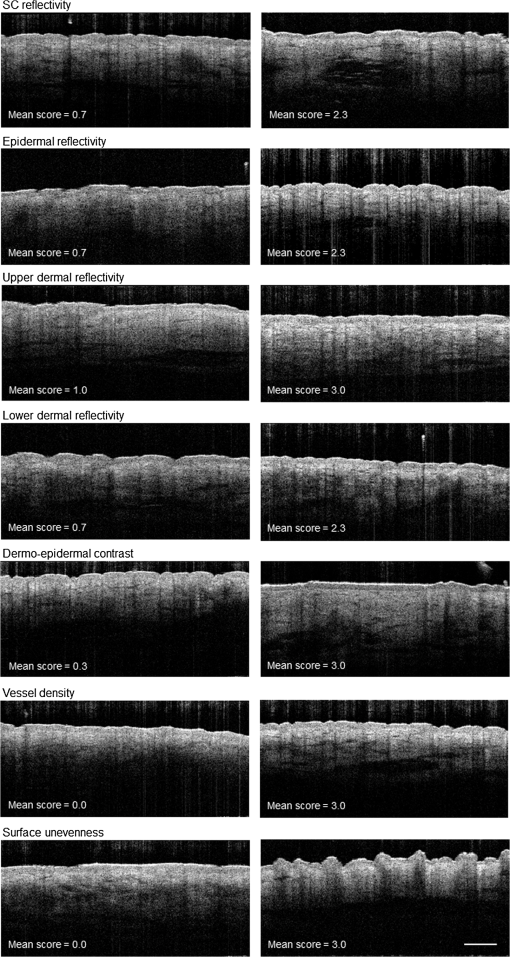 3.3.1.Construct validation: skin agingThe grading results of the OCT images are shown in Table 3. Median scores ranged from 0.8 for the item “surface unevenness” to 2.5 for the item “upper dermal reflectivity.” No floor and ceiling effects were observed. Table 3Grading results of the developed OCT items.
Note: x˜, median; IQR, interquartile range; min, minimum; max, maximum; po, interrater agreement; and SC, stratum corneum. Median “SC reflectivity” was higher in older subjects on all three skin areas with differences being statistically significant for the forearm sites. “Upper dermal reflectivity” was significantly lower in the old group on both forearm areas. “Dermoepidermal contrast” and “vessel density” were higher in the old group on the forearm and comparable on the inner upper arm. “Epidermal reflectivity” and “lower dermal reflectivity” were reduced in the old group without reaching statistical significance. “Surface unevenness” increased with age on the inner upper arm and volar forearm and decreased on the dorsal forearm. Differences between the skin areas were observed for “dermoepidermal contrast,” “vessel density,” and “surface unevenness.” Other item scores like “lower dermal reflectivity” and “upper dermal reflectivity” showed minor differences between the skin areas. 3.3.2.Interrater agreementInterrater agreement is presented in Table 3. The highest proportions of agreement were obtained for the items “SC reflectivity,” “upper dermal reflectivity,” “dermoepidermal contrast,” and “surface unevenness” (). The lowest proportion of agreement were observed for the item “epidermal reflectivity” (). 3.3.3.Criterion validation: skin microtopographyThe mean surface roughness parameters of the two age groups are presented in Table 4. ranged from on the volar forearm in the young group to on the dorsal forearm in the old group. ranged from on the volar forearm of the young group to on the dorsal forearm of the old group. SDs were always higher in the aged group. Differences between the age groups were statistically significant on the inner upper arm and volar forearm. In both age groups, surface roughness was highest on the dorsal forearm. Spearman correlation coefficients of the item scores of “surface unevenness” were highest with on the inner upper arm and on the volar forearm ( and ). The lowest correlation coefficients were observed for and on the dorsal forearm ( and ). Table 4Skin surface roughness parameters Rz (μm) and Rmax (μm) measured using Visioscan® VC98.
4.DiscussionThis study introduces additional characteristics besides ET to measure skin aging in OCT images. In a sample of young and aged women, different sun-exposed and sun-protected skin areas were measured. Based on the literature and our own experience with OCT imaging, we inductively developed seven potentially observable skin characteristics. The most common parameter measured in OCT images is ET, for which good correspondence with confocal laser scanning microscopy has been reported.15 The ET estimates obtained in our study were comparable with previously reported results.14,16 We were able to detect a slight thinning of the epidermis with age on the inner upper arm that confirms the results of previous studies.14,25 Nevertheless, ET thinning tends to occur considerably only up to the 30s16 and seems to be attenuated by the effects of photoaging.14,15,19 All newly proposed characteristics were detected and could be quantified in our sample. The items had an overall good distribution of the scores and showed no floor and ceiling effects. This indicates that the item scores were suitable to discriminate between different skin characteristics. Interrater agreement was high for the items “SC reflectivity,” “upper dermal reflectivity,” “dermoepidermal contrast,” and “surface unevenness,” indicating that these items were easier to grade leading to similar rating results. By contrast, agreement among the items “epidermal reflectivity” and “lower dermal reflectivity” was lower indicating that these items might be inadequate for assessing skin aging. Study results demonstrated that there are age- and site-dependent differences regarding the item scores. We have chosen the construct “skin aging” to evaluate the validity of the rating results. In intrinsically aged skin, the SC turnover rate is much slower26 and corneocytes are larger.27,28 Moreover, older subjects have a reduced amount of intercellular lipids.29 These age-dependent changes may lead to alterations in the optical properties of the SC by affecting the penetration of light through the SC,30 which might be a possible explanation for the higher “SC reflectivity” on both forearm sites of the old group. Whether the intensive surface reflection is really caused by the structure of the SC or by slightly different refractive indices between air and skin is ambiguous.31 However, we observed an obvious difference between young and older skin. Significant differences between the age groups were also observed on both forearm sites for “upper dermal reflectivity” and “dermoepidermal contrast,” which assumes that these parameters in OCT images do most clearly change with age. The decreased “upper dermal reflectivity” which was pronounced on both forearm sites likely reflects the deposition of disorganized and highly backscattering elastin from the papillary dermis to the reticular dermis and the loss of collagen content resulting in a signal attenuation of the upper dermis.32,33 In close relation to less “upper dermal reflectivity” on both forearm sites is the substantial increase in “dermoepidermal contrast.” The more the reflectivity of the upper dermis decreases the higher is the ability to distinguish between epidermis and dermis. “Lower dermal reflectivity” seemed to be neither related to age nor to sun exposure. This finding is likely to be explained by the limited penetration depth and lateral resolution of the OCT signal leading to an increased signal noise and a diffuse appearance of the deeper dermis. We expected to observe age- and site-dependent differences in “vessel density” because horizontal cutaneous vessels have been shown to be increased in the elderly.34 In addition, there is evidence that photodamaged skin exhibits reduced numbers of dermal vessels compared to intrinsically aged skin.35 On the other hand, an increased scattering coefficient of the dermis can lead to the reduction of dermal reflectivity thus affecting the ability to visualize blood vessels.36 However, an age- and site-dependent change in “vessel density” could be shown in our sample supporting the construct validity of this item. We observed an increase in “surface unevenness” in older subjects indicating a rougher and more folded skin surface in this age group. Epidermal changes and dermal degeneration processes during aging are considered to promote increased skin roughness.37,38 We could confirm this finding by Visioscan® measurements. and are regarded as the most reliable roughness parameters for quantification of the skin surface topography22 and were, therefore, chosen as reference standards for criterion validation in this study. Mean and estimates were similar to previous findings in young and old women.22,23 All measured roughness estimates increased with age in this study, which is in line with previously reported studies.39,40 Validity of the item “surface unevenness” was demonstrated by correlation with both roughness estimates. The item scores were associated with and on the inner upper arm and volar forearm, supporting that these estimates measure a similar concept on these sun-protected skin areas. In conjunction with a slightly lower surface unevenness score on the photoaged dorsal forearm skin in the old group, our results support the hypothesis that chronic UV exposure reduces the effect of intrinsic aging on this skin area,41 indicating that sun damage counteracts increasing roughness during aging. Furthermore, the limited range of und values at the dorsal forearm attenuates observed validity coefficients thus explaining the low correlation. 4.1.LimitationsThis explorative study was conducted with a small number of subjects to obtain first indicators about possible characteristics of OCT images that might be related to changes due to skin aging. Generalizability of the results is limited and larger confirmatory studies are needed. In order to reduce a possible bias due to gender and ethnicity, we included female Caucasians only. Finally, comparability with other OCT devices might be limited. 4.2.ConclusionThis study describes new quantifiable skin characteristics of OCT images besides ET. The items “SC reflectivity,” “upper dermal reflectivity,” “dermoepidermal contrast,” and “surface unevenness” have shown sufficient interrater agreement and the ability to differentiate between age groups, thus being considered as the best candidates for measuring skin aging on OCT images. ReferencesL. H. Kligman,
“Photoaging. Manifestations, prevention, and treatment,”
Clin. Geriatr. Med., 5
(1), 235
–251
(1989). 0749-0690 Google Scholar
J. Uitto,
“The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure,”
J. Drugs Dermatol., 7
(2 Suppl), s12
–16
(2008). JDDOBA 1545-9616 Google Scholar
M. A. Farage et al.,
“Intrinsic and extrinsic factors in skin ageing: a review,”
Int. J. Cosmet. Sci., 30
(2), 87
–95
(2008). http://dx.doi.org/10.1111/j.1468-2494.2007.00415.x IJCMDW 0142-5463 Google Scholar
M. Coma et al.,
“Methods for diagnosing perceived age on the basis of an ensemble of phenotypic features,”
Clin. Cosmet. Investig. Dermatol., 7 133
–137
(2014). http://dx.doi.org/10.2147/CCID.S52257 Google Scholar
A. Nkengne et al.,
“Influence of facial skin attributes on the perceived age of Caucasian women,”
J. Eur. Acad. Dermatol. Venereol., 22
(8), 982
–991
(2008). http://dx.doi.org/10.1111/jdv.2008.22.issue-8 JEAVEQ 0926-9959 Google Scholar
T. Omi et al.,
“Histological evidence for skin rejuvenation using a combination of pneumatic energy, broadband light, and growth factor therapy,”
J. Cosmet. Laser Ther., 12
(5), 222
–226
(2010). http://dx.doi.org/10.3109/14764172.2010.502459 1476-4172 Google Scholar
J. Lademann et al.,
“Optical investigations to avoid the disturbing influences of furrows and wrinkles quantifying penetration of drugs and cosmetics into the skin by tape stripping,”
J. Biomed. Opt., 10
(5), 054015
(2005). http://dx.doi.org/10.1117/1.2055507 JBOPFO 1083-3668 Google Scholar
E. Sattler, R. Kastle and J. Welzel,
“Optical coherence tomography in dermatology,”
J. Biomed. Opt., 18
(6), 061224
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061224 JBOPFO 1083-3668 Google Scholar
T. Gambichler, V. Jaedicke and S. Terras,
“Optical coherence tomography in dermatology: technical and clinical aspects,”
Arch. Dermatol. Res., 303
(7), 457
–473
(2011). http://dx.doi.org/10.1007/s00403-011-1152-x ADREDL 0340-3696 Google Scholar
N. S. Greaves et al.,
“Optical coherence tomography: a reliable alternative to invasive histological assessment of acute wound healing in human skin?,”
Br. J. Dermatol., 170
(4), 840
–850
(2014). http://dx.doi.org/10.1111/bjd.2014.170.issue-4 BJDEAZ 1365-2133 Google Scholar
V. R. Korde et al.,
“Using optical coherence tomography to evaluate skin sun damage and precancer,”
Lasers Surg. Med., 39
(9), 687
–695
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
L. M. Vasquez-Pinto et al.,
“Optical coherence tomography applied to tests of skin care products in humans—a case study,”
Skin Res. Technol., 21
(1), 90
–93
(2015). http://dx.doi.org/10.1111/srt.2014.21.issue-1 0909-752X Google Scholar
J. K. Barton et al.,
“Investigating sun-damaged skin and actinic keratosis with optical coherence tomography: a pilot study,”
Technol. Cancer Res. Treat., 2
(6), 525
–535
(2003). http://dx.doi.org/10.1177/153303460300200605 1533-0346 Google Scholar
T. Gambichler et al.,
“In vivo data of epidermal thickness evaluated by optical coherence tomography: effects of age, gender, skin type, and anatomic site,”
J. Dermatol. Sci., 44
(3), 145
–152
(2006). http://dx.doi.org/10.1016/j.jdermsci.2006.09.008 JDSCEI 0923-1811 Google Scholar
S. Neerken et al.,
“Characterization of age-related effects in human skin: A comparative study that applies confocal laser scanning microscopy and optical coherence tomography,”
J. Biomed. Opt., 9
(2), 274
–281
(2004). http://dx.doi.org/10.1117/1.1645795 JBOPFO 1083-3668 Google Scholar
T. Tsugita et al.,
“Positional differences and aging changes in Japanese woman epidermal thickness and corneous thickness determined by OCT (optical coherence tomography),”
Skin Res. Technol., 19
(3), 242
–250
(2013). http://dx.doi.org/10.1111/srt.12021 0909-752X Google Scholar
N. Aoi et al.,
“Ultrasound assessment of deep tissue injury in pressure ulcers: possible prediction of pressure ulcer progression,”
Plast. Reconstr. Surg., 124
(2), 540
–550
(2009). http://dx.doi.org/10.1097/PRS.0b013e3181addb33 PRSUAS 0032-1052 Google Scholar
J. Lademann et al.,
“Application of optical non-invasive methods in skin physiology: a comparison of laser scanning microscopy and optical coherent tomography with histological analysis,”
Skin Res. Technol., 13
(2), 119
–132
(2007). http://dx.doi.org/10.1111/srt.2007.13.issue-2 0909-752X Google Scholar
M. Mogensen et al.,
“Morphology and epidermal thickness of normal skin imaged by optical coherence tomography,”
Dermatology, 217
(1), 14
–20
(2008). http://dx.doi.org/10.1159/000118508 DERMEI 0742-3217 Google Scholar
M. Kuck et al.,
“Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing,”
Skin Res. Technol., 20
(1), 1
–7
(2014). http://dx.doi.org/10.1111/srt.12077 0909-752X Google Scholar
D. L. Streiner, G. R. Norman and J. Cairney,
“Scaling responses,”
Health Measurement Scales. A Practical Guide to Their Development and Use, 38
–73 Oxford University Press, Oxford
(2015). Google Scholar
C. Trojahn et al.,
“Reliability and validity of two in vivo measurements for skin surface topography in aged adults,”
Skin Res. Technol., 21
(1), 54
–60
(2014). http://dx.doi.org/10.1111/srt.12156 0909-752X Google Scholar
J. Kottner et al.,
“Comparison of two in vivo measurements for skin surface topography,”
Skin Res. Technol., 19
(2), 84
–90
(2013). http://dx.doi.org/10.1111/srt.12009 0909-752X Google Scholar
J. Kottner and D. L. Streiner,
“The difference between reliability and agreement,”
J. Clin. Epidemiol., 64
(6), 701
–702
(2011). http://dx.doi.org/10.1016/j.jclinepi.2010.12.001 JCEPEE 0895-4356 Google Scholar
C. Longo et al.,
“Skin aging: in vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy,”
J. Am. Acad. Dermatol., 68
(3), e73
–82
(2013). http://dx.doi.org/10.1016/j.jaad.2011.08.021 JAADDB 0190-9622 Google Scholar
G. L. Grove and A. M. Kligman,
“Age-associated changes in human epidermal cell renewal,”
J. Gerontol., 38
(2), 137
–142
(1983). http://dx.doi.org/10.1093/geronj/38.2.137 JOGEA3 0022-1422 Google Scholar
J. L. Leveque et al.,
“In vivo studies of the evolution of physical properties of the human skin with age,”
Int. J. Dermatol., 23
(5), 322
–329
(1984). http://dx.doi.org/10.1111/ijd.1984.23.issue-5 IJDEBB 0011-9059 Google Scholar
M. Kawai, G. Imokawa and M. Mizoguchi,
“Physiological analysis of the facial skin by corneocyte morphology and stratum corneum turnover,”
Nihon Hifuka Gakkai Zasshi, 99
(9), 999
–1006
(1989). Google Scholar
J. Rogers et al.,
“Stratum corneum lipids: the effect of ageing and the seasons,”
Arch. Dermatol. Res., 288
(12), 765
–770
(1996). http://dx.doi.org/10.1007/BF02505294 ADREDL 0340-3696 Google Scholar
J. L. Solan and K. Laden,
“Factors affecting the penetration of light through stratum corneum,”
J. Soc. Cosmet. Chem., 28
(3), 125
–137
(1977). JSCCA5 Google Scholar
J. Welzel et al.,
“Changes in function and morphology of normal human skin: evaluation using optical coherence tomography,”
Br. J. Dermatol., 150
(2), 220
–225
(2004). http://dx.doi.org/10.1111/bjd.2004.150.issue-2 BJDEAZ 1365-2133 Google Scholar
M. El-Domyati et al.,
“Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin,”
Exp. Dermatol., 11
(5), 398
–405
(2002). http://dx.doi.org/10.1034/j.1600-0625.2002.110502.x EXDEEY 0906-6705 Google Scholar
J. H. Chung et al.,
“Modulation of skin collagen metabolism in aged and photoaged human skin in vivo,”
J. Invest. Dermatol., 117
(5), 1218
–1224
(2001). http://dx.doi.org/10.1046/j.0022-202x.2001.01544.x JIDEAE 0022-202X Google Scholar
R. I. Kelly et al.,
“The effects of aging on the cutaneous microvasculature,”
J. Am. Acad. Dermatol., 33
(5 Pt 1), 749
–756
(1995). http://dx.doi.org/10.1016/0190-9622(95)91812-4 JAADDB 0190-9622 Google Scholar
J. H. Chung et al.,
“Differential effects of photoaging vs intrinsic aging on the vascularization of human skin,”
Arch. Dermatol., 138
(11), 1437
–1442
(2002). http://dx.doi.org/10.1001/archderm.138.11.1437 ARDEAC 0003-987X Google Scholar
T. Gambichler et al.,
“Applications of optical coherence tomography in dermatology,”
J. Dermatol. Sci., 40
(2), 85
–94
(2005). http://dx.doi.org/10.1016/j.jdermsci.2005.07.006 JDSCEI 0923-1811 Google Scholar
E. M. Wurm et al.,
“In vivo assessment of chronological ageing and photoageing in forearm skin using reflectance confocal microscopy,”
Br. J. Dermatol., 167
(2), 270
–279
(2012). http://dx.doi.org/10.1111/bjd.2012.167.issue-2 BJDEAZ 1365-2133 Google Scholar
S. G. Lagarrigue et al.,
“In vivo quantification of epidermis pigmentation and dermis papilla density with reflectance confocal microscopy: variations with age and skin phototype,”
Exp. Dermatol., 21
(4), 281
–286
(2012). http://dx.doi.org/10.1111/exd.2012.21.issue-4 EXDEEY 0906-6705 Google Scholar
K. De Paepe et al.,
“Microrelief of the skin using a light transmission method,”
Arch. Dermatol. Res., 292
(10), 500
–510
(2000). http://dx.doi.org/10.1007/s004030000166 ADREDL 0340-3696 Google Scholar
U. Jacobi et al.,
“In vivo determination of skin surface topography using an optical 3D device,”
Skin Res. Technol., 10
(4), 207
–214
(2004). http://dx.doi.org/10.1111/srt.2004.10.issue-4 0909-752X Google Scholar
C. Edwards, R. Heggie and R. Marks,
“A study of differences in surface roughness between sun-exposed and unexposed skin with age,”
Photodermatol. Photoimmunol. Photomed., 19
(4), 169
–174
(2003). http://dx.doi.org/10.1034/j.1600-0781.2003.00042.x PPPHEW 0905-4383 Google Scholar
BiographyCarina Trojahn received her diploma in human biology from the University of Marburg in 2010 and worked as a project manager of clinical studies in the field of dermatology and cosmetic science. In 2013, she started her PhD at the Clinical Research Center for Hair and Skin Science at the Department of Dermatology and Allergy at the Charité-Universitätsmedizin Berlin. Her research focuses on validation of methods for measuring structural and functional changes during skin aging. Gabor Dobos received his degree in medicine from the Semmelweis University of Budapest in 2012. In the same year, he started his PhD at the Clinical Research Center for Hair and Skin Science at the Department of Dermatology and Allergy at the Charité-Universitätsmedizin Berlin. His research focuses on skin diseases of the elderly, validation of measurement techniques, and clinical scores in the context of skin ageing. Claudia Richter received her master’s of English philology from the University of Halle-Wittenberg in 2007 and worked as study coordinator of clinical studies in the field of dermatology. In 2014, she started her PhD at the Clinical Research Center for Hair and Skin Science at the Department of Dermatology and Allergy at the Charité-Universitätsmedizin Berlin. Her research focuses on skin barrier function, skin physiological measurement techniques, and dermatological disorders, e.g., acne vulgaris. Ulrike Blume-Peytavi is a full university professor and the executive medical director of the Department of Dermatology and Allergy at the Charité-Universitätsmedizin Berlin. She is the director of the Clinical Research Center for Hair and Skin Science. In 2013, she became the president of the German Association of Pediatric Dermatology. Her research focuses on skin and hair physiology, pediatric dermatology, follicular targeting, and transcutaneous vaccination. Jan Kottner is the scientific director of the Clinical Research Center for Hair and Skin Science at the Department of Dermatology and Allergy at the Charité-Universitätsmedizin Berlin. He is an executive board member of the EPUAP. His research interests are skin barrier maintenance, protection, restoration, and skin problems and skin care interventions in aged and care-dependent subjects. He also has special expertise in the development and validation of clinical diagnoses, classifications, and scores. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

